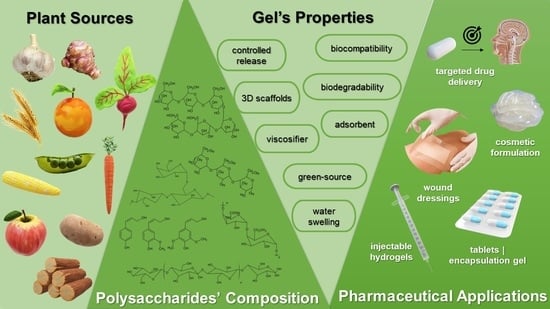
Plant Polysaccharides in Engineered Pharmaceutical Gels
Abstract
:1. Introduction
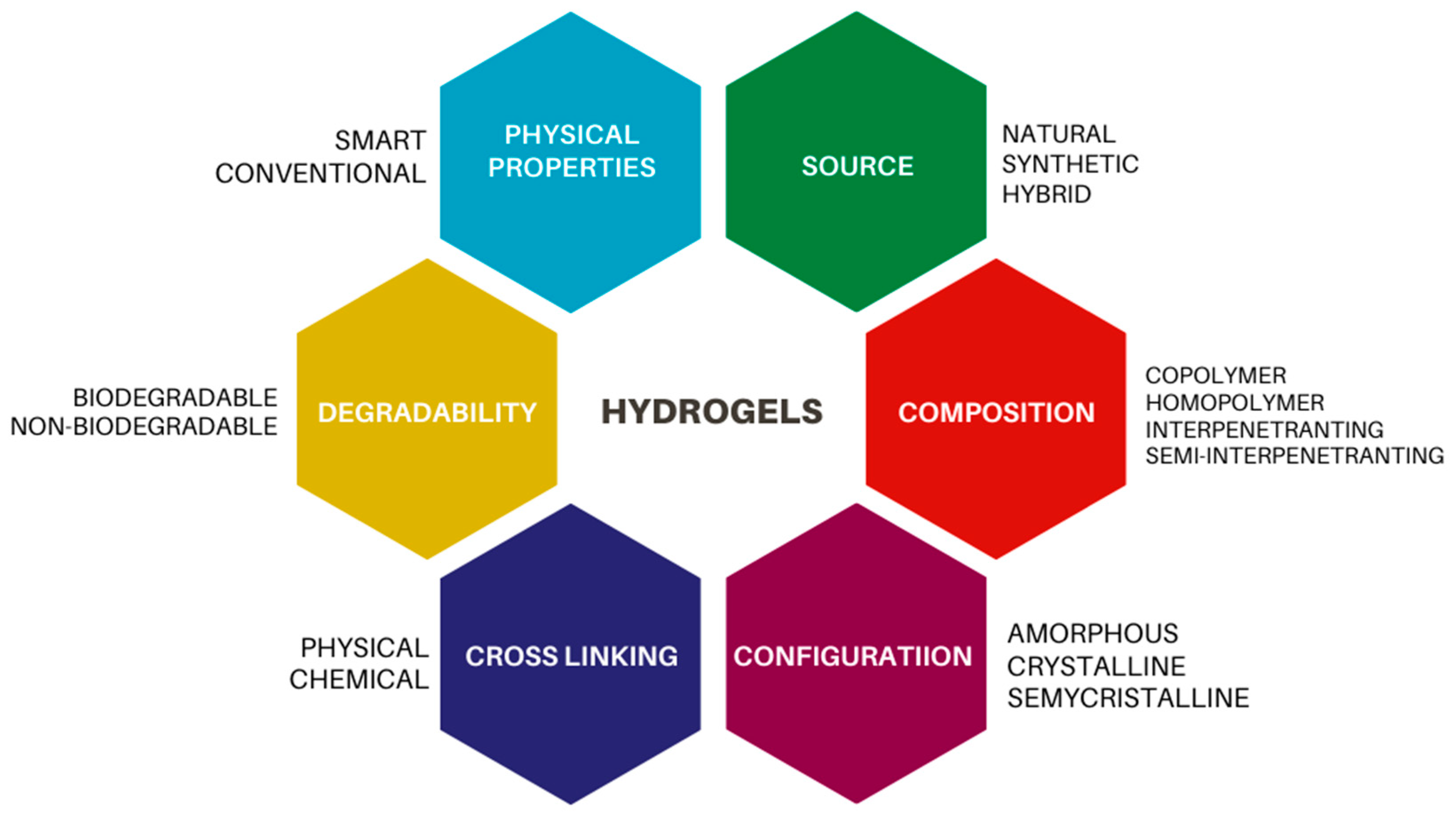
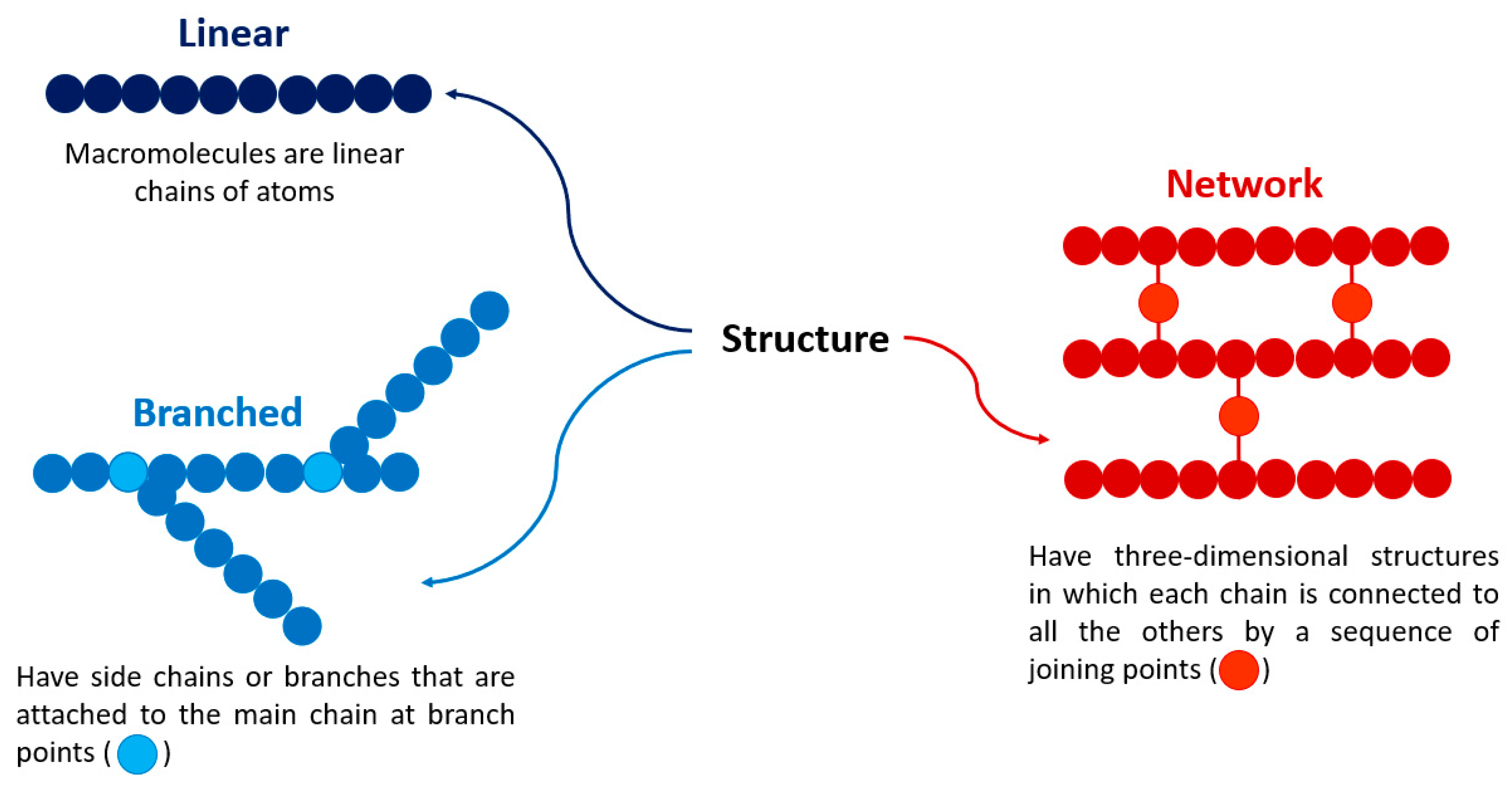
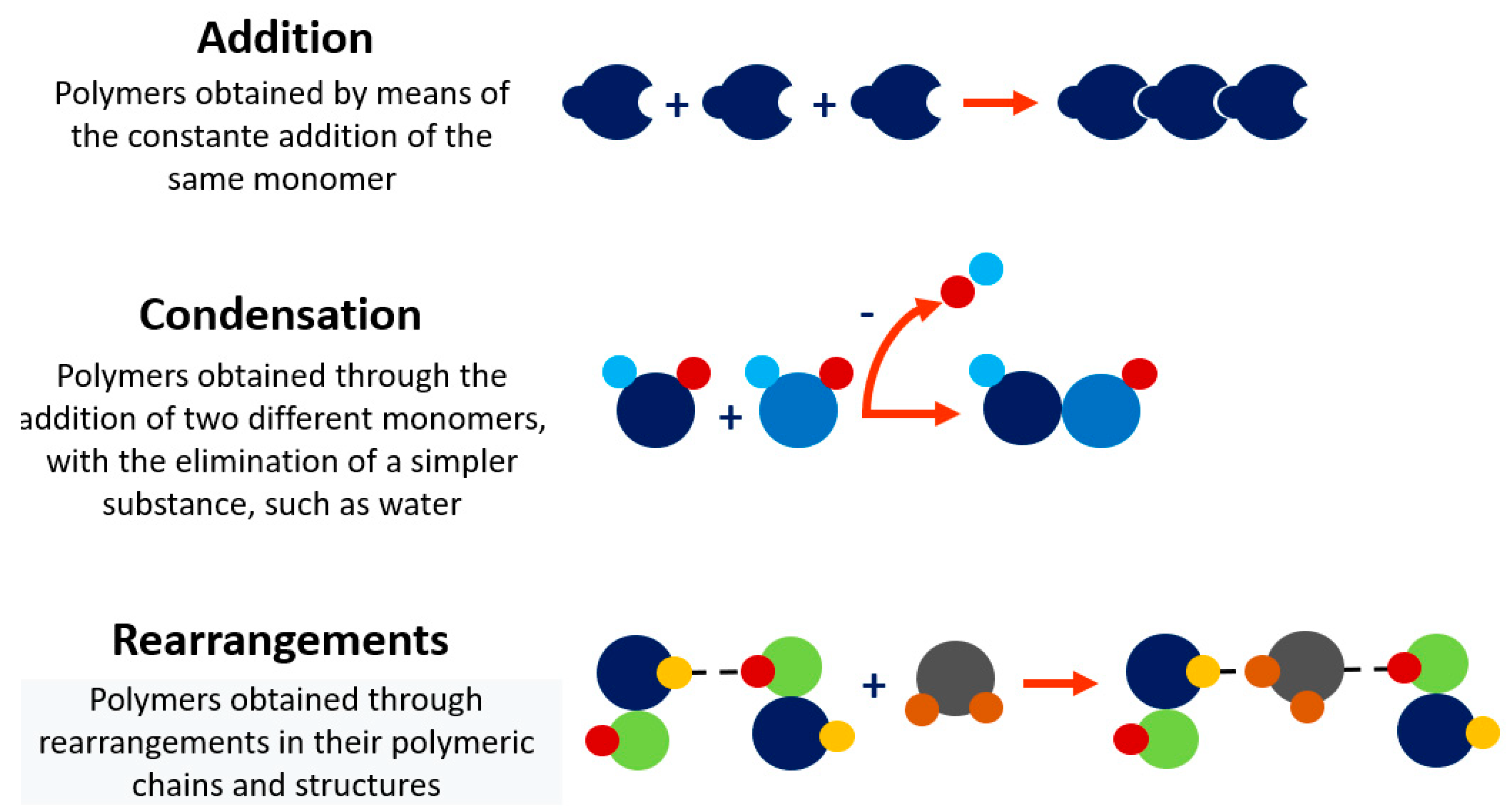
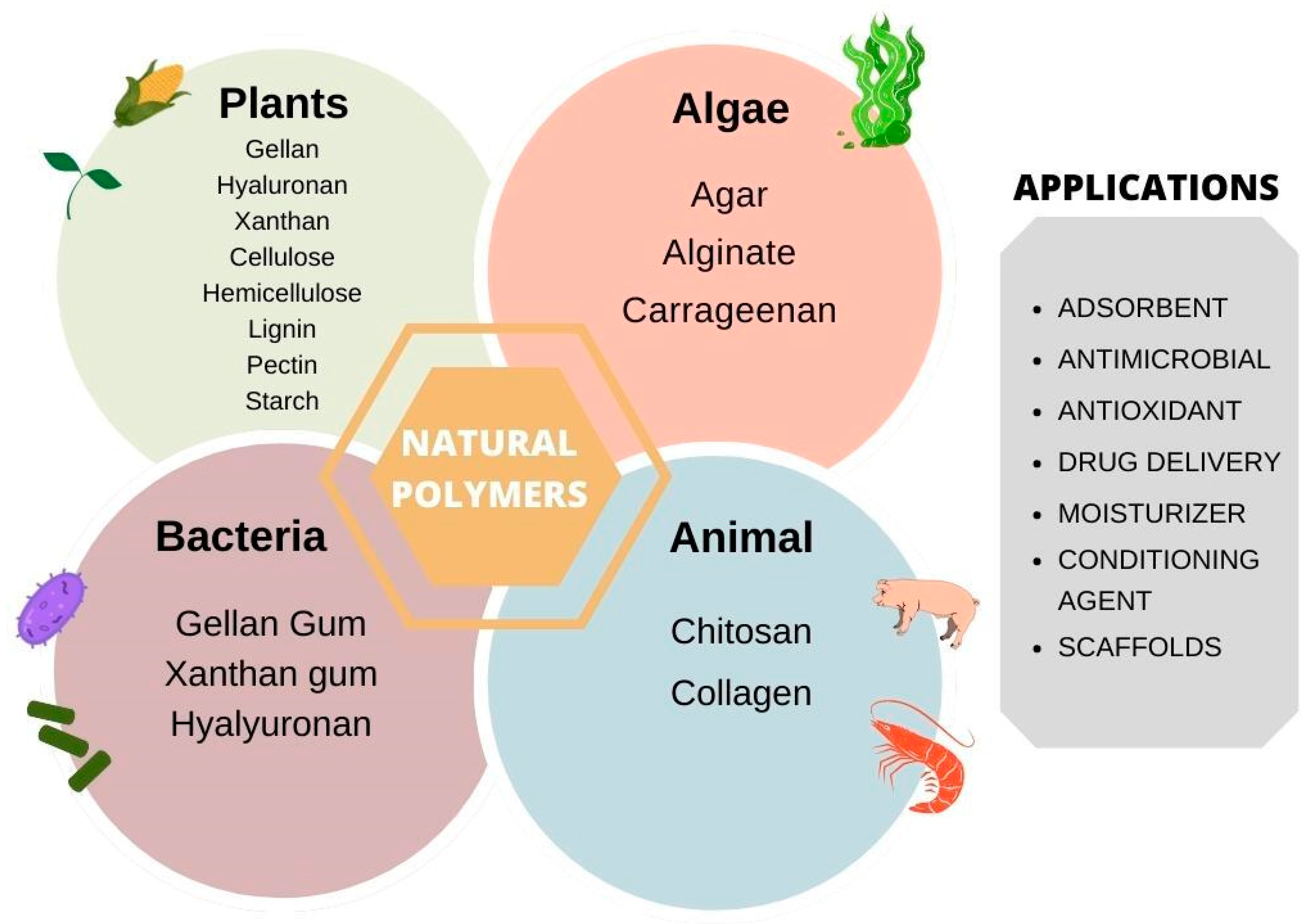
2. Polymers
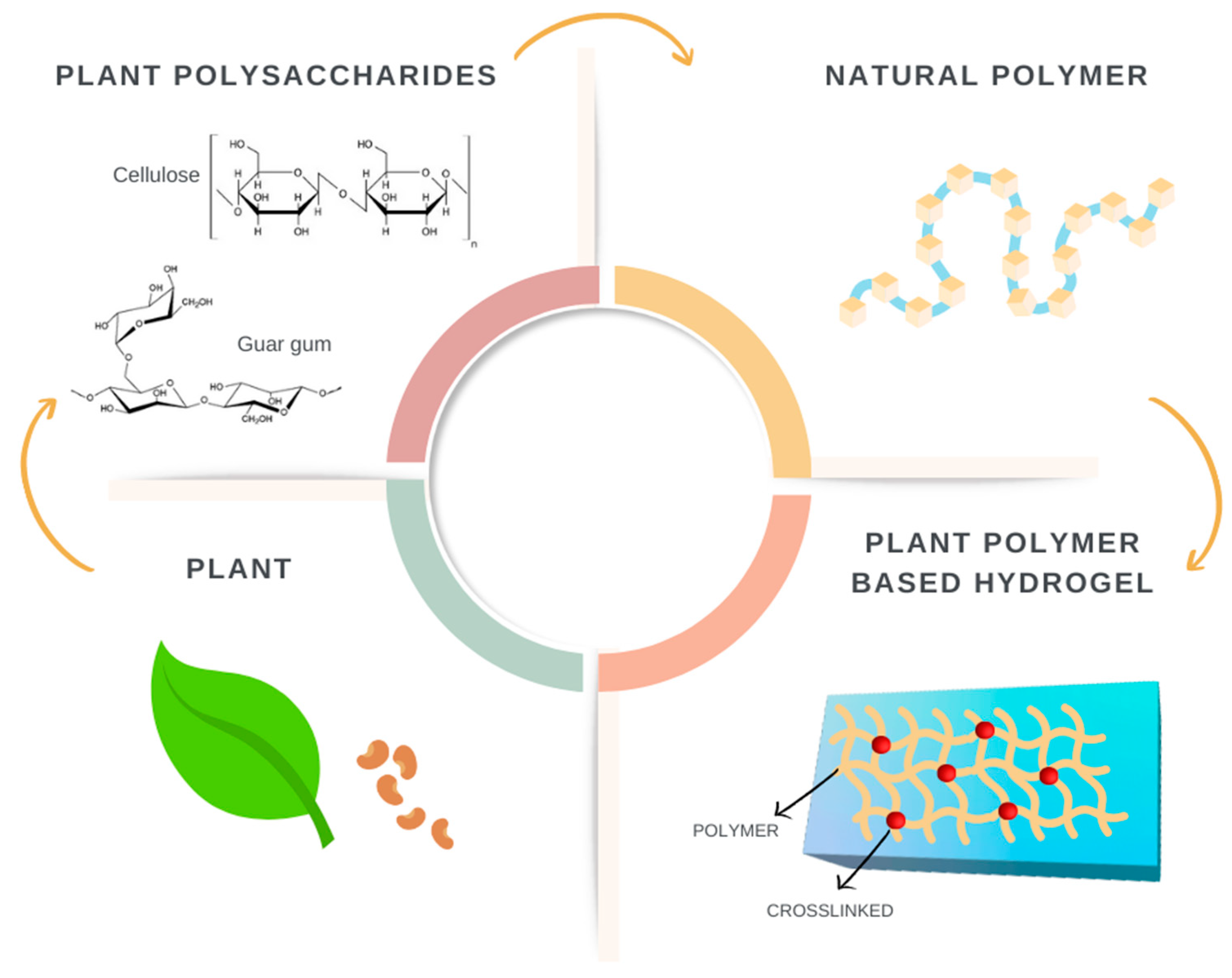
3. Plant-Derived Polymers
3.1. Developments, Characteristics, and Characterization
3.2. Cellulose
3.3. Hemicelluloses
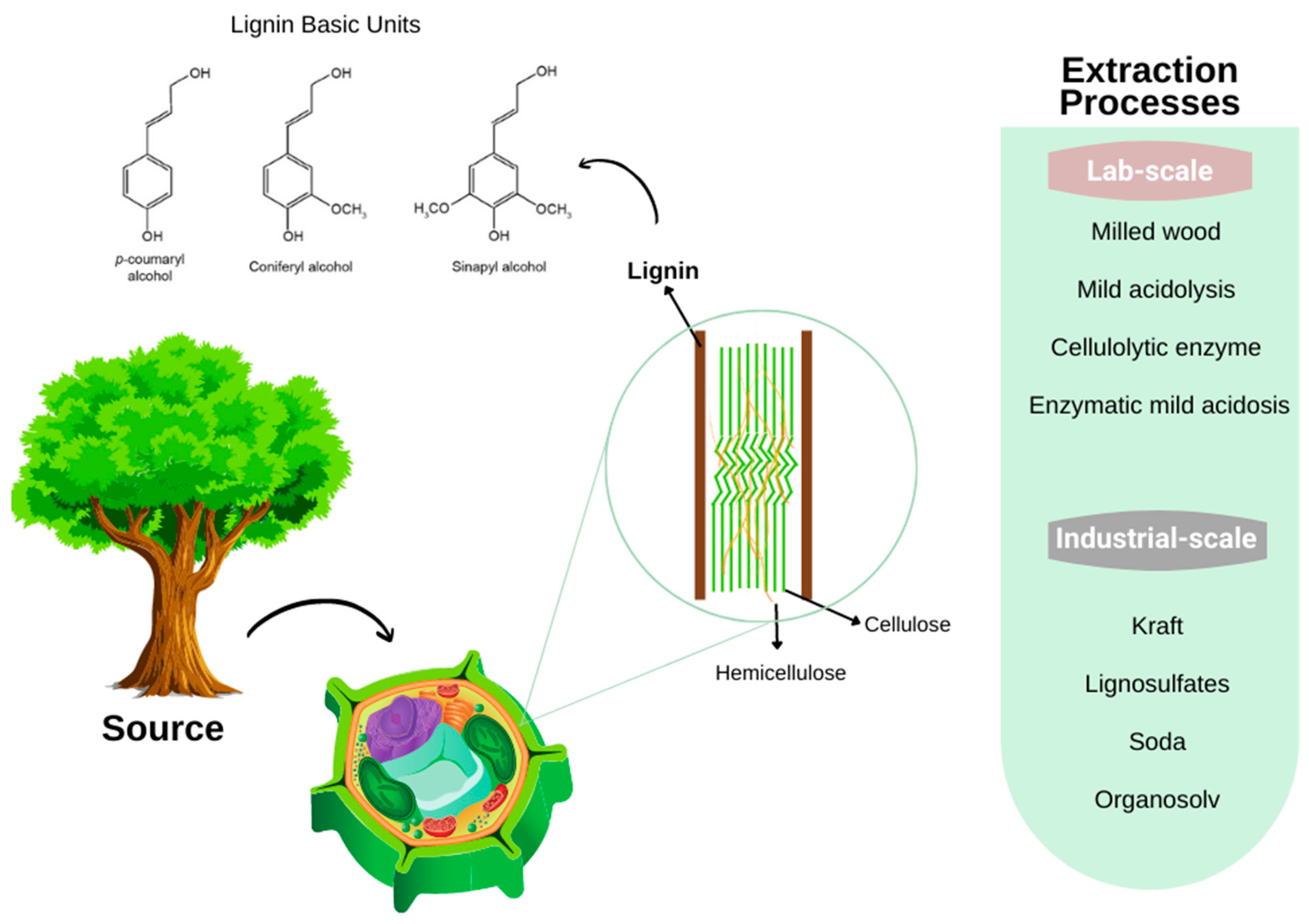
3.4. Lignin
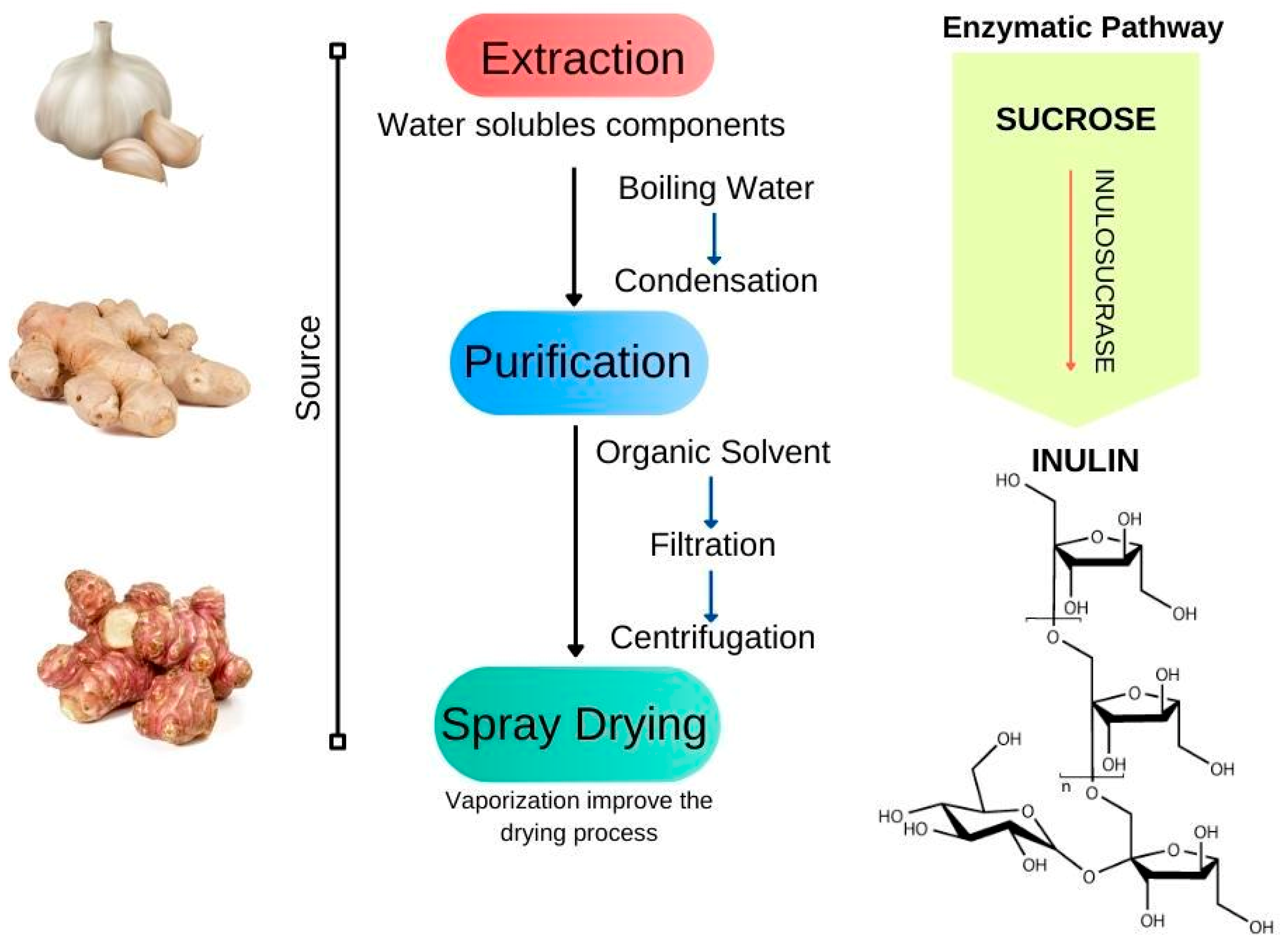
3.5. Inulin
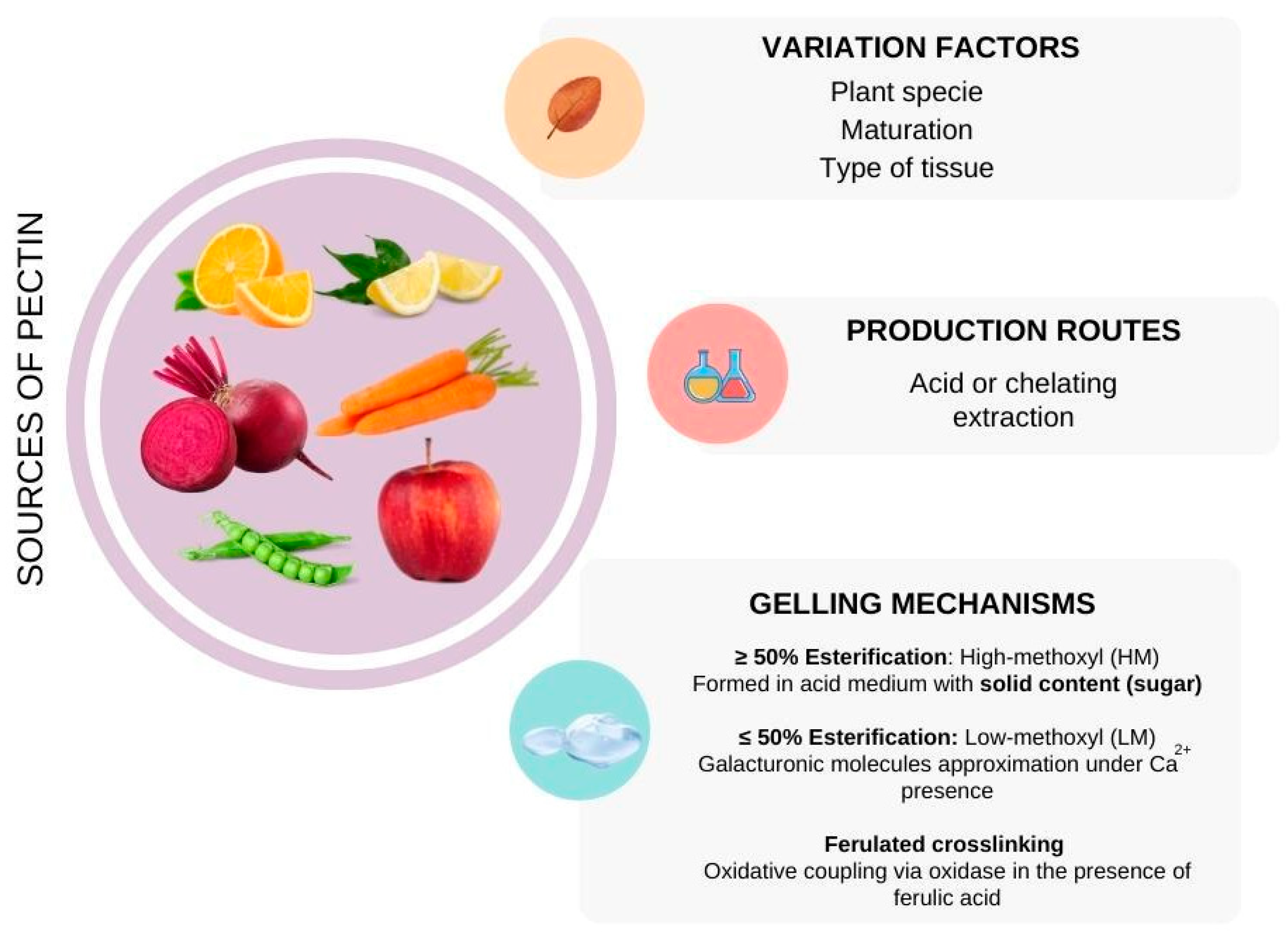
3.6. Pectin
3.7. Starch
3.8. Guar Gum
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jose, G.; Shalumon, K.; Chen, J.-P. Natural Polymers Based Hydrogels for Cell Culture Applications. Curr. Med. Chem. 2020, 27, 2734–2776. [Google Scholar] [CrossRef]
- Zielińska, A.; Eder, P.; Rannier, L.; Cardoso, J.C.; Severino, P.; Silva, A.M.; Souto, E.B. Hydrogels for modified-release drug delivery systems. Curr. Pharm. Des. 2021, 28, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Liu, Y.; Cao, Y. Formic acid: A versatile renewable reagent for green and sustainable chemical synthesis. Chin. J. Catal. 2015, 36, 1461–1475. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Takahashi, R. Interaction in polysaccharide solutions and gels. Curr. Opin. Colloid Interface Sci. 2003, 8, 396–400. [Google Scholar] [CrossRef]
- Eelkema, R.; Pich, A. Pros and cons: Supramolecular or macromolecular: What is best for functional hydrogels with advanced properties? Adv. Mater. 2020, 32, 1906012. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hokmabad, V.R.; Bakhshayesh, D.; Rahmani, A.; Asadi, N.; Salehi, R.; Nasrabadi, H.T. The application of hydrogels based on natural polymers for tissue engineering. Curr. Med. Chem. 2020, 27, 2658–2680. [Google Scholar] [CrossRef] [PubMed]
- Palivan, C.G.; Fischer-Onaca, O.; Delcea, M.; Itel, F.; Meier, W. Protein–polymer nanoreactors for medical applications. Chem. Soc. Rev. 2012, 41, 2800–2823. [Google Scholar] [CrossRef]
- Bačáková, L.; Novotná, K.; Pařízek, M. Polysaccharides as cell carriers for tissue engineering: The use of cellulose in vascular wall reconstruction. Physiol. Res. 2014, 63, S29. [Google Scholar] [CrossRef] [PubMed]
- Gawel, E.; Pannicke, N.; Hagemann, N. A Path Transition Towards a Bioeconomy—The Crucial Role of Sustainability. Sustainability 2019, 11, 3005. [Google Scholar] [CrossRef]
- Aeridou, E.; Díaz, D.D.; Alemán, C.; Pérez-Madrigal, M.M. Advanced Functional Hydrogel Biomaterials Based on Dynamic B–O Bonds and Polysaccharide Building Blocks. Biomacromolecules 2020, 21, 3984–3996. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni Vishakha, S.; Butte Kishor, D.; Rathod Sudha, S. Natural polymers—A comprehensive review. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 1597–1613. [Google Scholar]
- Rinaudo, M. Characterization and properties of some polysaccharides used as biomaterials. In Macromolecular Symposia; Wiley-VCH Verlag: Weinheim, Germany, 2006; Volume 245, pp. 549–557. [Google Scholar]
- Verma, D.; Sharma, S.K. Recent advances in guar gum based drug delivery systems and their administrative routes. Int. J. Biol. Macromol. 2021, 181, 653–671. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Auriemma, G.; Russo, P.; Del Gaudio, P.; García-González, C.A.; Landín, M.; Aquino, R.P. Technologies and Formulation Design of Polysaccharide-Based Hydrogels for Drug Delivery. Molecules 2020, 25, 3156. [Google Scholar] [CrossRef]
- Ferreira, N.; Ferreira, L.; Cardoso, V.; Boni, F.; Souza, A.; Gremião, M. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- De Oliveira, D.M.; Menezes, D.B.; Andrade, L.R.; Lima, F.D.C.; Hollanda, L.; Zielinska, A.; Sanchez-Lopez, E.; Souto, E.B.; Severino, P. Silver nanoparticles obtained from Brazilian pepper extracts with synergistic anti-microbial effect: Production, characterization, hydrogel formulation, cell viability, and in vitro efficacy. Pharm. Dev. Technol. 2021, 26, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Jayaramudu, T.; Ko, H.-U.; Kim, H.C.; Kim, J.W.; Muthoka, R.M.; Kim, J. Electroactive Hydrogels Made with Polyvinyl Alcohol/Cellulose Nanocrystals. Materials 2018, 11, 1615. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.Y.; Ahmad, I.; Amin, M.C.I.M. Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatin hydrogels for use in controlled drug delivery systems. Ind. Crops Prod. 2016, 93, 227–234. [Google Scholar] [CrossRef]
- Hu, L.; Du, M.; Zhang, J. Hemicellulose-Based Hydrogels Present Status and Application Prospects: A Brief Review. Open J. For. 2018, 8, 15–28. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Lignin-based hydrogels: A review of preparation, properties, and application. Int. J. Biol. Macromol. 2019, 135, 1006–1019. [Google Scholar] [CrossRef]
- Glasser, W.G. About Making Lignin Great Again—Some Lessons from the Past. Front. Chem. 2019, 7, 565. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Fouladian, P.; Parikh, A.; Barclay, T.G.; Song, Y.; Garg, S. Preparation and Characterization of Oxidized Inulin Hydrogel for Controlled Drug Delivery. Pharmaceutics 2019, 11, 356. [Google Scholar] [CrossRef]
- Florowska, A.; Hilal, A.; Florowski, T.; Mrozek, P.; Wroniak, M. Sodium Alginate and Chitosan as Components Modifying the Properties of Inulin Hydrogels. Gels 2022, 8, 63. [Google Scholar] [CrossRef]
- Kim, M.-S.; Chandika, P.; Jung, W.-K. Recent advances of pectin-based biomedical application: Potential of marine pectin. J. Mar. Biosci. Biotechnol. 2021, 13, 28–47. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Polym. Environ. 2022, 30, 19–50. [Google Scholar] [CrossRef]
- Chakraborty, I.; Pooja, N.; Mal, S.S.; Paul, U.C.; Rahman, M.H.; Mazumder, N. An Insight into the Gelatinization Properties Influencing the Modified Starches Used in Food Industry: A review. Food Bioprocess Technol. 2022, 15, 1195–1223. [Google Scholar] [CrossRef]
- Le, T.-A.; Zouheir, M.; Nikiforow, K.; Khatib, M.; Zohar, O.; Haick, H.; Huynh, T.-P. Synthesis, characterization, and humidity-responsiveness of guar gum xanthate and its nanocomposite with copper sulfide covellite. Int. J. Biol. Macromol. 2022, 206, 105–114. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y.; Xie, R.; Zhong, H.; Zhao, W.; Liu, Y.; Yang, H. Rapid Gelling of Guar Gum Hydrogel Stabilized by Copper Hydroxide Nanoclusters for Efficient Removal of Heavy Metal and Supercapacitors. Front. Chem. 2021, 9, 794755. [Google Scholar] [CrossRef] [PubMed]
- Azeera, M.; Vaidevi, S.; Ruckmani, K. Characterization Techniques of Hydrogel and Its Applications. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M., Ibrahim, H., Eds.; Springer International: Cham, Switzerland, 2019; pp. 737–761. [Google Scholar] [CrossRef]
- Ortega, E.O.; Hosseinian, H.; Meza, I.B.A.; López, M.J.R.; Vera, A.R.; Hosseini, S. Material Characterization Techniques and Applications, 15th ed.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Kang, H.; Liu, R.; Huang, Y. Cellulose-Based Gels. Macromol. Chem. Phys. 2016, 217, 1322–1334. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Zhang, J.; Du, X.; Wu, S.; Wang, H.; Wei, X. A novel ε-polylysine-modified microcrystalline cellulose based antibacterial hydrogel for removal of heavy metal. Int. J. Biol. Macromol. 2020, 163, 1915–1925. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; Liu, H.; Ma, L.; Huang, X.; Cai, Z.; Xu, X.; Shang, S.; Song, Z. Cellulose-based polymeric emulsifier stabilized poly(N-vinylcaprolactam) hydrogel with temperature and pH responsiveness. Int. J. Biol. Macromol. 2020, 143, 190–199. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Song, M.-H.; Pham, T.P.T.; Yun, Y.-S. Ionic liquid-assisted cellulose coating of chitosan hydrogel beads and their application as drug carriers. Sci. Rep. 2020, 10, 13905. [Google Scholar] [CrossRef]
- Llanes, L.; Dubessay, P.; Pierre, G.; Delattre, C.; Michaud, P. Biosourced Polysaccharide-Based Superabsorbents. Polysaccharides 2020, 1, 51–79. [Google Scholar] [CrossRef]
- Yan, G.; Chen, B.; Zeng, X.; Sun, Y.; Tang, X.; Lin, L. Recent advances on sustainable cellulosic materials for pharmaceutical carrier applications. Carbohydr. Polym. 2020, 244, 116492. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, R. Cellulose gels and microgels: Synthesis, service, and supramolecular interactions. In Supramolecular Polymer Networks and Gels; Seiffert, S., Ed.; Spinger: Cham, Switzerland, 2015; pp. 209–251. [Google Scholar]
- Sun, B.; Zhang, M.; Shen, J.; He, Z.; Fatehi, P.; Ni, Y. Applications of cellulose-based materials in sustained drug delivery systems. Curr. Med. Chem. 2019, 26, 2485–2501. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Komorowska, P.; Różański, J. Applications and Properties of Physical Gels Obtained on the Basis of Cellulose Derivatives. In Practical Aspects of Chemical Engineering; Springer: Cham, Switzerland, 2018; pp. 185–200. [Google Scholar]
- Cao, Y. Applications of cellulose nanomaterials in pharmaceutical science and pharmacology. Express Polym. Lett. 2018, 12, 768–780. [Google Scholar] [CrossRef]
- Bajerová, M.; Krejčová, K.; Rabišková, M.; Muselík, J.; Dvořáčková, K.; Gajdziok, J.; Masteiková, R. Oxycellulose beads with drug exhibiting pH-dependent solubility. AAPS PharmSciTech 2011, 12, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.S.; Ahmad, A.A. Diclofenac sodium loaded-cellulose acetate butyrate: Effect of processing variables on microparticles properties, drug release kinetics and ulcerogenic activity. J. Microencapsul. 2008, 25, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Tarrahi, R.; Khataee, A.; Karimi, A.; Yoon, Y. The latest achievements in plant cellulose-based biomaterials for tissue engineering focusing on skin repair. Chemosphere 2022, 288, 132529. [Google Scholar] [CrossRef]
- Krüger, M.; Oosterhoff, L.A.; van Wolferen, M.E.; Schiele, S.; Walther, A.; Geijsen, N.; De Laporte, L.; van der Laan, L.; Kock, L.M.; Spee, B. Cellulose Nanofibril Hydrogel Promotes Hepatic Differentiation of Human Liver Organoids. Adv. Health Mater. 2020, 9, 1901658. [Google Scholar] [CrossRef]
- Liu, R.; Dai, L.; Si, C.; Zeng, Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr. Polym. 2018, 195, 63–70. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, G.; Dufresne, A.; Lin, N. Fluorescent Aerogels Based on Chemical Crosslinking between Nanocellulose and Carbon Dots for Optical Sensor. ACS Appl. Mater. Interfaces 2019, 11, 16048–16058. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qamar, S.A.; Qamar, M.; Basharat, K.; Bilal, M. Engineered nanocellulose-based hydrogels for smart drug delivery applications. Int. J. Biol. Macromol. 2021, 181, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.; Mohammadi, M. The Use of Cellulose Nanocrystals for Potential Application in Topical Delivery of Hydroquinone. Chem. Biol. Drug Des. 2015, 86, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-Responsive Poly(N-Isopropylacrylamide)-Cellulose Nanocrystals Hybrid Hydrogels for Wound Dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Yang, J.; Han, C. Mechanically Viscoelastic Properties of Cellulose Nanocrystals Skeleton Reinforced Hierarchical Composite Hydrogels. ACS Appl. Mater. Interfaces 2016, 8, 25621–25630. [Google Scholar] [CrossRef]
- Rees, A.; Powell, L.C.; Chinga-Carrasco, G.; Gethin, D.T.; Syverud, K.; Hill, K.E.; Thomas, D.W. 3D bioprinting of carboxymethylated-periodate oxidized nanocellulose constructs for wound dressing applications. BioMed Res. Int. 2015, 2015, 925757. [Google Scholar] [CrossRef]
- Alonso, J.M.; Andrade del Olmo, J.; Perez Gonzalez, R.; Saez-Martinez, V. Injectable hydrogels: From laboratory to industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef]
- Jacquart, S.; Girod-Fullana, S.; Brouillet, F.; Pigasse, C.; Siadous, R.; Fatnassi, M.; Grimoud, J.; Rey, C.; Roques, C.; Combes, C. Injectable bone cement containing carboxymethyl cellulose microparticles as a silver delivery system able to reduce implant-associated infection risk. Acta Biomater. 2022, 145, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Zare-Akbari, Z.; Farhadnejad, H.; Furughi-Nia, B.; Abedin, S.; Yadollahi, M.; Khorsand-Ghayeni, M. PH-sensitive bionanocomposite hydrogel beads based on carboxymethyl cellulose/ZnO nanoparticle as drug carrier. Int. J. Biol. Macromol. 2016, 93, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohydr. Polym. 2020, 256, 117561. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Barsett, H.; Ebringerová, A.; Harding, S.; Heinze, T.; Hromádková, Z.; Muzzarelli, C.; Muzzarelli, R.; Paulsen, B.; El Seoud, O. Polysaccharides I: Structure, Characterization and Use; Heinze, T., Ed.; Spinger: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Dulie, N.W.; Woldeyes, B.; Demsash, H.D.; Jabasingh, A.S. An Insight into the Valorization of Hemicellulose Fraction of Biomass into Furfural: Catalytic Conversion and Product Separation. Waste Biomass-Valorization 2021, 12, 531–552. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.A.; Hubbe, M.; Taha, M.; Becquart, F.; Ayoub, A. A Review of Water-Resistant Hemicellulose-Based Materials: Processing and Applications. ChemSusChem 2017, 10, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.E.; Silva, A.E.; Júnior, T.N.; Gomes, M.C.S.; Aguiar, L.M.; Marcelino, H.R.; Araújo, I.B.; Bayer, M.P.; Ricardo, N.; de Oliveira, A.G. Xylan from corn cobs, a promising polymer for drug delivery: Production and characterization. Bioresour. Technol. 2010, 101, 5402–5406. [Google Scholar] [CrossRef] [PubMed]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.M. Hemicellulose biorefineries: A review on biomass pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864. [Google Scholar]
- Shiva; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; López-Sandin, I.; Morais, A.R.C.; Ruiz, H.A. Enzymatic Hydrolysis, Kinetic Modeling of Hemicellulose Fraction, and Energy Efficiency of Autohydrolysis Pretreatment Using Agave Bagasse. BioEnergy Res. 2022. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, S.; Ma, M.-G.; Ji, X.-X.; Choi, S.-E.; Si, C. The Kinetics Studies on Hydrolysis of Hemicellulose. Front. Chem. 2021, 9, 781291. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.; Zhang, R.; Xiao, L.; Yuan, T.; Shi, Q.; Sun, R. Evaluation of xylooligosaccharide production from residual hemicelluloses of dissolving pulp by acid and enzymatic hydrolysis. RSC Adv. 2018, 8, 35211–35217. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Dai, Q.; Gao, C.; Ren, J.; Liu, C.; Sun, R. Hemicellulose-based hydrogels and their potential application. In Polymer Gels; Thakur, V., Thakur, M., Eds.; Springer: Singapore, 2018; pp. 87–127. [Google Scholar]
- Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquah, M.K.; Bechelany, M. Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale 2020, 12, 22845–22890. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-F.; Wang, H.-h.; Jing, Z.-X.; Mohanathas, R. Hemicellulose-based pH-sensitive and biodegradable hydrogel for controlled drug delivery. Carbohydr. Polym. 2013, 92, 1357–1366. [Google Scholar] [CrossRef]
- Melandri, D.; De Angelis, A.; Orioli, R.; Ponzielli, G.; Lualdi, P.; Giarratana, N.; Reiner, V. Use of a new hemicellulose dressing (Veloderm®) for the treatment of split-thickness skin graft donor sites: A within-patient controlled study. Burns 2006, 32, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, M.; Alruwaili, N.; Alrowaili, Z.; Alomar, F.; Akhtar, S.; Alsaidan, O.; Alhakamy, N.; Zafar, A.; Elmowafy, M.; et al. Antibiotic-Loaded Psyllium Husk Hemicellulose and Gelatin-Based Polymeric Films for Wound Dressing Application. Pharmaceutics 2021, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Odelius, K.; Edlund, U.; Zhao, C.; Albertsson, A.-C. In Situ Synthesis of Magnetic Field-Responsive Hemicellulose Hydrogels for Drug Delivery. Biomacromolecules 2015, 16, 2522–2528. [Google Scholar] [CrossRef]
- Ferrari, E.; Ranucci, E.; Edlund, U.; Albertsson, A.-C. Design of renewable poly(amidoamine)/hemicellulose hydrogels for heavy metal adsorption. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Chowdhury, M.A. The controlled release of bioactive compounds from lignin and lignin-based biopolymer matrices. Int. J. Biol. Macromol. 2014, 65, 136–147. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Cárcamo-Martínez, Á.; Stewart, S.A.; Donnelly, R.F.; Larrañeta, E.; Borrega, M. Lignin for pharmaceutical and biomedical applications–Could this become a reality? Sustain. Chem. Pharm. 2020, 18, 100320. [Google Scholar] [CrossRef]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro-and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Rico-García, D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Hernández-Olmos, S.L.; Guerrero-Ramírez, G.L.; Vilas-Vilela, J.L. Lignin-based hydrogels: Synthesis and applications. Polymers 2020, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.; Martins, M.; Barros, A.; Gurikov, P.; Raman, S.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Gil-Chávez, J.; Gurikov, P.; Hu, X.; Meyer, R.; Reynolds, W.; Smirnova, I. Application of novel and technical lignins in food and pharmaceutical industries: Structure-function relationship and current challenges. Biomass-Convers. Biorefin. 2021, 11, 2387–2403. [Google Scholar] [CrossRef]
- Vásquez-Garay, F.; Carrillo-Varela, I.; Vidal, C.; Reyes-Contreras, P.; Faccini, M.; Mendonça, R.T. A Review on the Lignin Biopolymer and Its Integration in the Elaboration of Sustainable Materials. Sustainability 2021, 13, 2697. [Google Scholar] [CrossRef]
- Jung, H.Y.; Lee, J.S.; Han, H.T.; Jung, J.; Eom, K.; Lee, J.T. Lignin-Based Materials for Sustainable Rechargeable Batteries. Polymers 2022, 14, 673. [Google Scholar] [CrossRef]
- Brethauer, S.; Shahab, R.L.; Studer, M.H. Impacts of biofilms on the conversion of cellulose. Appl. Microbiol. Biotechnol. 2020, 104, 5201–5212. [Google Scholar] [CrossRef]
- Witzler, M.; Alzagameem, A.; Bergs, M.; El Khaldi-Hansen, B.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-Derived Biomaterials for Drug Release and Tissue Engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Mignard, N.; Taha, M.; Becquart, F.; Ayoub, A. Polysaccharides and lignin based hydrogels with potential pharmaceutical use as a drug delivery system produced by a reactive extrusion process. Int. J. Biol. Macromol. 2017, 104, 564–575. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent advances in green hydrogels from lignin: A review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef]
- Yew, P.; Zhu, D.; Lin, Q.; Jiang, L.; Chee, P.; Leong, H.; Dong, Z.; Guo, X.; Kai, D.; Loh, X. Synergistic UV protection effects of the lignin nanodiamond complex. Mater. Today Chem. 2021, 22, 100574. [Google Scholar] [CrossRef]
- Zhou, Y.; Qian, Y.; Wang, J.; Qiu, X.; Zeng, H. Bioinspired Lignin-Polydopamine Nanocapsules with Strong Bioadhesion for Long-Acting and High-Performance Natural Sunscreens. Biomacromolecules 2020, 21, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Dai, L.; Xu, C.; Wang, K.; Zheng, C.; Si, C. Lignin-Based Micro- and Nanomaterials and their Composites in Biomedical Applications. ChemSusChem 2020, 13, 4266–4283. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Larrañeta, E.; Imízcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Domínguez-Robles, J.; Rodríguez, A.; Donnelly, R.F. Synthesis and Characterization of Lignin Hydrogels for Potential Applications as Drug Eluting Antimicrobial Coatings for Medical Materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef]
- Sharma, S.; Kachhia, P.; Shukla, V.; Patel, A.; Rathod, V.; Narra, M. Valorization of lignin into nanoparticles and nanogel: Characterization and application. Bioresour. Technol. Rep. 2022, 18, 101041. [Google Scholar] [CrossRef]
- Anghel, N.; Dinu, V.; Verestiuc, L.; Spiridon, I. Transcutaneous Drug Delivery Systems Based on Collagen/Polyurethane Composites Reinforced with Cellulose. Polymers 2021, 13, 1845. [Google Scholar] [CrossRef]
- Han, J.; Liu, L.; Fan, Z.; Zhang, Z.; Yang, S.; Tang, Y. Grafting Cosmetic Active Ingredients for the Functionalization of Cosmetotextiles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 782, 022026. [Google Scholar] [CrossRef]
- Husemann, T. Pharmacographia. A History of the Principal Drugs of Vegetable Origin, Met with in Great Britain and British India, 2nd ed.; Flückiger, F.A., Hanbury, D., Eds.; Macmillan: New York, NY, USA, 1879; Volume 216, pp. 235–238. [Google Scholar] [CrossRef]
- Gupta, N.; Jangid, A.K.; Pooja, D.; Kulhari, H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. Int. J. Biol. Macromol. 2019, 132, 852–863. [Google Scholar] [CrossRef]
- Zhu, Z.; He, J.; Liu, G.; Barba, F.J.; Koubaa, M.; Ding, L.; Bals, O.; Grimi, N.; Vorobiev, E. Recent insights for the green recovery of inulin from plant food materials using non-conventional extraction technologies: A review. Innov. Food Sci. Emerg. Technol. 2016, 33, 1–9. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L. Inulin, a flexible oligosaccharide. II: Review of its pharmaceutical applications. Carbohydr. Polym. 2015, 134, 418–428. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and Its Application in Drug Delivery. Pharmaceuticals 2021, 14, 855. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, L.; Van den Mooter, G.; Augustijns, P.; Busson, R.; Toppet, S.; Kinget, R. Inulin hydrogels as carriers for colonic drug targeting: I. Synthesis and characterization of methacrylated inulin and hydrogel formation. Pharm. Res. 1997, 14, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Mandracchia, D.; Denora, N.; Franco, M.; Pitarresi, G.; Giammona, G.; Trapani, G. New biodegradable hydrogels based on inulin and α, β-polyaspartylhydrazide designed for colonic drug delivery: In vitro release of glutathione and oxytocin. J. Biomater. Sci. Polym. Ed. 2011, 22, 313–328. [Google Scholar] [CrossRef]
- Van den Mooter, G.; Vervoort, L.; Kinget, R. Characterization of methacrylated inulin hydrogels designed for colon targeting: In vitro release of BSA. Pharm. Res. 2003, 20, 303–307. [Google Scholar] [CrossRef]
- López-Molina, D.; Chazarra, S.; How, C.W.; Pruidze, N.; Navarro-Perán, E.; Garcia-Canovas, F.; Garcia-Ruiz, P.A.; Rojas-Melgarejo, F.; Rodríguez-López, J.N. Cinnamate of inulin as a vehicle for delivery of colonic drugs. Int. J. Pharm. 2015, 479, 96–102. [Google Scholar] [CrossRef]
- Von Baeckmann, C.; Riva, A.; Guggenberger, P.; Kählig, H.; Han, S.W.; Inan, D.; Del Favero, G.; Berry, D.; Kleitz, F. Targeting Gut Bacteria Using Inulin-Conjugated Mesoporous Silica Nanoparticles. Adv. Mater. Interfaces 2022, 9, 2270079. [Google Scholar] [CrossRef]
- Catenacci, L.; Sorrenti, M.; Perteghella, S.; Mandracchia, D.; Torre, M.L.; Trapani, A.; Milanese, C.; Tripodo, G. Combination of inulin and β-cyclodextrin properties for colon delivery of hydrophobic drugs. Int. J. Pharm. 2020, 589, 119861. [Google Scholar] [CrossRef]
- Giri, S.; Dutta, P.; Giri, T.K. Inulin-based carriers for colon drug targeting. J. Drug Deliv. Sci. Technol. 2021, 64, 102595. [Google Scholar] [CrossRef]
- Kermanian, M.; Sadighian, S.; Ramazani, A.; Naghibi, M.; Khoshkam, M.; Ghezelbash, P. Inulin-Coated Iron Oxide Nanoparticles: A Theranostic Platform for Contrast-Enhanced MR Imaging of Acute Hepatic Failure. ACS Biomater. Sci. Eng. 2021, 7, 2701–2715. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, H.; Khorasani, S.N.; Aminoroaya, A.; Molavian, M.R.; Allafchian, A.; Khalili, S. Facile preparation of chitosan-dopamine-inulin aldehyde hydrogel for drug delivery application. Int. J. Biol. Macromol. 2021, 185, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef] [PubMed]
- Bush, P.L. Pectin: Chemical Properties, Uses and Health Benefits; Nova Science: Hauppauge, NY, USA, 2014. [Google Scholar]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Liu, C.-M.; Li, T.; Liang, R.-H.; Luo, S.-J. Pectin modifications: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P. Chemistry of pectin and its pharmaceutical uses: A review. Silpakorn Univ. Int. J. 2003, 3, 206–228. [Google Scholar]
- Lupi, F.R.; Gabriele, D.; Seta, L.; Baldino, N.; De Cindio, B.; Marino, R. Rheological investigation of pectin-based emulsion gels for pharmaceutical and cosmetic uses. Rheol. Acta 2015, 54, 41–52. [Google Scholar] [CrossRef]
- Giusto, G.; Beretta, G.; Vercelli, C.; Valle, E.; Iussich, S.; Borghi, R.; Odetti, P.; Monacelli, F.; Tramuta, C.; Grego, E.; et al. Pectin-honey hydrogel: Characterization, antimicrobial activity and biocompatibility. Bio-Med. Mater. Eng. 2018, 29, 347–356. [Google Scholar] [CrossRef]
- Valle, K.Z.M.; Acuña, R.A.S.; Arana, J.V.R.; Lobo, N.; Rodriguez, C.; Cuevas-Gonzalez, J.C.; Tovar-Carrillo, K.L. Natural Film Based on Pectin and Allantoin for Wound Healing: Obtaining, Characterization, and Rat Model. BioMed Res. Int. 2020, 2020, 6897497. [Google Scholar] [CrossRef]
- Wu, X.; Sun, H.; Qin, Z.; Che, P.; Yi, X.; Yu, Q.; Zhang, H.; Sun, X.; Yao, F.; Li, J. Fully physically crosslinked pectin-based hydrogel with high stretchability and toughness for biomedical application. Int. J. Biol. Macromol. 2020, 149, 707–716. [Google Scholar] [CrossRef]
- Jantrawut, P.; Bunrueangtha, J.; Suerthong, J.; Kantrong, N. Fabrication and characterization of low methoxyl pectin/gelatin/carboxymethyl cellulose absorbent hydrogel film for wound dressing applications. Materials 2019, 12, 1628. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Choudhury, H.; D/O Segar Singh, S.K.; Chetty Annan, N.; Bhattamisra, S.K.; Gorain, B.; Mohd Amin, M.C.I. Budesonide-Loaded Pectin/Polyacrylamide Hydrogel for Sustained Delivery: Fabrication, Characterization and In Vitro Release Kinetics. Molecules 2021, 26, 2704. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Zafar, N.; Lebaz, N.; Mahmood, A.; Elaissari, A. Hydroxypropyl Methylcellulose-Based Hydrogel Copolymeric for Controlled Delivery of Galantamine Hydrobromide in Dementia. Processes 2020, 8, 1350. [Google Scholar] [CrossRef]
- Lemos, T.S.A.; de Souza, J.F.; Fajardo, A.R. Magnetic microspheres based on pectin coated by chitosan towards smart drug release. Carbohydr. Polym. 2021, 265, 118013. [Google Scholar] [CrossRef]
- De Souza Soares, L.; Gomes, B.T.; Miliao, G.L.; da Rocha, R.A.; de Carvalho Teixeira, A.V.N.; dos Reis Coimbra, J.S.; de Oliveira, E.B. Mixed starch/chitosan hydrogels: Elastic properties as modelled through simulated annealing algorithm and their ability to strongly reduce yellow sunset (INS 110) release. Carbohydr. Polym. 2021, 255, 117526. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.; Fernando, N.M.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.; Kulatunga, A.K. Development of starch-based materials using current modification techniques and their applications: A Review. Molecules 2021, 26, 6880. [Google Scholar] [CrossRef] [PubMed]
- Abdullah; Zou, Y.; Farooq, S.; Walayat, N.; Zhang, H.; Faieta, M.; Pittia, P.; Huang, Q. Bio-aerogels: Fabrication, properties and food applications. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Hemamalini, T.; Dev, V.R.G. Comprehensive review on electrospinning of starch polymer for biomedical applications. Int. J. Biol. Macromol. 2018, 106, 712–718. [Google Scholar] [CrossRef]
- Willfahrt, A.; Steiner, E.; Hötzel, J.; Crispin, X. Printable acid-modified corn starch as non-toxic, disposable hydrogel-polymer electrolyte in supercapacitors. Appl. Phys. A 2019, 125, 474. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer aerogels and foams: Chemistry, properties, and applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Quintanilla de Stéfano, J.C.; Abundis-Correa, V.; Herrera-Flores, S.D.; Alvarez, A.J. PH-sensitive starch-based hydrogels: Synthesis and effect of molecular components on drug release behavior. Polymers 2020, 12, 1974. [Google Scholar] [CrossRef] [PubMed]
- Junejo, S.A.; Flanagan, B.M.; Zhang, B.; Dhital, S. Starch structure and nutritional functionality – Past revelations and future prospects. Carbohydr. Polym. 2022, 277, 118837. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Jin, Z.; Jiao, A. Research progress of starch-based biodegradable materials: A review. J. Mater. Sci. 2021, 56, 11187–11208. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Shang, P.L.; Sulaiman, S.; Ariffin, F. A review: Interaction of starch/non-starch hydrocolloid blending and the recent food applications. Food Biosci. 2017, 19, 110–120. [Google Scholar] [CrossRef]
- Egharevba, H.O. Chemical properties of starch and its application in the food industry. In Chemical Properties of Starch; Books on Demand: Norderstedt, Germany, 2019; pp. 1–26. [Google Scholar]
- Dumitriu, S. Polysaccharides: Structural Diversity and Functional Versatility; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Maniglia, B.C.; Lima, D.C.; Junior, M.D.M.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Preparation of cassava starch hydrogels for application in 3D printing using dry heating treatment (DHT): A prospective study on the effects of DHT and gelatinization conditions. Food Res. Int. 2020, 128, 108803. [Google Scholar] [CrossRef]
- Noè, C.; Tonda-Turo, C.; Chiappone, A.; Sangermano, M.; Hakkarainen, M. Light Processable Starch Hydrogels. Polymers 2020, 12, 1359. [Google Scholar] [CrossRef]
- Abdollahi, Z.; Zare, E.; Salimi, F.; Goudarzi, I.; Tay, F.; Makvandi, P. Bioactive Carboxymethyl Starch-Based Hydrogels Decorated with CuO Nanoparticles: Antioxidant and Antimicrobial Properties and Accelerated Wound Healing In Vivo. Int. J. Mol. Sci. 2021, 22, 2531. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. Developments on carboxymethyl starch-based smart systems as promising drug carriers: A review. Carbohydr. Polym. 2021, 258, 117654. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Ravenelle, F.; Zhu, X.X. Polysaccharides for Drug Delivery and Pharmaceutical Applications; ACS: Washington, DC, USA, 2006. [Google Scholar]
- Elgaied-Lamouchi, D.; Descamps, N.; Lefevre, P.; Rambur, I.; Pierquin, J.Y.; Siepmann, F.; Siepmann, J.; Muschert, S. Starch-based controlled release matrix tablets: Impact of the type of starch. J. Drug Deliv. Sci. Technol. 2021, 61, 102152. [Google Scholar] [CrossRef]
- Ab’lah, N.; Wong, T.W. Chapter 9—Starch as oral colon-specific nano- and microparticulate drug carriers. In Polymer Science and Innovative Applications; Al Ali, A.-M.A., Ponnamma, D., Carignano, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 287–330. [Google Scholar] [CrossRef]
- Koev, T.T.; Harris, H.C.; Kiamehr, S.; Khimyak, Y.Z.; Warren, F.J. Starch hydrogels as targeted colonic drug delivery vehicles. Carbohydr. Polym. 2022, 289, 119413. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, M.; Chee, B.; Matos, M.; Cortese, Y.; Nugent, M.; De Lima, T.; Magalhães, W.; De Lima, G. Yerba Mate Extract in Microfibrillated Cellulose and Corn Starch Films as a Potential Wound Healing Bandage. Polymers 2020, 12, 2807. [Google Scholar] [CrossRef] [PubMed]
- Boyacı, D.; Kavur, P.B.; Gulec, S.; Yemenicioğlu, A. Physicochemical and Active Properties of Gelatine-Based Composite Gels Loaded with Lysozyme and Green Tea Polyphenols. Food Technol. Biotechnol. 2021, 59, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Daudt, R.M. Aplicação dos Componentes do Pinhão no Desenvolvimento de Produtos Inovadores Nas Indústrias Cosmética E de Alimentos. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2016. [Google Scholar]
- Mao, Y.; Pan, M.; Yang, H.; Lin, X.; Yang, L. Injectable hydrogel wound dressing based on strontium ion cross-linked starch. Front. Mater. Sci. 2020, 14, 232–241. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Nemati, S.; Kalateh, A. Nanocomposite hydrogel based on carrageenan-coated starch/cellulose nanofibers as a hemorrhage control material. Carbohydr. Polym. 2020, 251, 117013. [Google Scholar] [CrossRef]
- Edgar, K.J.; Marks, J.A. Green Hydrogels Based on Starch: Preparation Methods for Biomedical Applications. In Sustainability & Green Polymer Chemistry Volume 1: Green Products and Processes; American Chemical Society: Washington, DC, USA, 2020; Volume 1372, pp. 173–196. [Google Scholar]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Kumar, A.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Mola, G.T.; Stadler, F.J. Guar gum and its composites as potential materials for diverse applications: A review. Carbohydr. Polym. 2018, 199, 534–545. [Google Scholar] [CrossRef]
- Iqbal, D.N.; Shafiq, S.; Khan, S.M.; Ibrahim, S.M.; Abubshait, S.A.; Nazir, A.; Abbas, M.; Iqbal, M. Novel chitosan/guar gum/PVA hydrogel: Preparation, characterization and antimicrobial activity evaluation. Int. J. Biol. Macromol. 2020, 164, 499–509. [Google Scholar] [CrossRef]
- Bajpai, A.; Raj, V. Hydrophobically modified guar gum films for wound dressing. Polym. Bull. 2020, 78, 4109–4128. [Google Scholar] [CrossRef]
- Pal, R.R.; Kumar, D.; Raj, V.; Rajpal, V.; Maurya, P.; Singh, S.; Mishra, N.; Singh, N.; Singh, P.; Tiwari, N.; et al. Synthesis of pH-sensitive crosslinked guar gum-g-poly(acrylic acid-co-acrylonitrile) for the delivery of thymoquinone against inflammation. Int. J. Biol. Macromol. 2021, 182, 1218–1228. [Google Scholar] [CrossRef]
- Daud, H.; Ghani, A.; Iqbal, D.N.; Ahmad, N.; Nazir, S.; Muhammad, M.J.; Hussain, E.A.; Nazir, A.; Iqbal, M. Preparation and characterization of guar gum based biopolymeric hydrogels for controlled release of antihypertensive drug. Arab. J. Chem. 2021, 14, 103111. [Google Scholar] [CrossRef]
- Pandit, A.H.; Nisar, S.; Imtiyaz, K.; Nadeem, M.; Mazumdar, N.; Alam Rizvi, M.M.; Ahmad, S. Injectable, Self-Healing, and Biocompatible N,O-Carboxymethyl Chitosan/Multialdehyde Guar Gum Hydrogels for Sustained Anticancer Drug Delivery. Biomacromolecules 2021, 22, 3731–3745. [Google Scholar] [CrossRef] [PubMed]
- Dehghani Soltani, M.; Meftahizadeh, H.; Barani, M.; Rahdar, A.; Hosseinikhah, S.M.; Hatami, M.; Ghorbanpour, M. Guar (Cyamopsis tetragonoloba L.) plant gum: From biological applications to advanced nanomedicine. Int. J. Biol. Macromol. 2021, 193, 1972–1985. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.C.; dos Santos, L.V.; Santos, K.R.; Lima-Tenório, M.K.; Paludo, K.S.; Maurício, M.R.; Rubira, A.F.; Viana, A.G.; Tenório-Neto, E.T. Chemically crosslinked guar gum hydrogels: An investigation on the water transport and its relationship with hydrocortisone release. Int. J. Pharm. 2022, 617, 121626. [Google Scholar] [CrossRef] [PubMed]
- Talodthaisong, C.; Boonta, W.; Thammawithan, S.; Patramanon, R.; Kamonsutthipaijit, N.; Hutchison, J.; Kulchat, S. Composite guar gum-silver nanoparticle hydrogels as self-healing, injectable, and antibacterial biomaterials. Mater. Today Commun. 2020, 24, 100992. [Google Scholar] [CrossRef]
- De Paula, R.; Rodrigues, J. Composition and rheological properties of cashew tree gum, the exudate polysaccharide from Anacardium occidentale L. Carbohydr. Polym. 1995, 26, 177–181. [Google Scholar] [CrossRef]
- Olorunsola, E.O.; Bhatia, P.G.; Tytler, B.A.; Adedokun, M.O.; Adikwu, M.U. Dissolution and permeation characteristics of artemether tablets formulated with two gums of different surface activity. Trop. J. Pharm. Res. 2017, 16, 981–988. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S.; Pal, K.; Banerjee, I.; Pal, D. Gum-based hydrogels in drug delivery. In Biopolymer-Based Formulations; Elsevier: Amsterdam, The Netherlands, 2020; pp. 605–645. [Google Scholar]
- Aragão-Neto, A.C.; Soares, P.A.; Lima-Ribeiro, M.H.; Carvalho, E.J.; Correia, M.T.; Carneiro-Da-Cunha, M.G. Combined therapy using low level laser and chitosan-policaju hydrogel for wound healing. Int. J. Biol. Macromol. 2017, 95, 268–272. [Google Scholar] [CrossRef]
- Lustosa, A.K.M.F.; de Jesus Oliveira, A.C.; Quelemes, P.V.; Plácido, A.; Da Silva, F.V.; Oliveira, I.S.; De Almeida, M.P.; Amorim, A.d.G.N.; Delerue-Matos, C.; De Oliveira, R.D.C.M.; et al. In situ synthesis of silver nanoparticles in a hydrogel of carboxymethyl cellulose with phthalated-cashew gum as a promising antibacterial and healing agent. Int. J. Mol. Sci. 2017, 18, 2399. [Google Scholar] [CrossRef]
- Srivastava, N.; Choudhury, A.R. Recent advances in composite hydrogels prepared solely from polysaccharides. Colloids Surf. B Biointerfaces 2021, 205, 111891. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; classification, chemical properties, and future perspective applications in fields of pharmacology and biological medicine (a review of current applications and upcoming potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Loutfy, S.A.; Hussein, Y.; Kenawy, E.-R.S. Recent advances in PVA-polysaccharide based hydrogels and electrospun nanofibers in biomedical applications: A review. Int. J. Biol. Macromol. 2021, 187, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z. Recent advances in polysaccharide-based hydrogels for synthesis and applications. Aggregate 2021, 2, e21. [Google Scholar] [CrossRef]




| Polysaccharide | Chemical Characteristics | Gelling Behavior | References |
|---|---|---|---|
| Cellulose | Easy chemical modification; high degree of crystallinity; adequate mechanical properties, and great specific surface area | It forms a semi-interpenetrating polymer network | [23,24] |
| Hemicelluloses | A huge amount of hydroxyl groups allows chemical modifications | It presents film-forming properties due to gelation, with satisfactory mechanical properties | [25,26] |
| Lignin | Rich in phenolic and aliphatic hydroxyl groups that confer chemical versatility | It can form a continuous phase by gelation, with particulate-filled polymer networks | [27,28] |
| Inulin | Branched fructosyl units | Typically, inulin properties highly depend on their degree of polymerization | [29,30] |
| Pectin | Rich in carboxylate units (methyl esters) | The degree of esterification controls its gelling mechanism | [31,32] |
| Starch | It is formed by rich oxygenated units (amylose and amylopectin) | First, the starch is modified by physicochemical routes, and then, using hot water, the starch breaks down and swells the amorphous and semi-crystalline regions; smart starch gels can be obtained since it responds to stimulus | [33,34] |
| Guar Gum | Mainly composed of D-mannopyranose unit, with various hydroxyl groups | Usually involves slow gelling process time (low productivity) | [35,36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahú, J.O.; de Andrade, L.R.M.; de Melo Barbosa, R.; Crivellin, S.; da Silva, A.P.; Souza, S.D.A.; Cárdenas Concha, V.O.; Severino, P.; Souto, E.B. Plant Polysaccharides in Engineered Pharmaceutical Gels. Bioengineering 2022, 9, 376. https://doi.org/10.3390/bioengineering9080376
Bahú JO, de Andrade LRM, de Melo Barbosa R, Crivellin S, da Silva AP, Souza SDA, Cárdenas Concha VO, Severino P, Souto EB. Plant Polysaccharides in Engineered Pharmaceutical Gels. Bioengineering. 2022; 9(8):376. https://doi.org/10.3390/bioengineering9080376
Chicago/Turabian StyleBahú, Juliana O., Lucas R. Melo de Andrade, Raquel de Melo Barbosa, Sara Crivellin, Aline Pioli da Silva, Samuel D. A. Souza, Viktor O. Cárdenas Concha, Patrícia Severino, and Eliana B. Souto. 2022. "Plant Polysaccharides in Engineered Pharmaceutical Gels" Bioengineering 9, no. 8: 376. https://doi.org/10.3390/bioengineering9080376
APA StyleBahú, J. O., de Andrade, L. R. M., de Melo Barbosa, R., Crivellin, S., da Silva, A. P., Souza, S. D. A., Cárdenas Concha, V. O., Severino, P., & Souto, E. B. (2022). Plant Polysaccharides in Engineered Pharmaceutical Gels. Bioengineering, 9(8), 376. https://doi.org/10.3390/bioengineering9080376