Mesenchymal Stem Cell Use in Acute Tendon Injury: In Vitro Tenogenic Potential vs. In Vivo Dose Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Isolation, Culture, and Characterization of Rat Adipose-Derived MSCs
2.3. Immunophenotyping
2.4. Proliferation Assay
2.5. Tenogenic Differentiation of Rat Adipose-Derived MSCs
2.6. Immunofluorescence
2.7. Fibrin Gel
2.8. Surgery
2.9. Postoperative Management
2.10. Histological Analysis
2.11. Immunohistochemistry
2.12. Statistical Analysis
3. Results
3.1. Rat Mesenchymal Stem Cell Isolation, Characterization, and Tenogenic Differentiation
3.1.1. Isolation, Expansion, and Immunophenotyping of rAdMSCs and rBMSCs
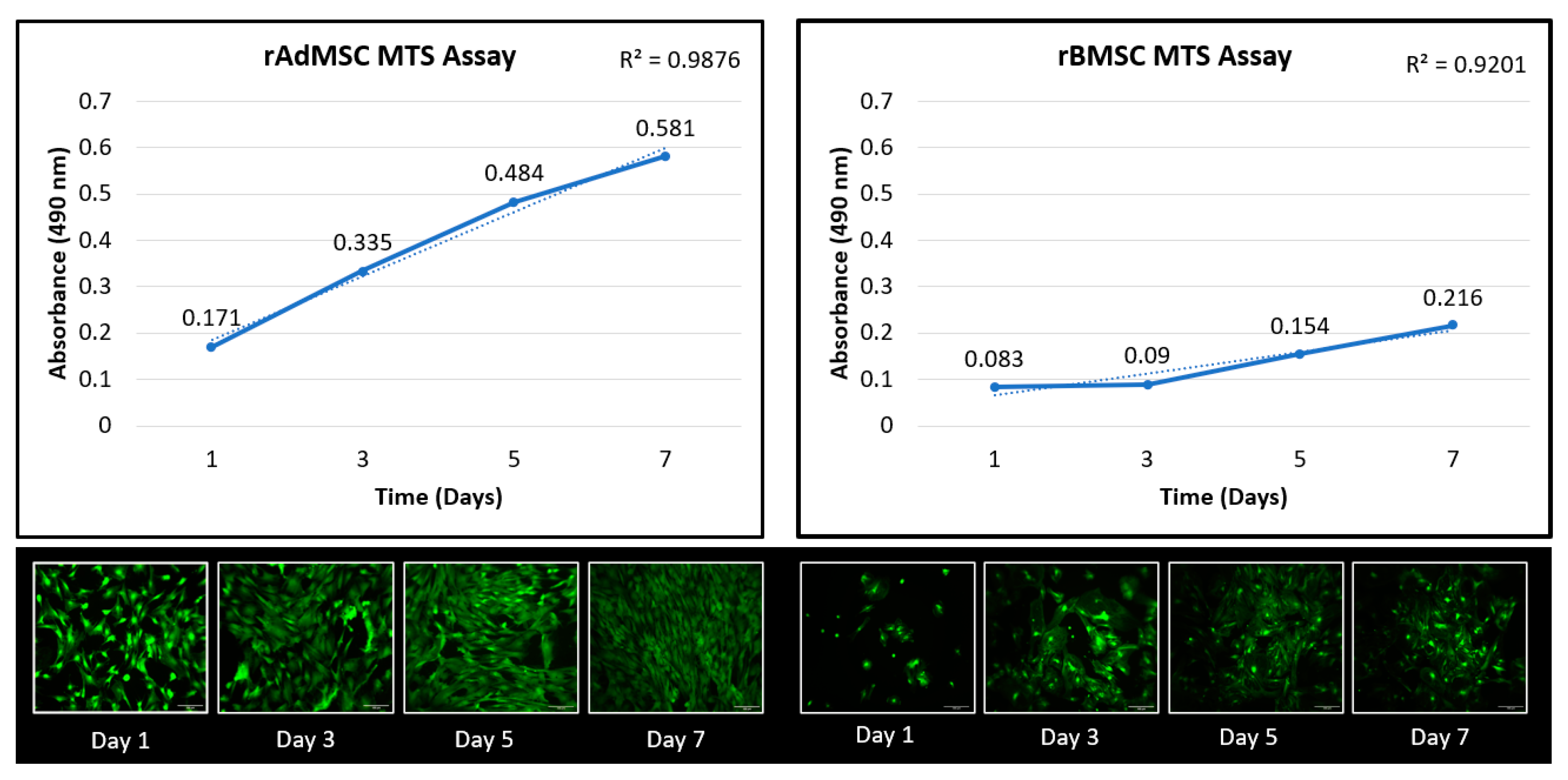
3.1.2. Cell Proliferation
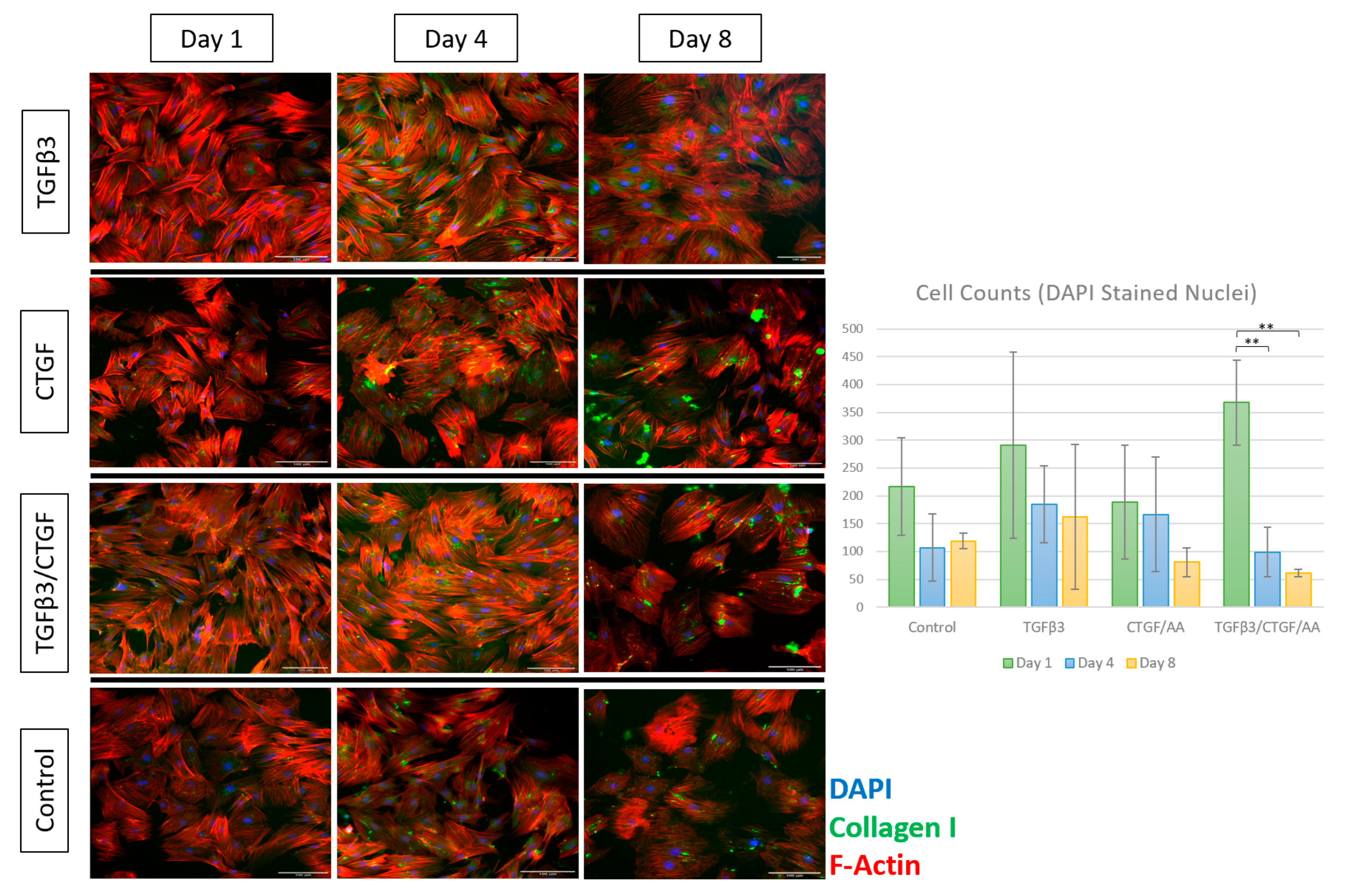
3.1.3. Tenogenic Differentiation—Morphological Changes
3.1.4. Immunofluorescence
3.2. Intralesional Rat Mesenchymal Stem Cell Use in Achilles Tendon Injury
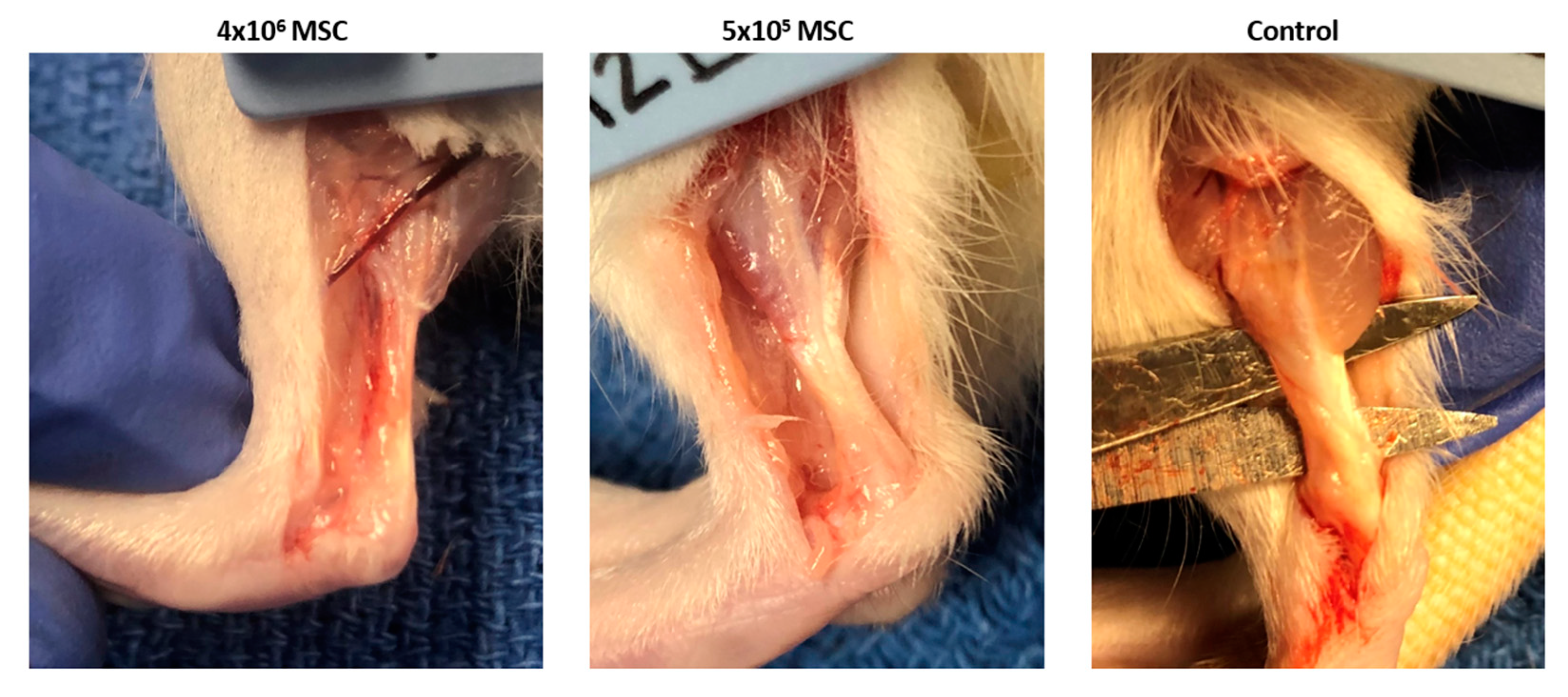
3.2.1. Gross Morphology
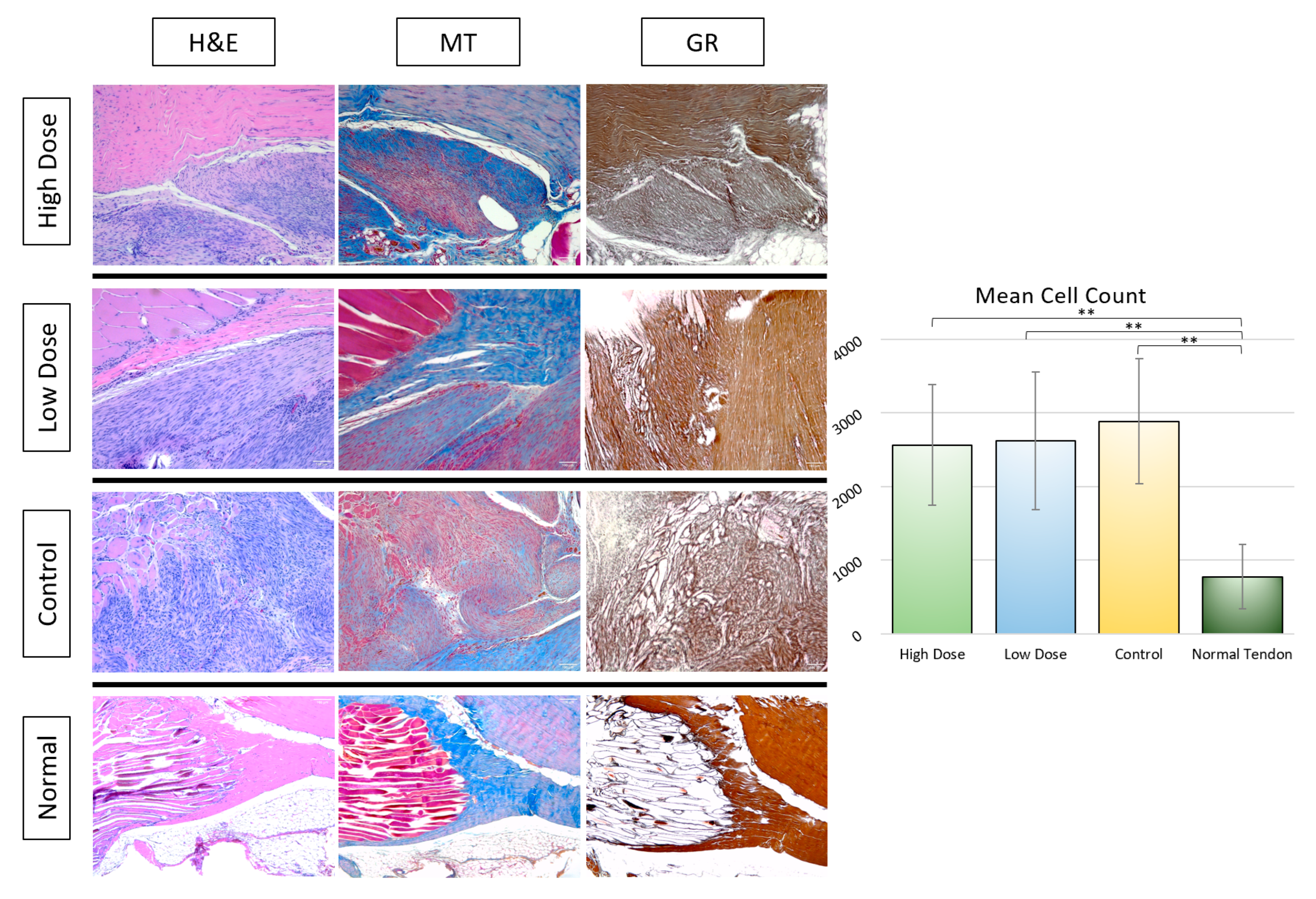
3.2.2. Histological Analysis
3.2.3. Immunohistochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schnabel, L.V.; Fortier, L.A.; McIlwraith, C.W.; Nobert, K.M. Therapeutic use of stem cells in horses: Which type, how, and when? Vet. J. 2013, 197, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Godwin, E.E.; Young, N.J.; Dudhia, J.; Beamish, I.C.; Smith, R.K.W. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet. J. 2012, 44, 25–32. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, B.; Bladon, B.; Parkin, T.; Fraser, B.; Lischer, C.J. An investigation of the relationship between race performance and superficial digital flexor tendonitis in the Thoroughbred racehorse. Equine Vet. J. 2010, 42, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S.J. Medical management of superficial digital flexor tendonitis: A comparative study in 219 horses (1992–2000). Equine Vet. J. 2004, 36, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Pacini, S.; Spinabella, S.; Trombi, L.; Fazzi, R.; Galimberti, S.; Dini, F.; Carlucci, F.; Petrini, M. Suspension of Bone Marrow–Derived Undifferentiated Mesenchymal Stromal Cells for Repair of Superficial Digital Flexor Tendon in Race Horses. Tissue Eng. 2007, 13, 2949–2955. [Google Scholar] [CrossRef] [Green Version]
- Al-Ani, M.K.A.; Xu, K.; Sun, Y.; Pan, L.; Xu, Z.; Yang, L. Study of Bone Marrow Mesenchymal and Tendon-Derived Stem Cells Transplantation on the Regenerating Effect of Achilles Tendon Ruptures in Rats. Stem Cells Int. 2015, 2015, 984146. [Google Scholar] [CrossRef]
- Oliva, F.; Maffulli, N.; Gissi, C.; Veronesi, F.; Calciano, L.; Fini, M.; Brogini, S.; Gallorini, M.; Passeri, C.A.L.; Bernardini, R.; et al. Combined ascorbic acid and T3 produce better healing compared to bone marrow mesenchymal stem cells in an Achilles tendon injury rat model: A proof of concept study. J. Orthop. Surg. Res. 2019, 14, 54. [Google Scholar] [CrossRef]
- Gissi, C.; Radeghieri, A.; Passeri, C.A.L.; Gallorini, M.; Calciano, L.; Oliva, F.; Veronesi, F.; Zendrini, A.; Cataldi, A.; Bergese, P.; et al. Extracellular vesicles from rat-bone-marrow mesenchymal stromal/stem cells improve tendon repair in rat Achilles tendon injury model in dose-dependent manner: A pilot study. PLoS ONE 2020, 15, e0229914. [Google Scholar] [CrossRef]
- Schnabel, L.V.; Lynch, M.E.; Van Der Meulen, M.C.H.; Yeager, A.E.; Kornatowski, M.A.; Nixon, A.J. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J. Orthop. Res. 2009, 27, 1392–1398. [Google Scholar] [CrossRef]
- Leong, N.L.; Kator, J.L.; Clemens, T.L.; James, A.; Dds, M.E.; Jiang, J. Tendon and Ligament Healing and Current Approaches to Tendon and Ligament Regeneration. J. Orthop. Res. 2020, 38, 7–12. [Google Scholar] [CrossRef]
- Bianco, S.T.; Moser, H.L.; Galatz, L.M.; Huang, A.H. Biologics and stem cell-based therapies for rotator cuff repair. Ann. N. Y. Acad. Sci. 2019, 1442, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Li, H.; Liu, Y.; Yu, X.; Zhang, X.; Chu, W.; She, Y.; Wang, D.; Chen, G. Fibroblast growth factor-2 promotes the function of tendon-derived stem cells in Achilles tendon restoration in an Achilles tendon injury rat model. Biochem. Biophys. Res. Commun. 2020, 521, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Brandt, L.; Schubert, S.; Scheibe, P.; Brehm, W.; Franzen, J.; Gross, C.; Burk, J. Tenogenic Properties of Mesenchymal Progenitor Cells Are Compromised in an Inflammatory Environment. Int. J. Mol. Sci. 2018, 19, 2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Miguel, M.P.; Fuentes-Julian, S.; Blazquez-Martinez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive Properties of Mesenchymal Stem Cells: Advances and Applications. Curr. Mol. Med. 2012, 12, 574–591. [Google Scholar] [CrossRef]
- Liu, L.; Hindieh, J.; Leong, D.J.; Sun, H.B. Advances of stem cell based-therapeutic approaches for tendon repair. J. Orthop. Transl. 2017, 9, 69–75. [Google Scholar] [CrossRef]
- Durgam, S.; Stewart, M. Cellular and Molecular Factors Influencing Tendon Repair. Tissue Eng. Part B Rev. 2017, 23, 307–317. [Google Scholar] [CrossRef]
- Liu, Y.; Suen, C.-W.; Zhang, J.-F.; Li, G. Current concepts on tenogenic differentiation and clinical applications. J. Orthop. Transl. 2017, 9, 28–42. [Google Scholar] [CrossRef]
- Grafe, I.; Alexander, S.; Peterson, J.R.; Snider, T.N.; Levi, B.; Lee, B.; Mishina, Y. TGF-β Family Signaling in Mesenchymal Differentiation. Cold Spring Harb. Perspect. Biol. 2018, 10, a022202. [Google Scholar] [CrossRef]
- Long, C.; Wang, Z.; Legrand, A.; Chattopadhyay, A.; Chang, J.; Fox, P.M. Tendon Tissue Engineering: Mechanism and Effects of Human Tenocyte Coculture with Adipose-Derived Stem Cells. J. Hand Surg. 2018, 43, 183.e1–183.e9. [Google Scholar] [CrossRef]
- Barsby, T.; Bavin, E.P.; Guest, D.J. Three-dimensional culture and transforming growth factor beta3 synergistically promote tenogenic differentiation of equine embryo-derived stem cells. Tissue Eng. Part A 2014, 20, 2604–2613. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Wang, X.-F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2008, 19, 71–88. [Google Scholar] [CrossRef]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Yin, Z.; Guo, J.; Wu, T.-Y.; Chen, X.; Xu, L.-L.; Lin, S.-E.; Sun, Y.-X.; Chan, K.-M.; Ouyang, H.; Li, G. Stepwise Differentiation of Mesenchymal Stem Cells Augments Tendon-Like Tissue Formation and Defect Repair In Vivo. Stem Cells Transl. Med. 2016, 5, 1106–1116. [Google Scholar] [CrossRef]
- Barsby, T.; Guest, D. Transforming growth factor beta3 promotes tendon differentiation of equine embryo-derived stem cells. Tissue Eng. Part A 2013, 19, 2156–2165. [Google Scholar] [CrossRef]
- Roth, S.P.; Schubert, S.; Scheibe, P.; Groß, C.; Brehm, W.; Burk, J. Growth Factor-Mediated Tenogenic Induction of Multipotent Mesenchymal Stromal Cells Is Altered by the Microenvironment of Tendon Matrix. Cell Transplant. 2018, 27, 1434–1450. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Rothrauff, B.B.; Lin, H.; Yu, S.; Tuan, R.S. Tendon-Derived Extracellular Matrix Enhances Transforming Growth Factor-β3-Induced Tenogenic Differentiation of Human Adipose-Derived Stem Cells. Tissue Eng. Part A 2017, 23, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Lee, S.Y.; Yang, E.-J.; Kim, H.Y.; Jo, I.; Shin, S.-J. Expression of tenocyte lineage-related factors from tonsil-derived mesenchymal stem cells. Tissue Eng. Regen. Med. 2016, 13, 162–170. [Google Scholar] [CrossRef]
- Saether, E.E.; Chamberlain, C.S.; Leiferman, E.M.; Kondratko-Mittnacht, J.R.; Li, W.-J.; Brickson, S.L.; Vanderby, R. Enhanced Medial Collateral Ligament Healing Using Mesenchymal Stem Cells: Dosage Effects on Cellular Response and Cytokine Profile. Stem Cell Rev. Rep. 2014, 10, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Awad, H.A.; Boivin, G.P.; Dressler, M.R.; Smith, F.N.L.; Young, R.G.; Butler, D.L. Repair of patellar tendon injuries using a cell–collagen composite. J. Orthop. Res. 2003, 21, 420–431. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, W.; Lim, C.; Chung, S.G. Treatment of Lateral Epicondylosis by Using Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Pilot Study. Stem Cells 2015, 33, 2995–3005. [Google Scholar] [CrossRef]
- Kwon, D.R.; Park, G.-Y.; Lee, S.C. Regenerative effects of mesenchymal stem cells by dosage in a chronic rotator cuff tendon tear in a rabbit model. Regen. Med. 2019, 14, 1001–1012. [Google Scholar] [CrossRef]
- Centeno, C.J. Clinical challenges and opportunities of mesenchymal stem cells in musculoskeletal medicine. PM&R 2014, 6, 70–77. [Google Scholar]
- Meirelles, S.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms Involved in the Therapeutic Properties of Mesenchymal Stem Cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef]
- Steinert, A.F.; Rackwitz, L.; Gilbert, F.; Nöth, U.; Tuan, R.S. Concise Review: The Clinical Application of Mesenchymal Stem Cells for Musculoskeletal Regeneration: Current Status and Perspectives. STEM CELLS Transl. Med. 2012, 1, 237–247. [Google Scholar] [CrossRef]
- Chamberlain, C.S.; Saether, E.E.; Vanderby, R.; Aktas, E. Mesenchymal Stem Cell Therapy on Tendon/Ligament Healing. J. Cytokine Biol. 2017, 2, 112. [Google Scholar] [CrossRef]
- Ursini, T.L.; Amelse, L.L.; Elkhenany, H.A.; Odoi, A.; Carter-Arnold, J.L.; Adair, H.S.; Dhar, M.S. Retrospective analysis of local injection site adverse reactions associated with 230 allogenic administrations of bone marrow-derived mesenchymal stem cells in 164 horses. Equine Vet. J. 2019, 51, 198–205. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal Stem Cells Avoid Allogenic Rejection. J. Inflam. 2005, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Hast, M.W.; Zuskov, A.; Soslowsky, L.J. The role of animal models in tendon research. Bone Jt. Res. 2014, 3, 193–202. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Thomopoulos, S.; Soslowsky, L.J. Animal Models of Tendon and Ligament Injuries for Tissue Engineering Applications. Clin. Orthop. Relat. Res. 1999, 367, S296–S311. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. 2015, 33, 832–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals. The National Academies Collection: Reports funded by National Institutes of Health. In Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Hu, X.; Zhang, X.; Zhu, J.; Zhang, J.; Fu, X.; Duan, X.; Ao, Y.; Zhou, C. Different tenogenic differentiation capacities of different mesenchymal stem cells in the presence of BMP-12. J. Transl. Med. 2015, 13, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labora, J.A.F.; Fernández-Pernas, P.; Fuentes, I.; De Toro, J.; Oreiro, N.; Sangiao-Alvarellos, S.; Mateos, J.; Arufe, M. Influence of age on rat bone-marrow mesenchymal stem cells potential. Sci. Rep. 2015, 5, 16765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghazali, K.M.; Newby, S.D.; Nima, Z.A.; Hamzah, R.N.; Watanabe, F.; Bourdo, S.E.; Masi, T.J.; Stephenson, S.M.; Anderson, D.E.; Dhar, M.S.; et al. Functionalized gold nanorod nanocomposite system to modulate differentiation of human mesenchymal stem cells into neural-like progenitors. Sci. Rep. 2017, 7, 16654. [Google Scholar] [CrossRef] [Green Version]
- Zayed, M.; Caniglia, C.; Misk, N.; Dhar, M.S. Donor-Matched Comparison of Chondrogenic Potential of Equine Bone Marrow- and Synovial Fluid-Derived Mesenchymal Stem Cells: Implications for Cartilage Tissue Regeneration. Front. Vet. Sci. 2017, 3, 121. [Google Scholar] [CrossRef] [Green Version]
- Carter-Arnold, J.L.; Neilsen, N.L.; Amelse, L.L.; Odoi, A.; Dhar, M.S. In vitroanalysis of equine, bone marrow-derived mesenchymal stem cells demonstrates differences within age- and gender-matched horses. Equine Vet. J. 2014, 46, 589–595. [Google Scholar] [CrossRef]
- Shojaee, A.; Parham, A. Strategies of tenogenic differentiation of equine stem cells for tendon repair: Current status and challenges. Stem Cell Res. Ther. 2019, 10, 181. [Google Scholar] [CrossRef]
- Newby, S.D.; Masi, T.; Griffin, C.D.; King, W.J.; Chipman, A.; Stephenson, S.; Anderson, D.E.; Biris, A.S.; Bourdo, S.E.; Dhar, M. Functionalized Graphene Nanoparticles Induce Human Mesenchymal Stem Cells to Express Distinct Extracellular Matrix Proteins Mediating Osteogenesis. Int. J. Nanomed. 2020, 15, 2501–2513. [Google Scholar] [CrossRef] [Green Version]
- Bottagisio, M.; Lopa, S.; Granata, V.; Talò, G.; Bazzocchi, C.; Moretti, M.; Lovati, A.B. Different combinations of growth factors for the tenogenic differentiation of bone marrow mesenchymal stem cells in monolayer culture and in fibrin-based three-dimensional constructs. Differentiation 2017, 95, 44–53. [Google Scholar] [CrossRef]
- Handala, L.; Fiore, T.; Rouillé, Y.; Helle, F. QuantIF: An ImageJ Macro to Automatically Determine the Percentage of Infected Cells after Immunofluorescence. Viruses 2019, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- Pemberton, K.; Mersman, B.; Xu, F. Using ImageJ to Assess Neurite Outgrowth in Mammalian Cell Cultures: Research Data Quantification Exercises in Undergraduate Neuroscience Lab. J. Undergrad. Neurosci. Educ. JUNE A Publ. FUN Fac. Undergrad. Neurosci. 2018, 16, A186–A194. [Google Scholar]
- Wu, X.; Ren, J.; Li, J. Fibrin glue as the cell-delivery vehicle for mesenchymal stromal cells in regenerative medicine. Cytotherapy 2012, 14, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Al-Ani, M.K.; Sun, Y.; Xu, W.; Pan, L.; Song, Y.; Xu, Z.; Pan, X.; Yang, L. Platelet-rich Plasma Activates Tendon-Derived Stem Cells to Promote Regeneration of Achilles Tendon Rupture in Rats. J. Tissue Eng. Regen. Med. 2017, 11, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Devana, S.K.; Kelley, B.V.; McBride, O.J.; Kabir, N.; Jensen, A.R.; Park, S.J.; Eliasberg, C.D.; Dar, A.; Mosich, G.M.; Kowalski, T.J.; et al. Adipose-derived Human Perivascular Stem Cells May Improve Achilles Tendon Healing in Rats. Clin. Orthop. Relat. Res. 2018, 476, 2091–2100. [Google Scholar] [CrossRef]
- Wagner, J.R.; Taguchi, T.; Cho, J.Y.; Charavaryamath, C.; Griffon, D.J. Evaluation of Stem Cell Therapies in a Bilateral Patellar Tendon Injury Model in Rats. J. Vis. Exp. 2018, 133, e56810. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, H.; Gao, S.; Yang, M.; Lyu, J.; Tang, K. Effect of Tendon Stem Cells in Chitosan/β-Glycerophosphate/Collagen Hydrogel on Achilles Tendon Healing in a Rat Model. Med. Sci. Monit. 2017, 23, 4633–4643. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, T.; Liu, F.; Qu, J.; Chen, Y.; Fan, S.; Chen, H.; Sun, L.; Zhao, C.; Hu, J.; et al. Effect of Low-Intensity Pulsed Ultrasound after Autologous Adipose-Derived Stromal Cell Transplantation for Bone-Tendon Healing in a Rabbit Model. Am. J. Sports Med. 2019, 47, 942–953. [Google Scholar] [CrossRef]
- de Girolamo, L.; Morlin Ambra, L.F.; Perucca Orfei, C.; McQuilling, J.P.; Kimmerling, K.A.; Mowry, K.C.; Johnson, K.A.; Phan, A.T.; Whited, J.L.; Gomoll, A.H. Treatment with Human Amniotic Suspension Allograft Improves Tendon Healing in a Rat Model of Collagenase-Induced Tendinopathy. Cells 2019, 8, 1411. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Wershof, E.; Park, D.; Barry, D.J.; Jenkins, R.P.; Rullan, A.; Wilkins, A.; Schlegelmilch, K.; Roxanis, I.; Anderson, K.I.; Bates, P.A.; et al. A FIJI macro for quantifying pattern in extracellular matrix. Life Sci. Alliance 2021, 4, e202000880. [Google Scholar] [CrossRef]
- Rojewski, M.T.; Weber, B.M.; Schrezenmeier, H. Phenotypic Characterization of Mesenchymal Stem Cells from Various Tissues. Transfus. Med. Hemotherapy 2008, 35, 168–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.-M.; Pan, J.; Zhang, Y.; Ning, L.-J.; Luo, J.-C.; Huang, F.-G.; Qin, T.-W. Bridging Repair of Large Rotator Cuff Tears Using a Multilayer Decellularized Tendon Slices Graft in a Rabbit Model. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Yamashita, T.; Toku, T.; Ju, Y. Optimization of differentiation time of mesenchymal-stem-cell to tenocyte under a cyclic stretching with a microgrooved culture membrane and selected measurement cells. Acta Bioeng. Biomech. 2018, 20, 3–10. [Google Scholar] [PubMed]
- Morita, Y.; Sato, T.; Higashiura, K.; Hirano, Y.; Matsubara, F.; Oshima, K.; Niwa, K.; Toku, Y.; Song, G.; Luo, Q.; et al. The optimal mechanical condition in stem cell-to-tenocyte differentiation determined with the homogeneous strain distributions and the cellular orientation control. Biol. Open 2019, 8, bio039164. [Google Scholar] [CrossRef] [Green Version]
- Kadzik, R.S.; Homa, K.E.; Kovar, D.R. F-Actin Cytoskeleton Network Self-Organization Through Competition and Cooperation. Annu. Rev. Cell Dev. Biol. 2020, 36, 35–60. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, A.; Richardson, D.W. Regulation of the tenogenic gene expression in equine tenocyte-derived induced pluripotent stem cells by mechanical loading and Mohawk. Stem Cell Res. 2019, 39, 101489. [Google Scholar] [CrossRef]
- Shukunami, C.; Takimoto, A.; Nishizaki, Y.; Yoshimoto, Y.; Tanaka, S.; Miura, S.; Watanabe, H.; Sakuma, T.; Yamamoto, T.; Kondoh, G.; et al. Scleraxis is a transcriptional activator that regulates the expression of Tenomodulin, a marker of mature tenocytes and ligamentocytes. Sci. Rep. 2018, 8, 3155. [Google Scholar] [CrossRef] [Green Version]
- Shukunami, C.; Takimoto, A.; Oro, M.; Hiraki, Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev. Biol. 2006, 298, 234–247. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.E.; Kopecki, Z.; Anderson, P.; Cowin, A.J. In vitro analysis of the effect of Flightless I on murine tenocyte cellular functions. J. Orthop. Surg. Res. 2020, 15, 170. [Google Scholar] [CrossRef]
- Gonçalves, A.; Rodrigues, M.; Lee, S.J.; Atala, A.; Yoo, J.J.; Reis, R.L.; Gomes, M.E. Understanding the Role of Growth Factors in Modulating Stem Cell Tenogenesis. PLoS ONE 2013, 8, e83734. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Hu, M.; Wu, H.; Ren, C.; Wang, J.; Cui, S. Tenascin-C expression and its associated pathway in BMSCs following co-culture with mechanically stretched ligament fibroblasts. Mol. Med. Rep. 2017, 15, 2465–2472. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. Sci. World J. 2015, 2015, 685690. [Google Scholar] [CrossRef]
- O’Brien, C.; Marr, N.; Thorpe, C. Microdamage in the equine superficial digital flexor tendon. Equine Vet. J. 2021, 53, 417–430. [Google Scholar] [CrossRef]
- Khan, M.R.; Dudhia, J.; David, F.H.; De Godoy, R.; Mehra, V.; Hughes, G.; Dakin, S.G.; Carr, A.J.; Goodship, A.E.; Smith, R.K.W. Bone marrow mesenchymal stem cells do not enhance intra-synovial tendon healing despite engraftment and homing to niches within the synovium. Stem Cell Res. Ther. 2018, 9, 169. [Google Scholar] [CrossRef]
- Khan, M.R.; Smith, R.K.; David, F.; Lam, R.; Hughes, G.; De Godoy, R.; Carr, A.J.; Goodship, A.E.; Dudhia, J. Evaluation of the Effects of Synovial Multipotent Cells on Deep Digital Flexor Tendon Repair in a Large Animal Model of Intra-Synovial Tendinopathy. J. Orthop. Res. 2020, 38, 128–138. [Google Scholar] [CrossRef]
- Ahrberg, A.B.; Horstmeier, C.; Berner, D.; Brehm, W.; Gittel, C.; Hillmann, A.; Josten, C.; Rossi, G.; Schubert, S.; Winter, K.; et al. Effects of mesenchymal stromal cells versus serum on tendon healing in a controlled experimental trial in an equine model. BMC Musculoskelet. Disord. 2018, 19, 230. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowers, K.; Amelse, L.; Bow, A.; Newby, S.; MacDonald, A.; Sun, X.; Anderson, D.; Dhar, M. Mesenchymal Stem Cell Use in Acute Tendon Injury: In Vitro Tenogenic Potential vs. In Vivo Dose Response. Bioengineering 2022, 9, 407. https://doi.org/10.3390/bioengineering9080407
Bowers K, Amelse L, Bow A, Newby S, MacDonald A, Sun X, Anderson D, Dhar M. Mesenchymal Stem Cell Use in Acute Tendon Injury: In Vitro Tenogenic Potential vs. In Vivo Dose Response. Bioengineering. 2022; 9(8):407. https://doi.org/10.3390/bioengineering9080407
Chicago/Turabian StyleBowers, Kristin, Lisa Amelse, Austin Bow, Steven Newby, Amber MacDonald, Xiaocun Sun, David Anderson, and Madhu Dhar. 2022. "Mesenchymal Stem Cell Use in Acute Tendon Injury: In Vitro Tenogenic Potential vs. In Vivo Dose Response" Bioengineering 9, no. 8: 407. https://doi.org/10.3390/bioengineering9080407
APA StyleBowers, K., Amelse, L., Bow, A., Newby, S., MacDonald, A., Sun, X., Anderson, D., & Dhar, M. (2022). Mesenchymal Stem Cell Use in Acute Tendon Injury: In Vitro Tenogenic Potential vs. In Vivo Dose Response. Bioengineering, 9(8), 407. https://doi.org/10.3390/bioengineering9080407






