Anticancer Activity, Mechanism, and Delivery of Allyl Isothiocyanate
Abstract
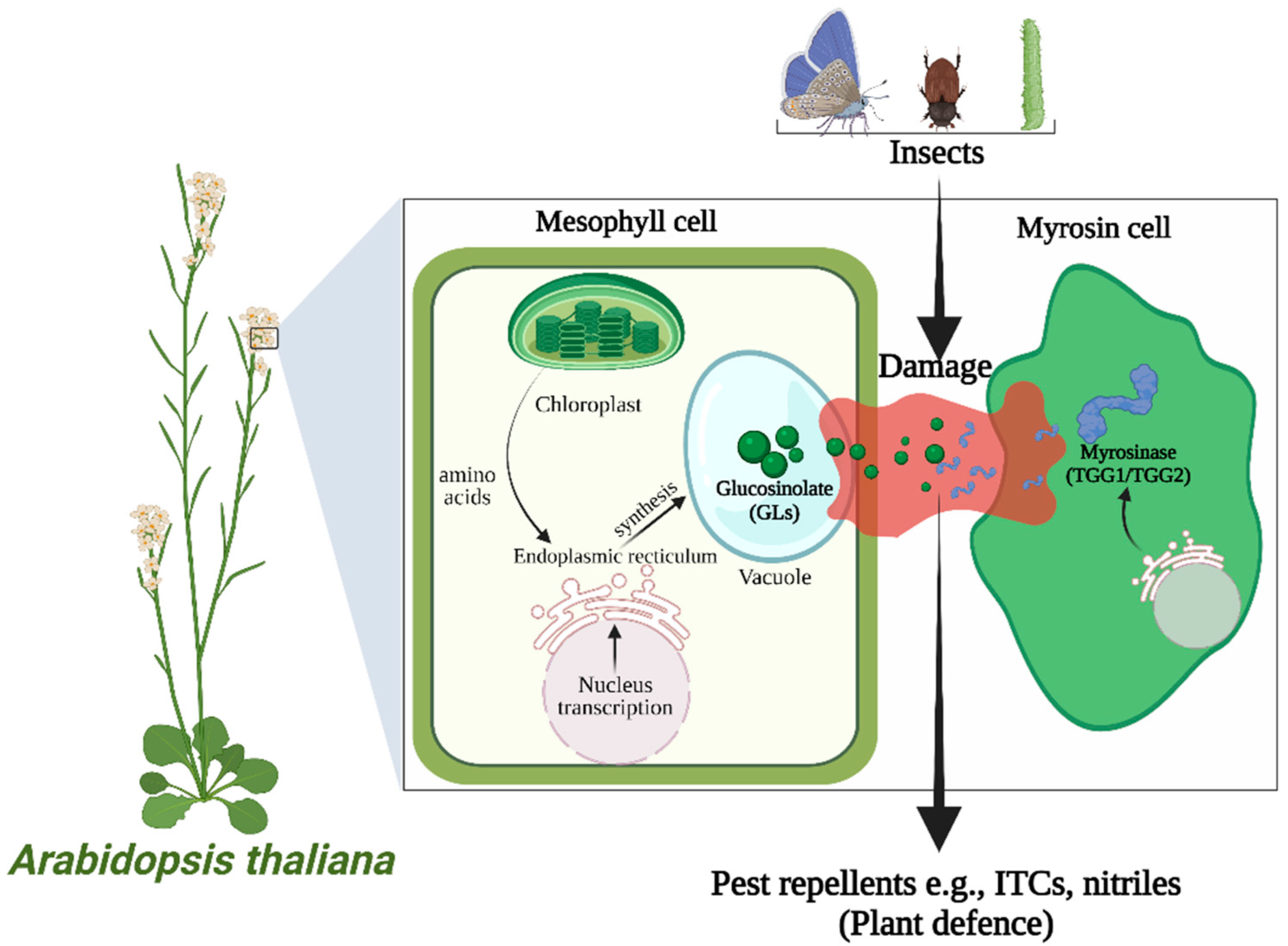
:1. Introduction
2. Anticancer Activity of AITC
2.1. Cervical Cancer
2.2. Malignant Glioma
2.3. Cisplatin-Resistant Oral Cancer Cells
2.4. Non-Small Lung Carcinomas
2.5. Breast Cancer
2.6. Ovarian Cancer
2.7. Bladder Cancer Cells
2.8. Prostate Cancer
2.9. Colorectal Cancer
2.10. Metastatic Melanoma Cells
2.11. Renal Cell Carcinomas
2.12. Leukemia
3. AITC Anticancer Mechanisms
3.1. Stimulation of Cell Cycle Arrest
3.2. Induction of Apoptosis
3.3. Suppression of Metastasis
3.4. AITC-Induced Autophagy
3.5. Antiangiogenetic Effect
3.6. Inhibition of Phase I and Induction of Phase II Enzymes
3.7. Induction of Replication Stress-Mediated DNA Damage
4. Delivery of AITC
4.1. AITC-Encapsulated Nanoemulsions
4.2. AIT-Loaded Polymeric Nanoparticles
4.3. AITC-Conjugated Silicon Quantum Dots
4.4. In Situ Delivery of AITC Using the Sinigrin–Myrosinase System
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; de Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in Brassica Vegetables: The Influence of the Food Supply Chain on Intake, Bioavailability and Human Health. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S219. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate Metabolism, Functionality and Breeding for the Improvement of Brassicaceae Vegetables. Breed. Sci. 2014, 64, 48. [Google Scholar] [CrossRef]
- McDanell, R.; McLean, A.E.M.; Hanley, A.B.; Heaney, R.K.; Fenwick, G.R. Chemical and Biological Properties of Indole Glucosinolates (Glucobrassicins): A Review. Food Chem. Toxicol. 1988, 26, 59–70. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Koutsidis, G.; Mavroudis, N.; Trafalis, D.T.; Botaitis, S.; Franco, R.; Zoumpourlis, V.; Amery, T.; Galanis, A.; Pappa, A.; et al. The Role of Isothiocyanates as Cancer Chemo-Preventive, Chemo-Therapeutic and Anti-Melanoma Agents. Antioxidants 2019, 8, 106. [Google Scholar] [CrossRef]
- Krul, C.; Humblot, C.; Philippe, C.; Vermeulen, M.; Van Nuenen, M.; Havenaar, R.; Rabot, S. Metabolism of Sinigrin (2-Propenyl Glucosinolate) by the Human Colonic Microflora in a Dynamic in Vitro Large-Intestinal Model. Carcinogenesis 2002, 23, 1009–1016. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Q.H.; Xu, K. Are Isothiocyanates Potential Anti-Cancer Drugs? Acta Pharmacol. Sin. 2009, 30, 501. [Google Scholar] [CrossRef]
- Gupta, P.; Kim, B.; Kim, S.H.; Srivastava, S.K. Molecular Targets of Isothiocyanates in Cancer: Recent Advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Kyriakou, S.; Anestopoulos, I.; Trafalis, D.T.; Deligiorgi, M.V.; Franco, R.; Pappa, A.; Panayiotidis, M.I. An Evaluation of the Anti-Carcinogenic Response of Major Isothiocyanates in Non-Metastatic and Metastatic Melanoma Cells. Antioxidants 2021, 10, 284. [Google Scholar] [CrossRef]
- Xu, C.; Shen, G.; Yuan, X.; Kim, J.H.; Gopalkrishnan, A.; Keum, Y.S.; Nair, S.; Kong, A.N.T. ERK and JNK Signaling Pathways Are Involved in the Regulation of Activator Protein 1 and Cell Death Elicited by Three Isothiocyanates in Human Prostate Cancer PC-3 Cells. Carcinogenesis 2006, 27, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Xiao, D.; Srivastava, S.K.; Lew, K.L.; Zeng, Y.; Hershberger, P.; Johnson, C.S.; Trump, D.L.; Singh, S.V. Allyl Isothiocyanate, a Constituent of Cruciferous Vegetables, Inhibits Proliferation of Human Prostate Cancer Cells by Causing G2/M Arrest and Inducing Apoptosis. Carcinogenesis 2003, 24, 891–897. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Xiao, D.; Lew, K.L.; Hershberger, P.; Kokkinakis, D.M.; Johnson, C.S.; Trump, D.L.; Singh, S.V. Allyl Isothiocyanate, a Constituent of Cruciferous Vegetables, Inhibits Growth of PC-3 Human Prostate Cancer Xenograft In Vivo. Carcinogenesis 2003, 24, 1665–1670. [Google Scholar] [CrossRef]
- Chen, H.E.; Lin, J.F.; Tsai, T.F.; Lin, Y.C.; Chou, K.Y.; Hwang, T.I.S. Allyl Isothiocyanate Induces Autophagy through the Up-Regulation of Beclin-1 in Human Prostate Cancer Cells. Am. J. Chin. Med. 2018, 46, 1625–1643. [Google Scholar] [CrossRef]
- Xu, K.; Thornalley, P.J. Studies on the Mechanism of the Inhibition of Human Leukaemia Cell Growth by Dietary Isothiocyanates and Their Cysteine Adducts in Vitro. Biochem. Pharmacol. 2000, 60, 221–231. [Google Scholar] [CrossRef]
- Hwang, E.S.; Lee, H.J. Induction of Quinone Reductase by Allylisothiocyanate (AITC) and the N-Acetylcysteine Conjugate of AITC in Hepa1c1c7 Mouse Hepatoma Cells. BioFactors 2006, 26, 7–15. [Google Scholar] [CrossRef]
- Liu, P.; Behray, M.; Wang, Q.; Wang, W.; Zhou, Z.; Chao, Y.; Bao, Y. Anti-Cancer Activities of Allyl Isothiocyanate and Its Conjugated Silicon Quantum Dots. Sci. Rep. 2018, 8, 1084. [Google Scholar] [CrossRef]
- Hwang, E.S.; Hyong, J.L. Allyl Isothiocyanate and Its N-Acetylcysteine Conjugate Suppress Metastasis via Inhibition of Invasion, Migration, and Matrix Metalloproteinase-2/-9 Activities in SK-Hep1 Human Hepatoma Cells. Exp. Biol. Med. 2006, 231, 421–430. [Google Scholar] [CrossRef]
- Chen, N.-G.; Chen, K.-T.; Lu, C.-C.; Lan, Y.-H.; Lai, C.-H.; Chung, Y.-T.; Yang, J.-S.; Lin, Y.-C. Allyl Isothiocyanate Triggers G2/M Phase Arrest and Apoptosis in Human Brain Malignant Glioma GBM 8401 Cells through a Mitochondria-Dependent Pathway. Oncol. Rep. 2010, 24, 449–455. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Li, Y.; Wade, K.L.; Paonessa, J.D.; Fahey, J.W.; Zhang, Y. Allyl Isothiocyanate-Rich Mustard Seed Powder Inhibits Bladder Cancer Growth and Muscle Invasion. Carcinogenesis 2010, 31, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Tang, L.; Li, Y.; Yang, L.; Choi, K.S.; Kazim, A.L.; Zhang, Y. Allyl Isothiocyanate Arrests Cancer Cells in Mitosis, and Mitotic Arrest in Turn Leads to Apoptosis via Bcl-2 Protein Phosphorylation. J. Biol. Chem. 2011, 286, 32259–32267. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Tang, L.; Li, Y.; Geng, F.; Paonessa, J.D.; Chen, S.C.; Wong, M.K.K.; Zhang, Y. Inhibition of Bladder Cancer Development by Allyl Isothiocyanate. Carcinogenesis 2010, 31, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Li, Y.; Geng, F.; Munday, R.; Zhang, Y. The Principal Urinary Metabolite of Allyl Isothiocyanate, N-Acetyl-S-(N-Allylthiocarbamoyl) Cysteine, Inhibits the Growth and Muscle Invasion of Bladder Cancer. Carcinogenesis 2012, 33, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Savio, A.L.V.; da Silva, G.N.; de Camargo, E.A.; Salvadori, D.M.F. Cell Cycle Kinetics, Apoptosis Rates, DNA Damage and TP53 Gene Expression in Bladder Cancer Cells Treated with Allyl Isothiocyanate (Mustard Essential Oil). Mutat. Res.-Fundam. Mol. Mech. Mutagenes. 2014, 762, 40–46. [Google Scholar] [CrossRef]
- Sávio, A.L.V.; da Silva, G.N.; Salvadori, D.M.F. Inhibition of Bladder Cancer Cell Proliferation by Allyl Isothiocyanate (Mustard Essential Oil). Mutat. Res.-Fundam. Mol. Mech. Mutagenes. 2015, 771, 29–35. [Google Scholar] [CrossRef]
- Chang, W.; Chen, B.; Inbaraj, B.S.; Chien, J. Preparation of Allyl Isothiocyanate Nanoparticles, Their Anti-inflammatory Activity towards RAW 264.7 Macrophage Cells and Anti-proliferative Effect on HT1376 Bladder Cancer Cells. J. Sci. Food Agric. 2019, 99, 3106–3116. [Google Scholar] [CrossRef]
- Tsai, S.C.; Huang, W.W.; Huang, W.C.; Lu, C.C.; Chiang, J.H.; Peng, S.F.; Chung, J.G.; Lin, Y.H.; Hsu, Y.M.; Amagaya, S.; et al. ERK-Modulated Intrinsic Signaling and G2/M Phase Arrest Contribute to the Induction of Apoptotic Death by Allyl Isothiocyanate in MDA-MB-468 Human Breast Adenocarcinoma Cells. Int. J. Oncol. 2012, 41, 2065–2072. [Google Scholar] [CrossRef]
- Bo, P.; Lien, J.C.; Chen, Y.Y.; Yu, F.S.; Lu, H.F.; Yu, C.S.; Chou, Y.C.; Yu, C.C.; Chung, J.G. Allyl Isothiocyanate Induces Cell Toxicity by Multiple Pathways in Human Breast Cancer Cells. Am. J. Chin. Med. 2016, 44, 415–437. [Google Scholar] [CrossRef]
- Rajakumar, T.; Pugalendhi, P.; Thilagavathi, S. Protective Effect of Allyl Isothiocyanate on Glycoprotein Components in 7,12-Dimethylbenz(a)Anthracene Induced Mammary Carcinoma in Rats. Indian J. Clin. Biochem. 2018, 33, 171–177. [Google Scholar] [CrossRef]
- Rajakumar, T.; Pugalendhi, P.; Thilagavathi, S. Dose Response Chemopreventive Potential of Allyl Isothiocyanate against 7,12-Dimethylbenz(a)Anthracene Induced Mammary Carcinogenesis in Female Sprague-Dawley Rats. Chem.-Biol. Interact. 2015, 231, 35–43. [Google Scholar] [CrossRef]
- Rajakumar, T.; Pugalendhi, P.; Thilagavathi, S.; Ananthakrishnan, D.; Gunasekaran, K. Allyl Isothiocyanate, a Potent Chemopreventive Agent Targets AhR/Nrf2 Signaling Pathway in Chemically Induced Mammary Carcinogenesis. Mol. Cell. Biochem. 2018, 437, 1–12. [Google Scholar] [CrossRef]
- Kumar, A.; D’Souza, S.S.; Tickoo, S.; Salimath, B.P.; Singh, H.B. Antiangiogenic and Proapoptotic Activities of Allyl Isothiocyanate Inhibit Ascites Tumor Growth In Vivo. Integr. Cancer Ther. 2009, 8, 75–87. [Google Scholar] [CrossRef]
- Tripathi, K.; Hussein, U.K.; Anupalli, R.; Barnett, R.; Bachaboina, L.; Scalici, J.; Rocconi, R.P.; Owen, L.B.; Piazza, G.A.; Palle, K. Allyl Isothiocyanate Induces Replication-Associated DNA Damage Response in NSCLC Cells and Sensitizes to Ionizing Radiation. Oncotarget 2015, 6, 5237–5252. [Google Scholar] [CrossRef] [PubMed]
- Rakariyatham, K.; Yang, X.; Gao, Z.; Song, M.; Han, Y.; Chen, X.; Xiao, H. Synergistic Chemopreventive Effect of Allyl Isothiocyanate and Sulforaphane on Non-Small Cell Lung Carcinoma Cells. Food Funct. 2019, 10, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Li, P.; Xue, Z. Effect of Allyl Isothiocyanate on the Viability and Apoptosis of the Human Cervical Cancer HeLa Cell Line in Vitro. Oncol. Lett. 2018, 15, 8756–8760. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.K.; Lund, E.K.; Parker, M.L.; Clarke, R.G.; Johnson, I.T. Allyl-Isothiocyanate Causes Mitotic Block, Loss of Cell Adhesion and Disrupted Cytoskeletal Structure in HT29 Cells. Carcinogenesis 2004, 25, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.C.; Lu, C.C.; Tang, Y.J.; Chiang, J.H.; Kuo, D.H.; Chen, F.A.; Chen, I.L.; Yang, J.S. Allyl Isothiocyanate Inhibits Cell Metastasis through Suppression of the MAPK Pathways in Epidermal Growth Factor-Stimulated HT29 Human Colorectal Adenocarcinoma Cells. Oncol. Rep. 2014, 31, 189–196. [Google Scholar] [CrossRef]
- Musk, S.R.R.; Johnson, I.T. Allyl Isothiocyanate Is Selectively Toxic to Transformed Cells of the Human Colorectal Tumour Line Ht29. Carcinogenesis 1993, 14, 2079–2083. [Google Scholar] [CrossRef]
- Lau, W.S.; Chen, T.; Wong, Y.S. Allyl Isothiocyanate Induces G2/M Arrest in Human Colorectal Adenocarcinoma SW620 Cells through down-Regulation of Cdc25B and Cdc25C. Mol. Med. Rep. 2010, 3, 1023–1030. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Mantso, T.; Trafalis, D.T.; Vasantha Rupasinghe, H.P.; Zoumpourlis, V.; Franco, R.; Botaitis, S.; Pappa, A.; Panayiotidis, M.I. Allyl Isothiocyanate Regulates Lysine Acetylation and Methylation Marks in an Experimental Model of Malignant Melanoma. Eur. J. Nutr. 2020, 59, 557–569. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, X.; Chang, K.; Liu, X.; Xiong, J. Allyl Isothiocyanate Inhibits the Proliferation of Renal Carcinoma Cell Line GRC-1 by Inducing an Imbalance between Bcl2 and Bax. Med. Sci. Monit. 2016, 22, 4283–4288. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.-Y.; Tsai, F.-J.; Bau, D.-T.; Hsu, Y.-M.; Yang, J.-S.; Tu, M.-G.; Chiang, S.-L. Potential Effects of Allyl Isothiocyanate on Inhibiting Cellular Proliferation and Inducing Apoptotic Pathway in Human Cisplatin-Resistant Oral Cancer Cells. J. Formos. Med. Assoc. 2021, 120, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, M.A.; Bracci, M.; Ciarapica, V.; Malavolta, M.; Provinciali, M.; Pieragostini, E.; Gaetani, S.; Monaco, F.; Lucarini, G.; Rapisarda, V.; et al. Allyl Isothiocyanate Exhibits No Anticancer Activity in MDA-MB-231 Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Barnett, R.; Tripathi, K.; Bachaboina, L.; Hussien, U.; Scalici, J.M.; Rocconi, R.P.; Palle, K. Dietary Isothiocyanates Inhibit Cancer Cell Growth by Inducing Replication Stress Mediated DNA Damage Response. Gynecol. Oncol. 2015, 137, 65. [Google Scholar] [CrossRef]
- Chiang, J.-H.; Lu, C.-C.; Yang, J.-S. Allyl Isothiocyanate Induces Apoptotic Mechanism via Endoplasmic Reticulum Stress and Mitochondrial Pathway in Human Colorectal Cancer HT29 Cells. In Proceedings of the 8th World Congress on Toxicology and Pharmacology, Dubai, United Arab Emirates, 13–15 April 2017; OMICS International: Dubai, United Arab Emirates, 2017; Volume 3. [Google Scholar] [CrossRef]
- Chiang, J.H.; Tsai, F.J.; Hsu, Y.M.; Yin, M.C.; Chiu, H.Y.; Yang, J.S. Sensitivity of Allyl Isothiocyanate to Induce Apoptosis via ER Stress and the Mitochondrial Pathway upon ROS Production in Colorectal Adenocarcinoma Cells. Oncol. Rep. 2020, 44, 1415. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, F.; Cao, X.; Chen, C.; Zhang, X.; Zhang, X.; Lin, W.; Wang, X.; Liang, C. Gab1 Regulates SDF-1-Induced Progression via Inhibition of Apoptosis Pathway Induced by PI3K/AKT/Bcl-2/BAX Pathway in Human Chondrosarcoma. Tumor Biol. 2016, 37, 1141–1149. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L.; Gonzalez, V. Selected Isothiocyanates Rapidly Induce Growth Inhibition of Cancer Cells. Mol. Cancer Ther. 2003, 2, 1045–1052. [Google Scholar]
- Norbury, C.; Nurse, P. Animal Cell Cycles and Their Control. Annu. Rev. Biochem. 1992, 61, 441–470. [Google Scholar] [CrossRef]
- Diaz-Moralli, S.; Tarrado-Castellarnau, M.; Miranda, A.; Cascante, M. Targeting Cell Cycle Regulation in Cancer Therapy. Pharmacol. Ther. 2013, 138, 255–271. [Google Scholar] [CrossRef]
- Zhang, Y. Cancer-Preventive Isothiocyanates: Measurement of Human Exposure and Mechanism of Action. Mutat. Res.-Fundam. Mol. Mech. Mutagenes. 2004, 555, 173–190. [Google Scholar] [CrossRef]
- Hasegawa, T.; Nishino, H.; Iwashima, A. Isothiocyanates Inhibit Cell Cycle Progression of HeLa Cells at G2/M Phase. Anti-Cancer Drugs 1993, 4, 273–279. [Google Scholar] [CrossRef]
- Maeda, T.; Nagaoka, Y.; Kawai, Y.; Takagaki, N.; Yasuda, C.; Yogosawa, S.; Sowa, Y.; Sakai, T.; Uesato, S. Inhibitory Effects of Cancer Cell Proliferation by Novel Histone Deacetylase Inhibitors Involve P21/WAF1 Induction and G2/M Arrest. Biol. Pharm. Bull. 2005, 28, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef] [PubMed]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular Basis for Chemoprevention by Sulforaphane: A Comprehensive Review. Cell. Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.L.; Li, F.; Lampe, J.W. Mechanisms of Action of Isothiocyanates in Cancer Chemoprevention: An Update. Food Funct. 2011, 2, 579. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Lai, E.; Teodoro, T.; Volchuk, A. Endoplasmic Reticulum Stress: Signaling the Unfolded Protein Response. Physiology 2007, 22, 193–201. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The Extracellular Matrix: A Dynamic Niche in Cancer Progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Kapral, M.; Wawszczyk, J.; Jurzak, M.; Dymitruk, D.; Weglarz, L. Evaluation of the Expression of Metalloproteinases 2 and 9 and Their Tissue Inhibitors in Colon Cancer Cells Treated with Phytic Acid—PubMed. Acta Pol. Pharm. 2010, 67, 625–629. [Google Scholar]
- Murray, D.; Morrin, M.; Mcdonnell, S. Increased Invasion and Expression of MMP-9 in Human Colorectal Cell Lines by a CD44-Dependent Mechanism. Anticancer Res. 2004, 24, 489–494. [Google Scholar]
- Hidalgo, M.; Eckhardt, S.G. Development of Matrix Metalloproteinase Inhibitors in Cancer Therapy. JNCI J. Natl. Cancer Inst. 2001, 93, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Herman-Antosiewicz, A.; Johnson, D.E.; Singh, S.V. Sulforaphane Causes Autophagy to Inhibit Release of Cytochrome c and Apoptosis in Human Prostate Cancer Cells. Cancer Res. 2006, 66, 5828–5835. [Google Scholar] [CrossRef] [Green Version]
- Fimognari, C.; Lenzi, M.; Hrelia, P. Chemoprevention of Cancer by Isothiocyanates and Anthocyanins: Mechanisms of Action and Structure-Activity Relationship. Curr. Med. Chem. 2008, 15, 440–447. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 Network Regulates Autophagy and Apoptosis. Cell Death Differ. 2011, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis and Apoptosis. Semin. Cancer Biol. 2003, 13, 159–167. [Google Scholar] [CrossRef]
- Cook, K.M.; Figg, W.D. Angiogenesis Inhibitors: Current Strategies and Future Prospects. CA Cancer J. Clin. 2010, 60, 222–243. [Google Scholar] [CrossRef]
- Thejass, P.; Kuttan, G. Inhibition of Endothelial Cell Differentiation and Proinflammatory Cytokine Production during Angiogenesis by Allyl Isothiocyanate and Phenyl Isothiocyanate. Integr. Cancer Ther. 2007, 6, 389–399. [Google Scholar] [CrossRef]
- Keum, Y.S.; Jeong, W.S.; Tony Kong, A.N. Chemoprevention by Isothiocyanates and Their Underlying Molecular Signaling Mechanisms. Mutat. Res. /Fundam. Mol. Mech. Mutagenesis 2004, 555, 191–202. [Google Scholar] [CrossRef]
- Talalay, P.; Fahey, J.W. Phytochemicals from Cruciferous Plants Protect against Cancer by Modulating Carcinogen Metabolism. J. Nutr. 2001, 131, 3027S–3033S. [Google Scholar] [CrossRef]
- Kensler, T.W. Chemoprevention by Inducers of Carcinogen Detoxication Enzymes. Environ. Health Perspect. 1997, 105 (Suppl. 4), 965. [Google Scholar] [CrossRef]
- Hayes, J.D.; McLellan, L.I. Glutathione and Glutathione-Dependent Enzymes Represent a Co-Ordinately Regulated Defence against Oxidative Stress. Free Radic. Res. 2009, 31, 273–300. [Google Scholar] [CrossRef]
- Talalay, P. Chemoprotection against Cancer by Induction of Phase 2 Enzymes. BioFactors 2000, 12, 5–11. [Google Scholar] [CrossRef]
- Munday, R.; Munday, C.M. Selective Induction of Phase II Enzymes in the Urinary Bladder of Rats by Allyl Isothiocyanate, a Compound Derived from Brassica Vegetables. Nutr. Cancer 2009, 44, 52–59. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair and Mutagenesis. Environ. Mol. Mutagenes. 2017, 58, 235. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.; Grilli, S.; Gürtler, R.; König, J.; Lambré, C.; Larsen, J.; Leblanc, J.; et al. Scientific Opinion on the Safety of Allyl Isothiocyanate for the Proposed Uses as a Food Additive. EFSA J. 2010, 8, 1943. [Google Scholar] [CrossRef]
- Zhang, Y. Allyl Isothiocyanate as a Cancer Chemopreventive Phytochemical. Mol. Nutr. Food Res. 2010, 54, 127–135. [Google Scholar] [CrossRef]
- Li, Y.; Teng, Z.; Chen, P.; Song, Y.; Luo, Y.; Wang, Q. Enhancement of Aqueous Stability of Allyl Isothiocyanate Using Nanoemulsions Prepared by an Emulsion Inversion Point Method. J. Colloid Interface Sci. 2015, 438, 130–137. [Google Scholar] [CrossRef]
- Encinas-Basurto, D.; Ibarra, J.; Juarez, J.; Burboa, M.G.; Barbosa, S.; Taboada, P.; Troncoso-Rojas, R.; Valdez, M.A. Poly(Lactic-Co-Glycolic Acid) Nanoparticles for Sustained Release of Allyl Isothiocyanate: Characterization, in Vitro Release and Biological Activity. J. Microencapsul. 2017, 34, 231–242. [Google Scholar] [CrossRef]
- Encinas-Basurto, D.; Juarez, J.; Valdez, M.A.; Burboa, M.G.; Barbosa, S.; Taboada, P. Targeted Drug Delivery Via Human Epidermal Growth Factor Receptor for Sustained Release of Allyl Isothiocyanate. Curr. Top. Med. Chem. 2018, 18, 1252–1260. [Google Scholar] [CrossRef]
- Tarar, A.; Alyami, E.M.; Peng, C.A. Eradication of Myrosinase-Tethered Cancer Cells by Allyl Isothiocyanate Derived from Enzymatic Hydrolysis of Sinigrin. Pharmaceutics 2022, 14, 144. [Google Scholar] [CrossRef]



| Cancer Cell Type | In Vivo/In Vitro Model, Cell Type | Concentration Range | Treatment Time | IC50/GC50/EC50 Values | References |
|---|---|---|---|---|---|
| Non-metastatic and metastatic melanoma cells | In vitro model, A375, B16F-10, VMM1, Hs294T, A431, HaCaT cells | 10 μM | 24 and 48 h | - | Mitsiogianni et al., 2021 [9] |
| Human prostate cancer cells | In vitro, PC-3 cells | 50–100 μM | 24 h and 48 h | Dose-dependent | Xu et al., 2005 [10] |
| In vitro, PC-3 cells, In vivo, PC-3 xenografts model | PC-3 xenografts (Bolus i.p. injection of 10 mmol AITC), PC-3 cells (0–9 μM) | PC-3 xenografts (three times per week), PC-3 cells (10 days) | IC50 of ~2.2 μM for PC-3 cells | Srivastava et al., 2003 [11,12] | |
| In vitro, PC-3 (androgen-independent) and LNCaP (androgen-dependent), PrEC cells | 20 μM AITC | 24, 48, or 72 h | IC50 of ~15–17 μM | Xiao et al., 2003 [11] | |
| In vitro, PC3 (CRL-1435), CWR22Rv1 (Rv1; CRL-2505), PrECs | 0–80 μM AITC | 24 h to 3 days | - | Chen et al., 2018 [13] | |
| Human cisplatin-resistant oral cancer cells | In vitro, CAL27 (CAR cells) | 0, 10, 20, 30, and 40 μM | 24 and 48 h | Dose-dependent | Chang et al., 2020 [13] |
| Human leukemia cell | In vitro, HL60 (p53-), ML1(p53+) | 100 nM–50 μM | 48 h | GC50 for ML-1 cells (2.41–3.22 μM), GC50 for HL60 cells (1.49–3.22 μM) | Xu et al., 2000 [14] |
| Human and mouse hepatoma cells | In vitro, mouse Hepa1c1c7 cells | AITC (0.1–20 μM), AITC-NAC (1 and 20 μM) | 24 h | Dose-dependent | Hwang et al., 2005 [15] |
| In vitro, HepG2, HHL5, murine MII perivascular M2 cells | AITC (0–320 μM), AITC-SiQDs (0–40 μM) | 0 to 24 h | Dose-dependent | Liu et al., 2018 [16] | |
| In vitro, SK-Hep l cells | AITC (0–20 μM), NAC-AITC (0–20 μM) | 24 and 72 h | Dose-dependent | Hwang et al., 2006 [17] | |
| Human brain malignant glioma cells | In vitro, GBM 8401 cells | 0.5, 1, 5, 10, and 20 μM | 24 h | IC50 (9.25 ± 0.69 μM) | Chen et al., 2010 [18] |
| Human bladder cancer cells | In vitro, UM-UC-3 cells, AY-27 cells, In vivo, orthotopic AY27 cells in a female F344 rat model | 13 and 26 μM for in vitro model, 9 or 90 μmol/kg bw* (71.5 or 715 mg MSP-1 per kg bw*) for in vivo model | In vitro model (24 and 72 h); in vivo (once daily for 3 weeks, started 1 day after cancer cell inoculation) | IC50 values of 10.8 and 8.6 μM for UM-UC-3, and AY-27 cells, respectively, 85.8 and 68.3 µg MSP- 1 per ml culture medium, respectively | Bhattacharya et al., 2010 [19] |
| In vitro, UM-UC-3 cells, UM-UC-6 cells, and T24 cells | 0, 7.5, 15, and 30 μM | 24 h | Dose-dependent | Geng et al., 2011 [20] | |
| In vitro, UM-UC-3 cells, AY-27, HUCs, in vivo, AY-27 cells were simultaneously inoculated both orthotopically and subcutaneously in a rat | In vitro (1–100 μM), In vivo (0, 10, 25, 50, 300 μmol/kg) | In vivo (once daily), in vitro (72 h) | IC50 of 2.7, 3.3, and 69.4 μM for UM-UC-3, AY-27, and HUC cells, respectively | Bhattacharya et al., 2009 [21] | |
| In vitro, UM-UC-3, AY-27 cell line; in vivo, female F344 rats | In vitro (NAC-AITC at 15 μM in UM-UC-3 and AY-27 cells); in vivo (at 10 μmol/kg body wt orally in rat bladder cancer model) | In vitro (24 h), in vivo (initiated 1 day after AY-27 cell inoculation and continued for 3 weeks) | IC50 of 7.4 and 9.1 μM for UM-UC-3, AY-27 cells, respectively | Bhattacharya et al., 2011 [22] | |
| In vitro, RT4 cell lines with a wild-type TP53 gene, T24 cell line with the TP53 allele | 5.0, 62.5, 72.5, 82.5, and 92.5 μM | 3 h | IC50 values of 310 and 350 μM for RT4 and T24 cells, respectively | Sávio et al., 2014 [23,24] | |
| In vitro HT1376 cells | AITC-equivalent doses of AITC-NPs (0.25, 0.50, 1.00, 1.43, 2.00, 2.50, and 3.34 g/L) | 4 to 24 h | IC50 of 1.15 g/L of AITC-NPs or 35.87 mg/L of AITC | Chang et al., 2018 [25] | |
| Macrophages | In vitro, RAW 264.7 cells | AITC-equivalent doses of AITC-NPs (0.25, 0.50, 1.00, 1.43, 2.00, 2.50, and 3.34 g/L) | 4 to 24 h | IC50 of 0.89 g/L of AITC-NPs and 31.1 mg/L of AITC | Chang et al., 2018 [25] |
| Human breast adenocarcinoma cells | In vitro MDA-MB-468 cells | 0,5, 10, and 20 μM | 24 and 48 h | IC50 of 10.26 ± 1.31 μM | Tsai et al., 2012 [26] |
| Human breast cancer Cells | In vitro MCF-7 (estrogen receptor positive), MDA-MB-231 (estrogen receptor negative) cells | 0, 1.5625, 3.125, 6.25, 12.5, and 25 μM | 48 h | Dose-dependent | Bo et al., 2016 [27] |
| Human and mouse mammary carcinoma | In vivo, female Sprague–Dawley rats | 10, 20, and 40 mg/kg bw* | once a day by starting one week before the exposure to the carcinogen | Dose-dependent | Thangarasu et al., 2018 and 2015 [28,29,30] |
| In vitro, EAT (Ehrlich ascites tumor) cells; in vivo, EAT cells were injected into 8-week-old Swiss Albino mice | 1, 5, 10, and 15 μM | 24, 48, and 72 h | Dose-dependent | Kumar et al., 2009 [31] | |
| Human non-small cell lung cancer (NSCLC) cells | In vitro, A549 cells, H1299, HBECs cells | 5, 10, and 20 μM | 6, 16, 24, and 48 h | IC50 values of 10 and 5 μM for A549 and H1299 cells, respectively | Tripathi et al., 2015 [32] |
| In vitro, A549 cells | AITC (2.5–12.5 μM), AITC + SFN (6.25 μM AITC with 5 μM SFN) | 72 h | IC50 values of 12.64 μM for AITC, IC50 of 5.53 μM AITC, and 4.43 μM SFN for the combined treatment | Rakariyatham et al., 2019 [33] | |
| Human cervical cancer cells | In vitro, HeLa cells | 0, 5, 15, and 45 μM AITC | 24, 48, and 72 h | Dose-dependent | Qin et al., 2017 [34] |
| Human colorectal adenocarcinoma cells | In vitro, HT-29 cells | 12 μM | 7 and 24 h | - | Smith et al., 2004 [35] |
| In vitro, HT29 cells | 5 and 10 μM of AITC | 24 h | Dose-dependent | Lai et al., 2013 [36] | |
| In vitro, HT29 cells | 1.2–1.6 µg/ml | 24 h | Dose-dependent | Musk et al., 1993 [37] | |
| In vitro, Hs68, Caco-2, COLO 201, SW620 cells; in vivo, SW620 xenograft | In vitro (0–150 μM), SW620 xenografts (5 and 10 μmol) | 24 and 72 h | Dose-dependent | Lau et al., 2010 [38] | |
| Malignant melanoma cells | In vitro, A375, B16-F10, VMM1, Hs294T, A431, HaCaT cells | 2.5 and 50 μM | 24 and 48 h | EC50 of 15.6 and 21.7 μM for A375 and Hs294T cells, respectively, after 24 h and 12.0, 43.4, 21.3, and 14.9 μM for A375, A431, Hs294T, and B16-F10 cells, respectively, after 48 h | Mitsiogianni et al., 2020 [39] |
| Renal carcinoma cell line (RCC) | In vitro, GRC-1 cells | 0, 7.5, 15, and 30 μM | 24, 48, and 72 h | Dose-dependent | Jiang et al., 2016 [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarar, A.; Peng, S.; Cheema, S.; Peng, C.-A. Anticancer Activity, Mechanism, and Delivery of Allyl Isothiocyanate. Bioengineering 2022, 9, 470. https://doi.org/10.3390/bioengineering9090470
Tarar A, Peng S, Cheema S, Peng C-A. Anticancer Activity, Mechanism, and Delivery of Allyl Isothiocyanate. Bioengineering. 2022; 9(9):470. https://doi.org/10.3390/bioengineering9090470
Chicago/Turabian StyleTarar, Ammar, Sarah Peng, Soha Cheema, and Ching-An Peng. 2022. "Anticancer Activity, Mechanism, and Delivery of Allyl Isothiocyanate" Bioengineering 9, no. 9: 470. https://doi.org/10.3390/bioengineering9090470
APA StyleTarar, A., Peng, S., Cheema, S., & Peng, C.-A. (2022). Anticancer Activity, Mechanism, and Delivery of Allyl Isothiocyanate. Bioengineering, 9(9), 470. https://doi.org/10.3390/bioengineering9090470







