Abstract
Winemaking is one of the oldest biotechnology techniques in the world. The wine industry generates 20 million tons of by-products, such as wastewater, stalk, lees, pomace, and stems, each year. The objective of this research project is to valorize wine industry by-products by producing a functional beverage via the fermentation of grape pomace with the kombucha consortium. In this study, grape pomace kombucha was produced under different conditions, and the concentration of the added sucrose in addition to the fermentation duration and temperature were varied. Overall, fermentation was characterized by the consumption of sugars and the production of organic acids and ethanol. An improvement in the concentrations of the total polyphenols and anthocyanins was observed in the developed product (i.e., up to 100%). Moreover, an enhancement of the antioxidant potential by 100%, as well as increases of 50 to 75% in the anti-inflammatory and antidiabetic activities, was noted.
1. Introduction
In recent decades, there has been a growing interest in gaining value from agro-industrial wastes that is driven by both environmental concerns and economic considerations. According to the International Organization of Vine and Wine (OIV), worldwide wine production in 2023 was estimated to be 250 million hl. The wine industry produces significant amounts of waste annually such as grape pomace, vine shoots, stalks, lees, and filtration cakes, thereby averaging 0.3–0.5 kg of by-products per liter of wine made [1]. Therefore, the global production of waste is estimated between 7.5 and 12.5 million kg. These winery wastes are characterized by an acidic pH, a notable polyphenol content, and low levels of micronutrients and heavy metals. Due to their complex chemical composition, these wine by-products can undergo various treatments and have multiple beneficial applications. They can be repurposed for composting, animal feed, the extraction of bioactive compounds, and the creation of biodegradable packaging, as well as serve as additives in food and cosmetic industries, among other possibilities [2].
Kombucha is a traditional beverage originally prepared by the fermentation of sweetened tea leaves with a symbiotic consortium of yeasts and bacteria (SCOBY). During kombucha production, several microbial interactions take place where sugars (glucose and fructose) are fermented into the ethanol by the yeasts, and the alcohol is converted into acetic acid by the acetic acid bacteria. This process results in the formation of a characteristic biofilm on the drink’s surface, which is attributed to the microorganisms synthetizing the cellulose; hence, the name “tea fungus”, which is associated with kombucha. The microbial composition of kombucha varies based on factors like the inoculum’s type and source, the substrate, and processing conditions [3]. Recently, kombucha microbiota have been reviewed, which include yeasts such as Brettanomyces, Saccharomyces, and Candida; lactic acid bacteria such as Lactobacillus, Lactococcus, and Oenococcus; and acetic acid bacteria such as Acetobacter, Gluconoacetobacter, Gluconobacter, and Komagataeibacter [4,5].
Renowned for its rich composition in bioactive compounds, kombucha has demonstrated various interesting biological activities including antioxidant, antimicrobial, antihypertensive, and anticarcinogenic properties. The interest in this industry has been actively growing during the past few years, with market estimations reaching USD 3.5 to 5 billion by 2025 [6]. The therapeutic potential of the beverage along with the desire to valorize agro-industrial residues has led researchers to explore the fermentation of non-tea substrates with the kombucha inoculum. These substrates encompass fruits, vegetables, plants, herbs, dairy, baked goods, and industrial by-products. Scientists have investigated the fermentation of leaves, peels, cascara, kernels, broth, and wastewater by kombucha cultures, which has resulted in improved phenolic profiles and the enhancement of some biological activities [6].
However, studies exploring kombucha production from grapes and grape by-products remain limited. Ayed et al. [7] examined the fermentation of red grape juice using kombucha cultures, which resulted in a product displaying antioxidant and antimicrobial activities. Additionally, the valorization of winery effluents by the kombucha consortium was evaluated, showing that the resulting kombucha was rich in organic acids, total polyphenols, contained vitamin C, and exhibited strong antioxidant activity [8,9].
Grape pomace, a commonly overlooked wine by-product, has not previously been explored as a substrate for kombucha fermentation. Consequently, our study aimed to explore the potential of utilizing grape pomace for kombucha fermentation with the purpose of creating a healthful beverage. Different fermentation conditions were tested in order to optimize the process: the quantity of added sucrose, fermentation duration, and temperature.
2. Materials and Methods
2.1. Chemicals and Reagents
The chemicals and reagents used were of chemical grade and were obtained from SIGMA-Aldrich (Saint-Louis, MO, USA), Fisher Scientific (Asin, Pergain-Taillac, France), Becton Dickinson & Company (Franklin Lakes, NJ, USA).
2.2. Kombucha Inoculum and the Fermentation Process
The kombucha inoculum and was reproduced by the backslopping method as described by Villarreal-Soto et al. [10]. The microbial composition of this inoculum was determined in accordance with the method used by Villarreal-Soto et al. [11]. The most represented bacteria and yeasts genera were Gluconoacetobacter, Gluconobacter, Komagataeibacter, Brettanomyces, and Schizosaccharomyces. Water was heated to 40 °C and sucrose was added at a concentration of 70 g/L. Once the temperature reached 80 °C, 10 g/L of black tea (Ceylan) was infused for 15 min. The mixture was cooled to 20 °C, and 980 mL was poured into 2 L beakers and inoculated with 20 g of kombucha biofilm and 20 mL of fermented kombucha tea. Next, 10 mL of apple cider vinegar was added, and then fermentation was carried out for 14 days at 25 °C in an incubator (Binder series KT) [10].
2.3. Grape Pomace Kombucha
Grape pomace was collected from a local winery in Lebanon. It was made of different grape varieties including Cabernet Sauvignon, Cabernet Franc, Syrah, and Merlot. The material was dried using an air dryer chamber at a low temperature (30 °C), and it was grinded using Robot Coupe R5 plus and stored in polyethylene bags at −18 °C.
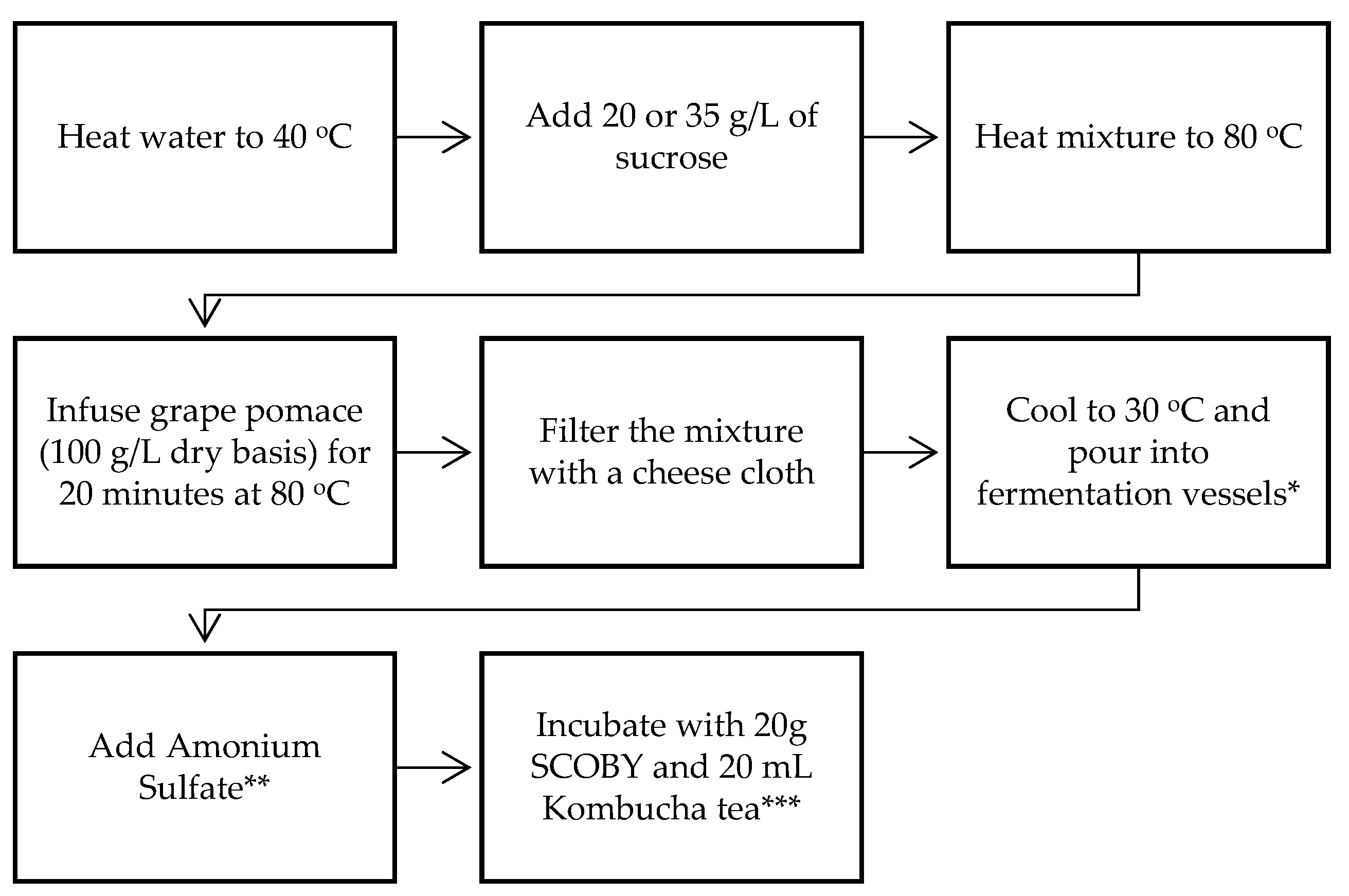
Different batches of grape pomace kombucha were prepared by varying the following three parameters: the sucrose concentration, the fermentation duration, and temperature. The same procedure used in the preparation of tea kombucha was also followed to produce grape pomace kombucha, with a few modifications. The procedure is illustrated in Figure 1. Pomace (100 g/L dry weight) was added to the heated and sweetened water (20 or 35 g/L sucrose), and it was infused at 80 °C for 15–20 min. After cooling to 30 °C, ammonium sulfate (0.20–0.25 g/L), kombucha biofilm (SCOBY), and kombucha tea were added to the infusion. Fermentation was carried out at 20 or 25 °C for 7 or 10 days depending on the sample. The concentrations of the products and processing parameters are presented in Figure 1.
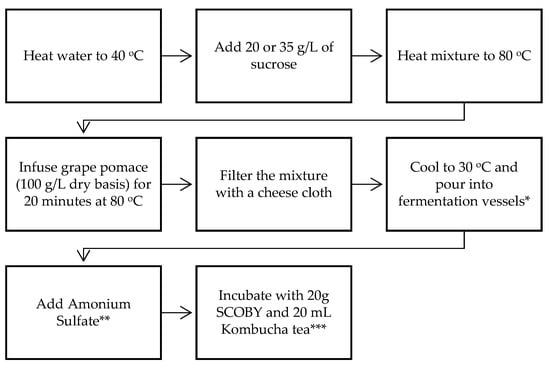
Figure 1.
Grape pomace kombucha production flowchart. * 2 L glass beakers (diameter = 6.5 cm) were used. ** 0.2 g/L of (NH4)2SO4 was added for the samples prepared with 20 g/L of sucrose, and 0.25 g/L of (NH4)2SO4 was added for the samples prepared with 35 g/L of sucrose. *** Fermentation was carried for 7 or 10 days, at 20 and 25 °C.
2.4. Chemical Composition Analysis of the Grape Pomace Kombucha Samples
2.4.1. pH, Titratable Acidity, Brix Level, Sugar, and Acid Analysis
The pH was determined using a pH meter (Eutech Instruments-pH700). Titratable acidity was obtained based on the OIV-MA-S313-01 method, where the samples were titrated with 0.1 N of NaOH. The brix value was measured by a refractometer (ATAGO pocket refractometer).
The concentrations of glucose, fructose, sucrose, malic acid, tartaric acid, acetic acid, lactic acid, gluconic acid, and ethanol were determined by High Performance Liquid Chromatography (HPLC, Thermoscientific, Asnières-sur-Seine, France) using an HPX87H Aminex Biorad column (300 × 7.8 mm) that was thermostated at 50 °C. Both refractive index and UV detectors were used and regulated at 40 °C. Samples were centrifuged at 10,000 rpm for 5 min (Fisherbrand Centrifuge, Waltham, MA, USA). The supernatant was then filtered through a 0.45 μm membrane filter (Fisherbrand, PTFE) into vials and diluted with deionized water (1:10). The elution was conducted with a constant flow rate of 0.6 mL/min using a 10 mM sulfuric acid solution as a solvent (pH 2.2). Next, 20 μL of the sample was automatically injected into the equipment for analysis. Compounds were quantified through standard curves (expressed in g/L). Standard solutions were prepared with de-ionized water at concentrations ranging between 0 and 15 g/L.
2.4.2. Polyphenol Analysis
The concentration of total polyphenols was determined by following the Folin–Ciocalteu colorimetric method [12]. Samples were diluted (1:10) with distilled water. Standard solutions of gallic acid were prepared at concentrations of 0, 10, 25, 50, and 80 mg/L. The analysis was carried in a 96-well microplate. The procedure included adding 20 µL of standard solution or sample and 10 µL of Folin–Ciocalteu reagent, which were placed on the microplate in the microplate reader (BMG-Labtech Spectrostar-Nano, Champigny-sur-Marne, France) and agitated for 10 s. Then, 170 µL of Na2CO3 (2.36%, w/w) was added, and the microplate was agitated for 10 s. The absorbance was read at 760 nm at 45 °C for a reaction time of 45 min. The concentration of total polyphenols was obtained by plotting the absorbance of the sample on the gallic acid calibration curve, which was expressed in GAE mg/L.
The phenolic compounds were identified through HPLC analysis in an ultimate 3000 pump-Dionex and a DAD detector (ThermoFisher Scientific, Waltham, MA, USA). Separation was achieved on an RPC18 reversed-phase column (Phenomenex, Le Pecq, France), which was of a 25 cm × 4.6 mm size, and the particles of a 5 μm size were thermostated at 25 °C in accordance with the method used by Villarreal-Soto et al. [9] with some alterations. The elution was performed at a flow rate of 1.2 mL/min using a mobile phase consisting of acidified MilliQ water (acetic acid, pH = 2.6) (Solvent A) and acidified water/Acetonitrile (20:80 v/v) (Solvent B). The gradient followed was from 12% B to 30% B for 35 min, from 30% B to 50% for 5 min, from 50% B to 88% B for 5 min, and from 88% B to 12% B for 15 min. The extracts were prepared at a concentration of 20 mg/L in a mixture of acidified water/Acetonitrile (80:20 v/v) and filtered with a Millex-HA 0.45 µm syringe filter (Sigma Aldrich, Saint-Louis, MO, USA). The injection volume was 20 μL. The detection was performed at 280 nm. The phenolic compounds were identified by contrasting the retention time of the detected peaks to those of the known standards.
The total anthocyanin concentration was estimated by the sulfur dioxide discoloration method that was used by Ghanem et al. [13] with a few modifications.
2.5. Antioxidant Activity
The antioxidant potential of the kombucha beverage was evaluated by measuring the scavenging activity against the 1,1-diphenyl-2-picrylhydrazyl free radicals (DPPH) with the method used by Dawra et al. [14]. The DPPH solution was prepared by adding 4.8 mg of DPPH reagent to 50 mL of ethanol (96%). The solution was covered by aluminum film to prevent any damage that could be caused by light exposure and stored in the fridge. The mixture was diluted with ethanol to provide absorbance measurements of 0.7 to 0.9. Then, 40 to 120 µL of the diluted sample was added to 150 µL of ethanol. The volume was completed to 300 µL with ethanol. After an incubation period of 25 min at room temperature, the absorbance was read for a duration of 45 min (3 cycles of 15 min) at 516 nm. The antioxidant activity was expressed by IC50 (mL/L) as different concentrations of the samples were tested.
2.6. Anti-Inflammatory and Anti-Diabetic Activities
The anti-inflammatory and antidiabetic activities were conducted on grape pomace kombucha ethanolic extracts at a concentration of 50 mg/L. The kombucha was first evaporated through a vacuum rotary evaporator at 35 °C to obtain a dry extract. The dry extract was collected, weighed, and stored at ambient temperature. An ethanolic extraction was conducted by dissolving 1 g of dry extract in 20 mL of ethanol, and the mixture was placed once again in a vacuum rotary evaporator (35 °C) until dryness was achieved to obtain the final ethanolic extract. The anti-inflammatory potential was evaluated with the method described by Villarreal-Soto et al. [10], where the capacity of inhibiting the activity of 15-lipoxygenase enzyme (15-lox) was measured. Nordihydroguaiaretic acid (NDGA) was used as a control.
The antidiabetic activity of grape pomace kombucha was evaluated by measuring the inhibitory assays of α-amylase and α-glucosidase enzymes with the method described by Ghanem et al. [15]. The α-amylase enzyme solution was prepared by dissolving 3 mg of porcine pancreatic α-amylase powder in 5 mL of potassium phosphate buffer solution—which contained 80.1 g of sodium phosphate monobasic dihydrate (NaH2PO4, 2H2O) in 450 mL of distilled water—and 78.1 g of disodium phosphate (Na2HPO4) in 550 mL of distilled water (pH = 6.9). The absorbance was read at 540 nm after adding DNS reagent (0.109 g of 3,5-Dinitrosalicylic acid and 2.28 g of sodium potassium tartrate (C4H4KNaO6)) in 50 mL of NaOH (2M) to evaluate the degradation of the 1% potato starch by the different extracts.
The determination of the inhibited α-glucosidase was conducted by measuring the absorbance (405 nm) of the solutions, which were prepared by adding the enzyme (1 U/mL buffer) to the samples and then adding 5 mM of 4-Nitrophenyl-β-D-glucopyranoside (PNP-G). Acarbose was used as a standard inhibitor for both assays.
2.7. Statistical Analysis
All measurements were conducted in triplicates. The means, standard deviations, curves, and their equations were obtained through Microsoft Excel (version 16.0).
IBM SPSS Statistics (version 26) was used to perform an analysis of variance (ANOVA), Tukey’s honestly significant difference (HSD) test, and to determine the Pearson’s correlation coefficient.
3. Results
3.1. Chemical Analysis
At the beginning and end of fermentation, the grape pomace kombucha samples were analyzed for their pH levels, brix degrees, total acidity, total sugar content, and concentrations of acetic acid and ethanol. The results are presented in Table 1.

Table 1.
Chemical analysis of the grape pomace kombucha samples.
Brix measurements provide a general idea on the concentration of sugars. On Day 0, the brix values were found to be between 3.9 and 5.3. The brix levels decreased in all samples during fermentation, thereby indicating a consumption of sugars. The drop ranged between 43 and 54% depending on the sample. The initial pH of the different grape pomace kombucha samples ranged between 3.23 and 3.38. In parallel, the titratable acidity (initially ranging between 3.4 and 3.8 g/L) increased in all samples. The highest concentration was recorded in the beverages prepared with 35 g/L of sucrose and fermented for 10 days at 25 °C (12.41 g/L).
On Day 0, the concentration of total sugars ranged between 37 and 55 g/L depending on the sample and the sugar added during the preparation. At the end of fermentation, the quantity of the residual sugars was significantly different in the majority of the samples. However, it was noticed that the beverages were rich in sugars; thus, lowering the amount of added sucrose might have been beneficial and provided an economical advantage. On the other hand (in terms of acetic acid), we perceived that the most acidic samples (10.98, 11.58, and 13.03 g/L of acetic acid) were significantly similar. Moreover, statistical resemblances were found between the other samples, regardless of the fermentation conditions. Finally, with regard to ethanol, the sample prepared with 35 g/L of sucrose and fermented for 7 days at 25 °C had the highest concentration (9.30 g/L) and was significantly different than all the other samples (p < 0.05).
3.2. Total Polyphenol, Anthocyanin, and Antioxidant Activity of the Grape Pomace Kombucha
In order to assess the effect of the kombucha fermentation on grape pomace, the samples were analyzed for their concentrations of total polyphenols and anthocyanins. The results are presented in Table 2.

Table 2.
Concentrations of the total polyphenols, total anthocyanins and the antioxidant activity, at the beginning and at the end of fermentation in the grape pomace kombucha.
As shown in Table 2, an increase in the concentration of the total polyphenols, ranging between 5 and 140%, was noted at the end of fermentation. The richest sample was the one prepared with 20 g/L of added sucrose and fermented for 7 days at 20 °C. This sample was also significantly different from all others (p < 0.05). The grape pomace kombucha made with 35 g/L of added sugar and fermented for 7 days at 20 °C scored the second highest concentration of polyphenols, and it was also statistically different (p < 0.05). With regard to anthocyanins, the results suggest a similar pattern to polyphenols with respect to fermentation temperature. As shown in Table 2, the highest content of anthocyanins was found when fermentation was conducted at 20 °C. In fact, all samples fermented at 20 °C were statistically similar (p < 0.05), as well as those fermented for 7 days at 25 °C. Moreover, we noticed that increasing the fermentation duration to 10 days would only cause a smaller concentration at 25 °C.
IC50 is defined as the half maximal inhibitory concentration; in other terms, it is the amount of sample needed to scavenge 50% of the DPPH radicals. A lower IC50 value indicates a stronger antioxidant activity. Initially, on Day 0, the IC50 values of the unfermented grape pomace ranged between 2.45 and 2.68 mL/L. As shown in Table 2, the IC50 values decreased with fermentation time, pointing to an enhancement of the antioxidant ability of the beverage. In essence, this ability was doubled. The highest antioxidant potential (i.e., the lowest IC50 value) was noted in the sample prepared with 20 g/L of sucrose and fermented for 7 days at 20 °C (which was also the richest sample in polyphenols). The same recipe fermented at 25 °C ranked second, which suggests that time, temperature, and added sucrose had the most impact on the inhibition of DPPH radicals.
The grape pomace kombucha extracts were screened for their polyphenol content. The results of the HPLC analysis are presented in Table 3. The results showed that grape pomace kombucha contains a broad spectrum of phenolic acids, especially phenolic acids such as gallic acid, hydroxybenzoate, ethyl trans-2-hydroxycinnamate, and cinnamyl-3,4-dihydroxy-A-cyanocinnamate. The flavonoid family includes several compounds such as quercetin, myricetin, taxifolin, dihydroxyflavone, and dimethoxyflavone. The presence of these compounds depends on the fermentation conditions.

Table 3.
The identified phenolic compounds and their derivatives in grape pomace kombucha.
3.3. Biological Activities: Antidiabetic and Anti-Inflammatory Potential
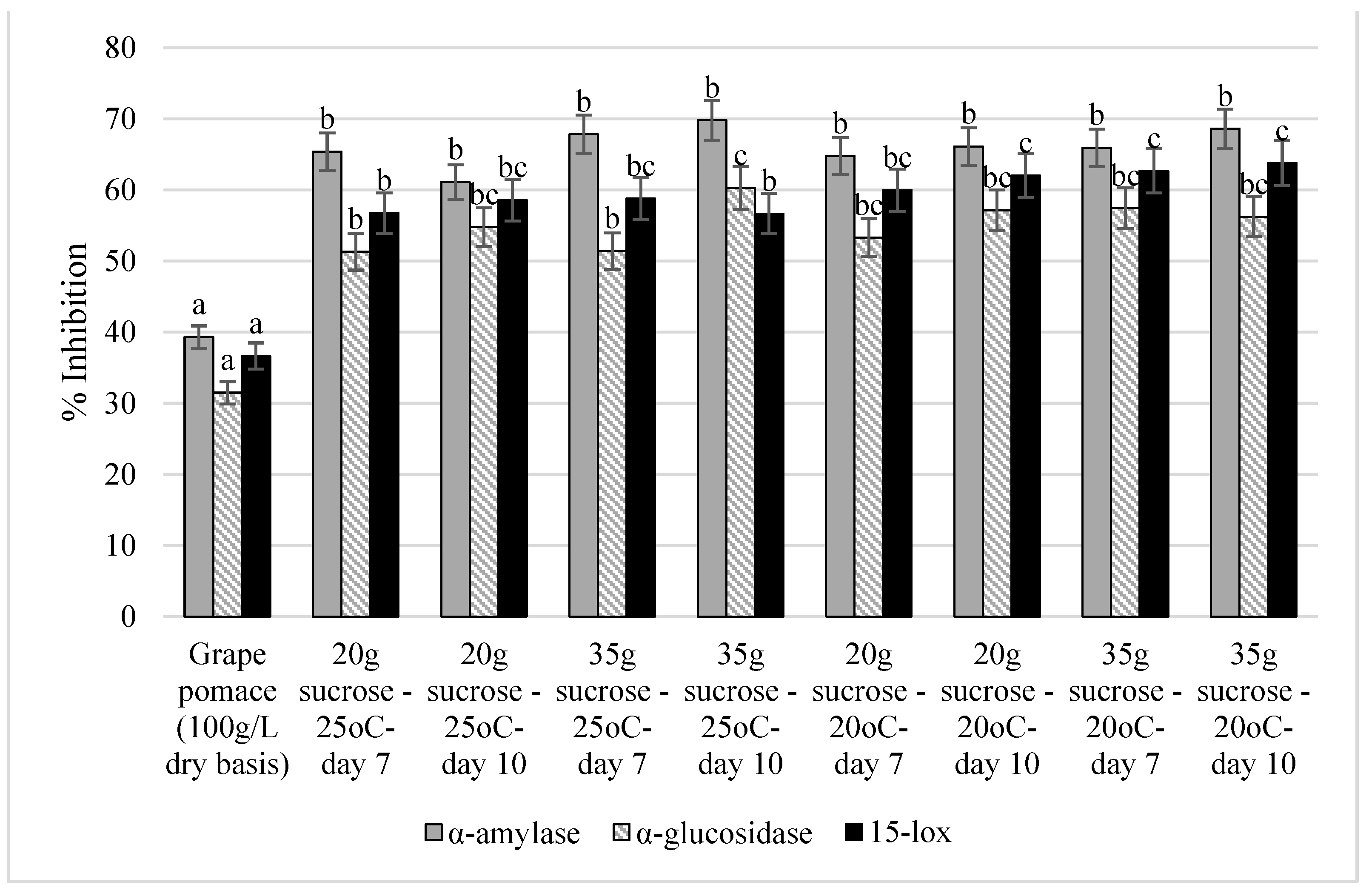
The antidiabetic potential of the grape pomace kombucha extracts was investigated by measuring the inhibition of the α-amylase and α-glucosidase enzymes (in vitro). Those enzymes are involved in the metabolism of carbohydrates. The anti-inflammatory potential of the grape pomace kombucha extracts was assessed by measuring the inhibition of 15-lipoxygenase (15-lox), which is one of the key enzymes involved in the synthesis of pro-inflammatory prostaglandins. The results are portrayed in Figure 2.
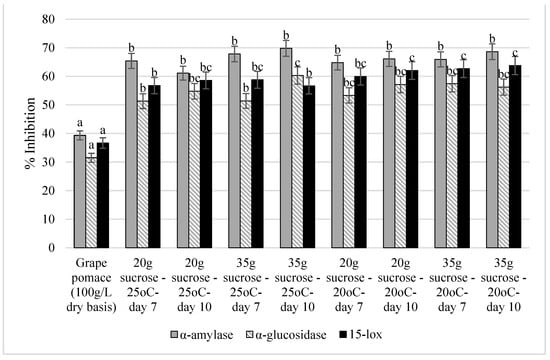
Figure 2.
The antidiabetic and anti-inflammatory activities of the grape pomace kombucha extracts measured by the inhibition of α-amylase, α-glucosidase, and 15-lipoxygenase enzymes. Values with different lower-case letters represent significant differences (p < 0.05).
As shown in Figure 2, all the grape pomace-fermented samples were statistically similar; however, they were different from the unfermented grape pomace in terms of α-amylase inhibition. A significant increase (55–77%) in the inhibition of α-amylase was detected in all extracts after fermentation. A similar pattern was obtained for α-glucosidase; however, the increase was higher (62–91%). Additionally, more resemblances were found among the samples. For example, the sample that scored the highest inhibition rate (i.e., the sample prepared by adding 35 g/L of sucrose and fermented at 25 °C for 10 days) was statistically similar to some of the samples while differing from others.
The inhibition rate of 15-lox was originally around 36% in the unfermented grape pomace. The anti-inflammatory activity increased significantly in all grape pomace kombucha samples (by 55–74%), reaching an inhibition of up to 63% in some cases.
3.4. Biological Activities: Antidiabetic and Anti-Inflammatory Potential
In order to assess the effect of the fermentation and processing conditions on the chemical composition and biological activities of grape pomace kombucha, the Pearson correlation coefficients were calculated, as displayed in Table 4. This table gathers all the coefficients and provides a brief summary on the relationships obtained. The results show that the fermentation time positively influenced the total acidity and the antioxidant activity while negatively impacting the total polyphenol content. Fermentation temperature was negatively correlated with pH, total polyphenol content, and total anthocyanin content but positively correlated with total acidity. The total acidity demonstrated a positive correlation with the antioxidant activity, but it negatively impacted the total polyphenol content.

Table 4.
Pearson correlation coefficients.
4. Discussion
The dynamism of kombucha fermentation is characterized by the consumption of sugars and the production of organic acids and ethanol. The enhancement of the titratable acidity in this study was most probably due to the production of acetic acid during fermentation. Moreover, the percentage increase was high, reaching more than 200% in some cases. However, it was close to what was found in other studies. For instance, a 240% increase in acidity was noted after 10 days of fermentation in the grape juice kombucha [7].
The observed pH variations in the samples might be attributed to the dissimilar ratios of grape varieties in the Lebanese pomace. In fact, the pomace was collected in bulk and was probably unevenly mixed. As shown in Table 1, a decrease in pH values (5 to 12%) was observed after fermentation and was most likely due to the production of the organic acids by the bacteria embedded in the SCOBY.
Sucrose hydrolysis to glucose and fructose is usually ensured by yeasts through the enzyme invertase. However, in the case of grape pomace kombucha, the hydrolysis of sucrose is secured earlier due to the low pH of the substrate (when compared to the pH of tea), the high temperature during infusion (80 °C), and the addition of kombucha tea during production. A competition for sugars (glucose, fructose, and sucrose) occurs between the microorganisms of the microbial community of SCOBY. In fact, not only do yeasts utilize those sources of carbon (to generate ethanol and CO2), but also some bacteria such as Komagataeibacter or lactic acid bacteria consume sugars [16]. The production of organic acids is typically guaranteed by acetic acid bacteria and lactic acid bacteria. At the end of fermentation, the quantity of residual sugars was significantly different in the majority of the samples. However, it was noticed that the samples were rich in sugars, and thus lowering the amount of added sucrose might be beneficial and provide an economical advantage. Therefore, reducing the fermentation time to less than 7 days would probably be advantageous for the taste of the beverages as they would logically contain less acetic acid. Additionally, shortening the fermentation duration would be more efficient and profitable in terms of production, and it would consequently yield a less alcoholic beverage. With regard to the effect of fermentation temperature on the chemical composition of grape pomace kombucha, it seemed that a lower temperature resulted in a slower consumption of sugars and therefore a smaller production of acetic acid. Grape pomace is one of the main by-products generated during winemaking. Polyphenols are partially transferred from the berries into the wine during fermentation and maceration, and a large proportion is retained in the residues. Different factors might impact the phenolic content of grape pomace such as grape variety, degree of maturity, cultivation practices, and winemaking techniques [17]. The increase in the concentration of total polyphenols might be explained by the biotransformation of phenolic compounds. The reduction in IC50 values was already demonstrated in various studies and was not surprising considering the richness of grape pomace in polyphenols [2]. Fermentation is known to modify the structures of polyphenols due to different reactions that can take place. Polyphenolic compounds can undergo different transformations such as decarboxylation, hydroxylation, and esterification [18]. They are considered to be among the most important molecules in grapes due to their biological activities.
Polyphenolic compounds exhibit an antioxidant potential by acting as reducing agents, hydrogen donors, metal chelators, and singlet oxygen quenchers [19]. Fermentation is associated with an improvement in the concentration of polyphenols in plant-based foods; therefore, it can be linked to an enhancement in the antioxidant activity. This is achieved through microbial hydrolysis and the production of organic acids [20]. Kombucha-focused research has reported an increase in phenolic content and antioxidant potential after inoculating different substrates with SCOBY [21]. Anthocyanins belong to the category of flavonoids; they are responsible for the red color of wine and their consumption is associated with many health benefits [22]. Anthocyanin content increases as a result of kombucha fermentation in Zijuan tea, thereby resulting in a change in color of the beverage from yellowish brown to salmon pink [23]. A solid negative correlation has been established between fermentation temperature and the final concentration of polyphenols (r = −0.644). However, it has been shown that a higher fermentation temperature when producing laver kombucha will result in a higher quantity of phenols [24]. In our case, the samples fermented for a longer duration (10 days) were poorer in polyphenols, which might be attributed to their adsorption by the biofilm and to their biotransformation into larger molecules, which might be favored at higher temperatures. This was also confirmed by the Pearson correlation coefficient, as a strong negative link was obtained between the concentration of total polyphenols and fermentation duration (r = −0.741). Different studies have reported that a shorter fermentation period results in a richer concentration of polyphenols in kombucha products [7]. The concentration of anthocyanins has solidly been negatively correlated to fermentation temperature (r = −0.834) in addition to being fairly linked to fermentation duration (r = −0.289).
According to some authors, fermentation time is the most influential parameter on antioxidant potential [25]. A similar observation can be noted in the case of grape pomace kombucha where, in terms of processing parameters, duration has been found to be the most significant (r = 0.504). With regard to chemical composition, it was found that brix levels impacted the scavenging potential of DPPH radicals [26], which was not the case in grape pomace kombucha as a medium correlation was obtained. As a matter of fact, titratable acidity and acetic acid concentration have mostly been linked to a better antioxidant activity (r = 0.644 and 0.641, respectively), whereas total phenolic content has been negatively correlated with this activity (r = −0.571). This might be explained by the fact that not all the polyphenols present exhibit an antioxidant potential.
Determining the exact impact of fermentation parameters on the identified polyphenols is complex as no discernible logical pattern can be established. For instance, caffeic acid was only detected in one of the extracts. However, this molecule has been extensively examined in both grape products and in the kombucha literature due to its antioxidant, anti-inflammatory, and anticarcinogenic activities [19]. Moreover, it is worth mentioning that caffeic acid can be converted into many molecules such as ferulic acid and coumaric acid (which are also known to exhibit biological activities) [27].
The detection pattern of ferulic acid is relatively similar to that of A-Cyano-4-Hydroxycinnamic acid. However, it is important to mention that ferulic acid can be transformed to hydroxystyrenes by Brettanomyces anomalus through the action of hydroxycinnamic acid decarboxylase [28]. The presence of Brettanomyces in the SCOBY of tea kombucha was reported by Villarreal-Soto et al. [11]. Therefore, this might be one of the reasons behind the detection of this compound during the earlier stages of fermentation and for its absence later. (−)-Chicoric acid has been reported to have numerous health benefits, it can act as an antioxidant, antimicrobial, and anti-inflammatory agent [29].
Flavonoids are the largest and most studied category of polyphenols. They are divided into the following subclasses depending on their molecular structure: flavones, flavanones, flavonols, isoflavones, anthocyanidins, and flavanols. They are associated with a broad spectrum of health benefits because of their antioxidative, anti-inflammatory, anticarcinogenic, and anti-mutagenic capacities [30]. They also exhibit key cellular enzyme functions and are potent inhibitors for quite a few enzymes. Taxifolin, also known as dihydroquercetin, is characterized by its anti-inflammatory and antioxidant action. Dihydroquercetin is considered to be a relatively unstable polyphenol and can be easily subjected to polymerization and degradation [31].
Villareal-Soto et al. showed that the quantity of rutin in tea kombucha decreased as a result of fermentation [10]. The reduction in rutin, or its absence in some grape pomace kombucha samples, might be attributed to enzymatic polymerization, which was found to increase its antioxidant potential.
Myricitrin, a reputable antioxidant, was only detected in half of the grape pomace kombucha extracts. This molecule was originally present in the unfermented grape pomace but failed to remain stable in different fermentation conditions. For instance, it was only present in two of the samples made with 35 g/L of added sugar, regardless of the fermentation duration and temperature, like taxifolin. Most of the work in the literature is focused on studying myricetin and not myricitrin. In fact, both compounds are structurally similar and differ only in the radicals bound to them. Myricetin, a structurally related molecule to different phenolic compounds, is associated with anti-inflammatory, analgesic, anticarcinogenic, hepatoprotective, and antidiabetic activities. It was also detected in some kombucha and follows a analogous pattern to myricitrin (with only a few exceptions) [32].
Baicalein is usually associated with the roots of a Chinese herb (Scutellaria baicalensis Georgi), yet it has been quantified in grape products by some authors. This compound exhibits health advantages by playing antioxidant and anti-inflammatory roles [33]. Baicalein can undergo different types of transformation depending on the surrounding environment. It can be a product of baicalin hydrolysis or can bind itself to different molecules such as glycosides [34]. The absence of pure baicalein in some grape pomace kombucha extracts is therefore probably due to the biotransformation of the molecule in high-sugar mediums at lower fermentation temperatures.
Flavones are antioxidants with antiaging, anti-inflammatory, antimicrobial, and anticancer activities. They are known to go through many series of biotransformation through microbial enzymes. When examining the structures of the different flavones found in all extracts, it can be noticed that they only differed by the type and position of the groups bound to the phenyl and heterocyclic rings. This was probably due to the polymerization of the flavones during fermentation. No pattern was detected with regard to the effect of the processing conditions on the identity of the flavones detected in the extracts.
Resveratrol belongs to the stilbenes category of polyphenols, with trans resveratrol being the biologically active isomer. Resveratrol acts as an antioxidant, anti-inflammatory, antidiabetic, anticancer, and anti-Alzheimer agent [35]. Different modifications in the chemical structure of resveratrol can take place, leading to various derivatives of the compound. It is possible that certain structural changes were responsible for the absence of resveratrol in some of the extracts. In fact, polydatin (the glycoside form of resveratrol), for example, was initially present in the unfermented grape pomace. The conversion of polydatin to resveratrol can occur as a result of acid hydrolysis and enzymatic and microbial transformation [36]. On the other hand, rhapontigenin is one of the numerous derivatives of resveratrol [37], thereby signifying that the absence of rhapontin is some samples was also due to a structural change in the compound. Fermentation parameters seemed to impact the presence of scopoletin, where the occurrence of the compound was mostly dependent on both the fermentation temperature and concentration of added sucrose. Coumarins are known to go undergo numerous permutations through the reactions of substitutions and conjugations, with 7-hydroxycoumarin being the primary metabolite [38]. Their detection in some of extracts might be attributed to the fact that they were subjected to numerous kinds of biotransformation; however, additional investigation is required to deduce how fermentation conditions influence the type of compound obtained.
The antidiabetic potential of kombucha products is one of the beneficial effects associated with the beverage [6]. Some resemblances were found among the samples. For example, the sample that scored the highest inhibition rate (i.e., the sample prepared by adding 35 g/L of sucrose and fermented at 25 °C for 10 days) was statistically similar to some samples while differing from others. The improvement of the antidiabetic potential of kombucha products has been investigated by different authors. Watawana et al. examined the effect of fermentation with SCOBY on the activity of starch hydrolase enzyme in coffee kombucha [39]. The α-amylase and α-glucosidase inhibitory activities increased after the fifth day of fermentation, and a better inhibition was noticed for α-amylase. Furthermore, the antidiabetic activity was enhanced after inoculation with tea fungus in soymilk kombucha and green and black tea kombucha [6]. Determining the effect of fermentation parameters on the inhibition of α-amylase was not possible since all extracts were statistically similar. However, the inhibition of α-glucosidase was mostly impacted by fermentation duration (r = 0.490). A strong correlation with the concentration of total polyphenols in grape pomace kombucha extracts was found (r = 0.599). Polyphenols are known to be good regulators of carbohydrate metabolism; they have strong chelating capacities and can limit the function of enzymes by altering their structures [40].
Kombucha fermentation has been proven to improve the anti-inflammatory potential of different substrates. A two-time increase in the inhibition of 15-lox was obtained after inoculating yerba mate with SCOBY [6]. In tea kombucha extracts, a notable improvement was also obtained. The inhibition rate of 15-lox resembled the effect of nordihydroguaiaretic acid (NDGA), a natural anti-inflammatory molecule [10]. The values obtained were close to one another; however, we noticed that the samples fermented at 20 °C delivered better anti-inflammatory activity. The Pearson correlation coefficient was calculated to determine which parameters were linked with the inhibition of 15-lox in the grape pomace kombucha extracts. Fermentation temperature had a strong negative impact (r = −0.752), whereas the quantity of added sucrose (r = 0.192) and fermentation duration (r = 0.120) had a low positive impact. Interestingly, the pH of the beverage and the anthocyanin content were strongly correlated with the anti-inflammatory activity of grape pomace kombucha (r = 0.653 and r = 0.672, respectively).
5. Conclusions
The concept of valorizing grape pomace with kombucha consortium fermentation is promising. The dynamics of the fermentation of grape pomace kombucha are relatively similar to traditional tea kombucha and to kombucha analogs that have a decrease in the concentration of sugars correlated with fermentation duration and an increase in total acidity due to the production of organic acids.
An improvement in the phenolic profile of grape pomace was detected at the end of fermentation, where the total phenolic and anthocyanin contents were enhanced. Consequently, the grape pomace kombucha exhibited a stronger antioxidant potential and higher antidiabetic and anti-inflammatory activities as a result of the fermentation.
To summarize, fermentation duration was among the most influential parameters as it affected the chemical composition, antioxidant, and antidiabetic activities of the beverage. Temperature mostly shaped the anthocyanin concentration, anti-inflammatory activity, and antioxidant potential of the samples. Finally, the quantity of added sucrose seemed to have a less of an impact on grape pomace kombucha as it essentially affected the final concentration of sugars in the beverage. Therefore, it would be interesting to explore additional production parameters such as different grape pomace varieties, lower sucrose concentration, and shorter fermentation duration to assess their impact on the characteristics of the beverage. Moreover, conducting sensory analysis and examining the aromas can be beneficial. Finally, performing in vivo trials is important to better evaluate the health benefits of grape pomace kombucha.
Author Contributions
Conceptualization of the project P.T., Y.E.R., J.B., S.B., N.B. and Z.R.; methodology, P.T., Y.E.R., J.B., S.B. and N.B; formal analysis, N.B.; investigation, P.T., Y.E.R., J.B., S.B. and N.B. writing—original draft preparation, N.B.; writing—review and editing, P.T., Y.E.R., J.B., S.B., N.B. and Z.R.; supervision, P.T. and Y.E.R.; funding acquisition, P.T. and Y.E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the Chemical Engineering Laboratory (Laboratoire de Génie Chimique—LGC), the Holy Spirit University of Kaslik university (USEK), and the Lebanese Agricultural Research Institute (LARI) for providing essential resources and support that facilitated the progress of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nanni, A.; Parisi, M.; Colonna, M. Wine By-Products as Raw Materials for the Production of Biopolymers and of Natural Reinforcing Fillers: A Critical Review. Polymers 2021, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of Grape Pomace: An Approach That Is Increasingly Reaching Its Maturity—A Review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Içen, H.; Corbo, M.R.; Sinigaglia, M.; Korkmaz, B.I.O.; Bevilacqua, A. Microbiology and antimicrobial effects of kombucha, a short overview. Food Biosci. 2023, 56, 103270. [Google Scholar] [CrossRef]
- Huang, X.; Xin, Y.; Lu, T. A systematic, complexity-reduction approach to dissect the kombucha tea microbiome. eLife 2022, 11, e76401. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.; Beaufort, S.; Rizk, Z.; Bouajila, J.; Taillandier, P.; El Rayess, Y. Kombucha Analogues around the World: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 10105–10129. [Google Scholar] [CrossRef] [PubMed]
- Ayed, L.; Ben Abid, S.; Hamdi, M. Development of a Beverage from Red Grape Juice Fermented with the Kombucha Consortium. Ann. Microbiol. 2017, 67, 111–121. [Google Scholar] [CrossRef]
- Vitas, J.S.; Vukmanović, S.Z.; Malbaša, R.V.; Tepić Horecki, A.N. Influence of Process Temperature on Ethanol Content in Kombucha Products Obtained by Fermentation of Flotated Must Effluent. Acta Period. Technol. 2019, 50, 311–315. [Google Scholar] [CrossRef]
- Vukmanović, S.; Vitas, J.; Malbaša, R. Valorization of Winery Effluent Using Kombucha Culture. J. Food Process. Preserv. 2020, 44, e14627. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of Fermentation Conditions on the Production of Bioactive Compounds with Anticancer, Anti-Inflammatory and Antioxidant Properties in Kombucha Tea Extracts. Process Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Bouajila, J.; Pace, M.; Leech, J.; Cotter, P.D.; Souchard, J.-P.; Taillandier, P.; Beaufort, S. Metabolome-Microbiome Signatures in the Fermented Beverage, Kombucha. Int. J. Food Microbiol. 2020, 333, 108778. [Google Scholar] [CrossRef] [PubMed]
- Dawra, M.; El Rayess, Y.; El Beyrouthy, M.; Nehme, N.; El Hage, R.; Taillandier, P.; Bouajila, J. Biological activities and chemical characterization of the Lebanese endemic plant Origanum ehrenbergii Boiss. Flavour Fragr. J. 2021, 36, 339–351. [Google Scholar] [CrossRef]
- Ghanem, C.; Taillandier, P.; Rizk, Z.; Nehme, N.; Souchard, J.P.; El Rayess, Y. Evolution of polyphenols during syrah grapes maceration: Time versus temperature effect. Molecules 2019, 24, 2845. [Google Scholar] [CrossRef] [PubMed]
- Dawra, M.; Bouajila, J.; El Beyrouthy, M.; Abi Rizk, A.; Taillandier, P.; Nehme, N.; El Rayess, Y. Chemical Characterization and Antioxidant, Antibacterial, Antiacetylcholinesterase and Antiproliferation Properties of Salvia fruticosa Miller Extracts. Molecules 2023, 28, 2429. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, C.; Bouajila, J.; Rizk, Z.; El Beyrouthy, M.; Sadaka, C.; Gürer, E.S.; Sharifi-Rad, J.; Nehme, N.; El Rayess, Y. Comparative analysis of pre-fermentation treatments on phenolic compounds and bioactivity in Vitis Vinifera var. Syrah and var. Cabernet Sauvignon grapes. Nutrire 2023, 48, 25. [Google Scholar] [CrossRef]
- Barbosa, C.D.; Uetanabaro, A.P.T.; Santos, W.C.R.; Caetano, R.G.; Albano, H.; Kato, R.; Cosenza, G.P.; Azeredo, A.; Góes-Neto, A.; Rosa, C.A.; et al. Microbial–physicochemical integrated analysis of kombucha fermentation. LWT 2021, 148, 111788. [Google Scholar] [CrossRef]
- Guerra-Rivas, C.; Gallardo, B.; Mantecón, Á.R.; del Álamo-Sanza, M.; Manso, T. Evaluation of Grape Pomace from Red Wine By-Product as Feed for Sheep. J. Sci. Food Agric. 2017, 97, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Tinikul, R.; Chenprakhon, P.; Maenpuen, S.; Chaiyen, P. Biotransformation of Plant-Derived Phenolic Acids. Biotechnol. J. 2018, 13, e1700632. [Google Scholar] [CrossRef] [PubMed]
- Cosme, F.; Pinto, T.; Vilela, A. Phenolic Compounds and Antioxidant Activity in Grape Juices: A Chemical and Sensory View. Beverages 2018, 4, 22. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of Fermentation on the Antioxidant Activity in Plant-Based Foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Leal, J.M.; Suárez, L.V.; Jayabalan, R.; Oros, J.H.; Escalante-Aburto, A. A Review on Health Benefits of Kombucha Nutritional Compounds and Metabolites. CYTA–J. Food 2018, 16, 390–399. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Loira, I.; López, C.; Palomero, F.; González, C. Emerging Non-thermal Technologies for the Extraction of Grape Anthocyanins. Antioxidants 2021, 10, 1863. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Li, R.-Y.; Chen, J.-X.; Wang, F.; Gao, Y.; Fu, Y.-Q.; Xu, Y.-Q.; Yin, J.-F. Zijuan tea- based kombucha: Physicochemical, sensorial, and antioxidant profile. Food Chem. 2021, 363, 130322. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Eun, J.-B. Impact of Time and Temperature on the Physicochemical, Microbiological, and Nutraceutical Properties of Laver Kombucha (Porphyra dentata) during Fermentation. LWT 2022, 154, 112643. [Google Scholar] [CrossRef]
- Muzaifa, M.; Andini, R.; Sulaiman, M.I.; Abubakar, Y.; Rahmi, F. Novel Utilization of Coffee Processing By-Products: Kombucha Cascara Originated from “Gayo-Arabica”. IOP Conf. Ser. Earth Environ. Sci. 2021, 644, 012048. [Google Scholar] [CrossRef]
- Zubaidah, E.; Dewantari, F.J.; Novitasari, F.R.; Srianta, I.; Blanc, P.J. Potential of Snake Fruit (Salacca Zalacca (Gaerth.) Voss) for the Development of a Beverage through Fermentation with the Kombucha Consortium. Biocatal. Agric. Biotechnol. 2018, 13, 198–203. [Google Scholar] [CrossRef]
- Newair, E.F.; Abdel-Hamid, R.; Kilmartin, P.A. Electrochemical Determination of the Antioxidant Activity in Echinacea Purpurea Roots Using Square Wave Voltammetry. Electroanalysis 2017, 29, 1131–1140. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea—A Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Sun, Q.; Park, Y. The Bioactive Effects of Chicoric Acid as a Functional Food Ingredient. J. Med. Food 2019, 22, 645–652. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Khlupova, M.; Vasil’eva, I.; Shumakovich, G.; Zaitseva, E.; Chertkov, V.; Shestakova, A.; Morozova, O.; Yaropolov, A. Enzymatic Polymerization of Dihydroquercetin (Taxifolin) in Betaine-based Deep Eutectic Solvent and Product Characterization. Catalysts 2021, 11, 639. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Neuroprotective and Cognitive Enhancement Potentials of Baicalin: A Review. Brain Sci. 2018, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, Y.-D.; Park, H.; Moon, K.-O.; Ha, K.-T.; Baek, N.-I.; Park, C.-S.; Joo, M.; Cha, J. Synthesis and Biological Evaluation of a Novel Baicalein Glycoside as an Anti-Inflammatory Agent. Eur. J. Pharmacol. 2015, 744, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Fiod Riccio, B.V.; Fonseca-Santos, B.; Colerato Ferrari, P.; Chorilli, M. Characteristics, Biological Properties and Analytical Methods of Trans-Resveratrol: A Review. Crit. Rev. Anal. Chem. 2020, 50, 339–358. [Google Scholar] [CrossRef]
- Chen, M.; Li, D.; Gao, Z.; Zhang, C. Enzymatic Transformation of Polydatin to Resveratrol by Piceid-β-D-Glucosidase from Aspergillus Oryzae. Bioprocess Biosyst. Eng. 2014, 37, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Lee, H.L.; Song, J.; Lee, Y.; Kim, B.-G.; Mok, H.; Ahn, J.-H. Biosynthesis of Resveratrol Derivatives and Evaluation of Their Anti-Inflammatory Activity. Appl. Biol. Chem. 2021, 64, 1–10. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Enhancement of the Functional Properties of Coffee Through Fermentation by “Tea Fungus” (Kombucha). J. Food Process. Preserv. 2015, 39, 2596–2603. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).