Pigmented Native Maize: Unlocking the Potential of Anthocyanins and Bioactive Compounds from Traditional to Functional Beverages
Abstract
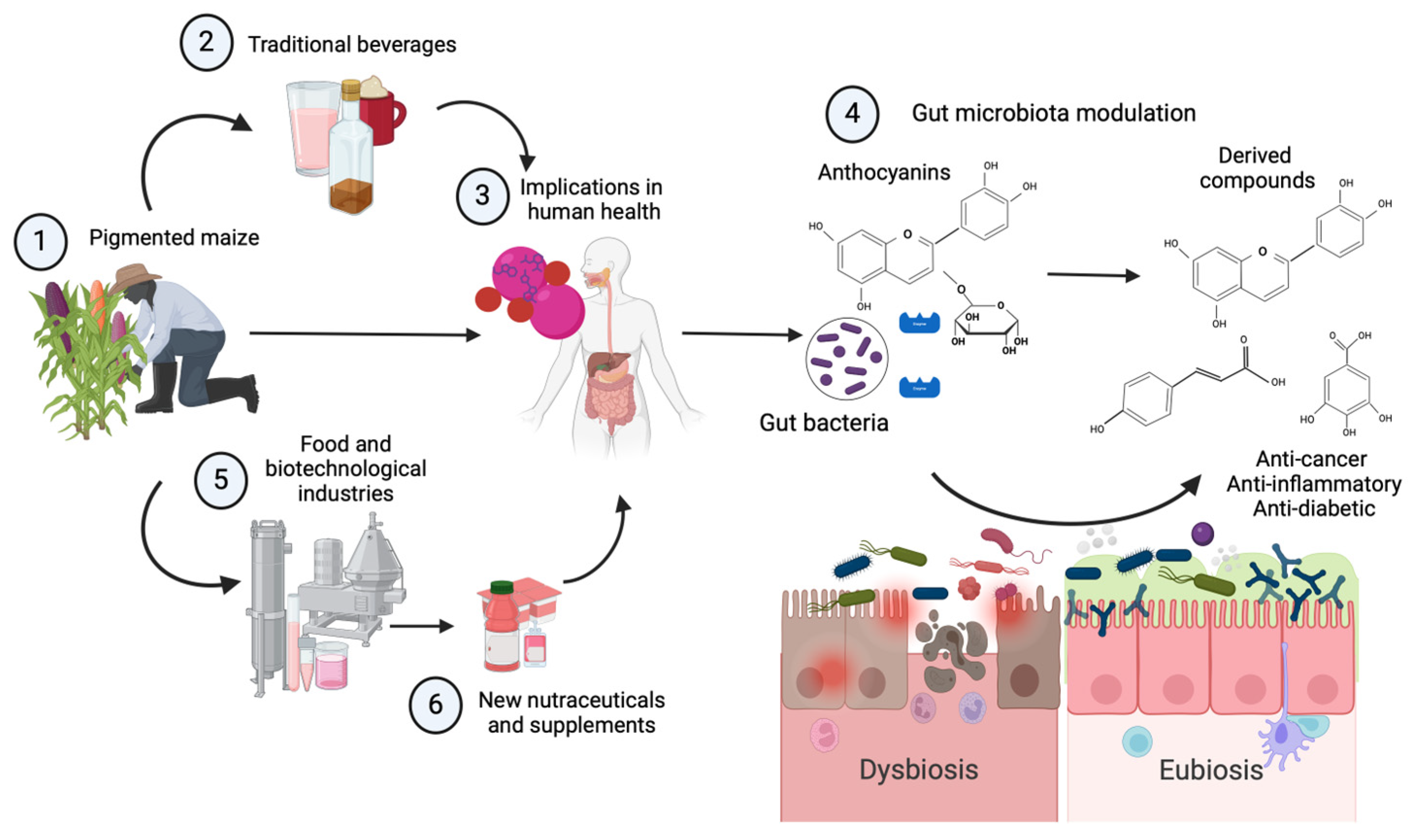
1. Introduction
2. Principal Antioxidant Compounds Reported in Pigmented Maize
3. Bioactive Compounds in Maize and Health Effects
4. Gut Microbiota Modulation by Vegetal Anthocyanins
5. Mexican Traditional Beverages
6. Anthocyanins from Pigmented Maize as Potential Ingredients for the Development of New Functional Beverages
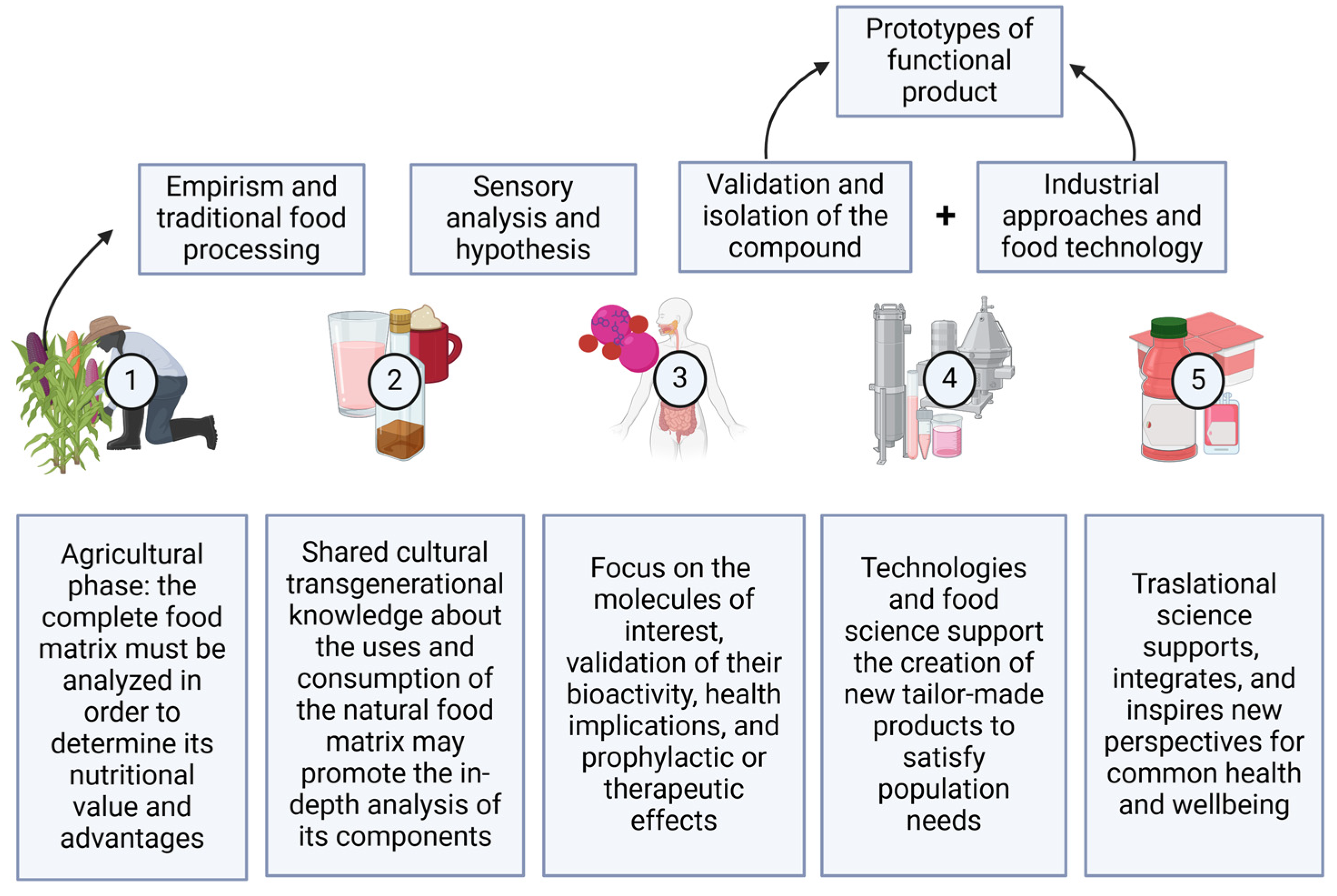
7. Discussion and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD, Food, Nations AOotU. OECD-FAO Agricultural Outlook 2023–2032; OECD: Paris, France, 2023. [Google Scholar]
- Vallebueno-Estrada, M.; Rodríguez-Arévalo, I.; Rougon-Cardoso, A.; Martínez González, J.; García Cook, A.; Montiel, R.; Vielle-Calzada, J.-P. The earliest maize from San Marcos Tehuacán is a partial domesticate with genomic evidence of inbreeding. Proc. Natl. Acad. Sci. USA 2016, 113, 14151–14156. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Valdivia, I.; Perkins, A.C.; Schneider, H.M.; Vallebueno-Estrada, M.; Burridge, J.D.; González-Orozco, E.; Montufar, A.; Montiel, R.; Lynch, J.P.; Vielle-Calzada, J.-P. Gradual domestication of root traits in the earliest maize from Tehuacán. Proc. Natl. Acad. Sci. USA 2022, 119, e2110245119. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.J. Why we need GMO crops in agriculture. Mo. Med. 2014, 111, 492–507. [Google Scholar] [PubMed]
- Broa Rojas, E.; Vázquez Carrillo, M.G.; Estrella Chulím, N.G.; Hernández Salgado, J.H.; Ramírez Valverde, B.; Bahena Delgado, G. Características fisicoquímicas y calidad de la proteína de maíces nativos pigmentados de Morelos en dos años de cultivo. Rev. Mex. de Cienc. Agrícolas 2019, 10, 683–697. [Google Scholar] [CrossRef]
- Halbwirth, H.; Martens, S.; Wienand, U.; Forkmann, G.; Stich, K. Biochemical formation of anthocyanins in silk tissue of Zea mays. Plant Sci. 2003, 164, 489–495. [Google Scholar] [CrossRef]
- Cappellini, F.; Marinelli, A.; Toccaceli, M.; Tonelli, C.; Petroni, K. Anthocyanins: From Mechanisms of Regulation in Plants to Health Benefits in Foods. Front. Plant Sci. 2021, 12, 748049. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vega, H.; Vázquez-Carrillo, G.; Muñóz-Rosales, G.M.; Martínez-Loperena, R.; Heredia-Nava, D.; Martínez-Sifuentes, J.Á.; Anaya-Esparza, L.M.; Gómez-Rodríguez, V.M. Physical and Chemical Characteristics of Native Maize from the Jalisco Highlands and Their Influence on the Nixtamalization Process. Agriculture 2022, 12, 1293. [Google Scholar] [CrossRef]
- Lopez-Martinez, L.X.; Parkin, K.L.; Garcia, H.S. Phase II-Inducing, Polyphenols Content and Antioxidant Capacity of Corn (Zea mays L.) from Phenotypes of White, Blue, Red and Purple Colors Processed into Masa and Tortillas. Plant Foods Hum. Nutr. 2011, 66, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Ruiz, G.; Guyot, J.P.; Ruiz-Teran, F.; Morlon-Guyot, J.; Wacher, C. Microbial and physiological characterization of weakly amylolytic but fast-growing lactic acid bacteria: A functional role in supporting microbial diversity in pozol, a Mexican fermented maize beverage. Appl. Environ. Microbiol. 2003, 69, 4367–4374. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) Lam.). Sci. Rep. 2018, 8, 5018. [Google Scholar] [CrossRef]
- Liu, D.; Ji, Y.; Wang, K.; Guo, Y.; Wang, H.; Zhang, H.; Li, L.; Li, H.; Cui, S.W.; Wang, H. Purple sweet potato anthocyanin extract regulates redox state related to gut microbiota homeostasis in obese mice. J. Food Sci. 2022, 87, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Guzzon, F.; Arandia Rios, L.W.; Caviedes Cepeda, G.M.; Céspedes Polo, M.; Chavez Cabrera, A.; Muriel Figueroa, J.; Medina Hoyos, A.E.; Jara Calvo, T.W.; Molnar, T.L.; Narro León, L.A.; et al. Conservation and Use of Latin American Maize Diversity: Pillar of Nutrition Security and Cultural Heritage of Humanity. Agronomy 2021, 11, 172. [Google Scholar] [CrossRef]
- Cui, L.; Gao, R.; Dong, S.; Zhang, J.; Liu, P.; Zhang, H.; Meng, J.; Shi, D. Effects of ear shading on the anthocyanin contents and quality of kernels in various genotypes of maize. Aust. J. Crop Sci. 2012, 6, 704–710. [Google Scholar]
- Zilić, S.; Serpen, A.; Akıllıoğlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D. The chemistry and biological effects of flavonoids and phenolic acids. Ann. N. Y. Acad. Sci. 1998, 854, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kondo, T. Structure and Molecular Stacking of Anthocyanins—Flower Color Variation. Angew. Chem. Int. Ed. Engl. 1991, 30, 17–33. [Google Scholar] [CrossRef]
- Mazza, G.J.; Miniati, E. Anthocyanins in Fruits Vegetables & Grains; CRC-Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Jing, P.; Noriega, V.; Schwartz, S.J.; Giusti, M.M. Effects of growing conditions on purple corncob (Zea mays L.) anthocyanins. J. Agric. Food Chem. 2007, 55, 8625–8629. [Google Scholar] [CrossRef]
- de la Parra, C.; Saldivar, S.O.; Liu, R.H. Effect of processing on the phytochemical profiles and antioxidant activity of corn for production of masa, tortillas, and tortilla chips. J. Agric. Food Chem. 2007, 55, 4177–4183. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Aulis, F.; Hernandez-Vazquez, L.; Aguilar-Osorio, G.; Arrieta-Baez, D.; Navarro-Ocana, A. Extraction and Identification of Anthocyanins in Corn Cob and Corn Husk from Cacahuacintle Maize. J. Food Sci. 2019, 84, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.G.; Edna Alarcón, A.; Oscar García, B.; Jose, C.S.; Tania, A.Z. Chemical, Antioxidant, and Cytotoxic Properties of Native Blue Corn Extract. In Natural Products and Cancer Drug Discovery; Farid, A.B., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 67–77. [Google Scholar]
- Salinas Moreno, Y.; Sánchez, G.S.; Hernández, D.R.; Lobato, N.R. Characterization of Anthocyanin Extracts from Maize Kernels. J. Chromatogr. Sci. 2005, 43, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Ge-Zhang, S.; Song, M. Anthocyanins in metabolites of purple corn. Front. Plant Sci. 2023, 14, 1154535. [Google Scholar] [CrossRef] [PubMed]
- Carrera, E.J.; Cejudo-Bastante, M.J.; Hurtado, N.; Heredia, F.J.; González-Miret, M.L. Revalorization of Colombian purple corn Zea mays L. by-products using two-step column chromatography. Food Res. Int. 2023, 169, 112931. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhu, Y.; Wang, Y.; Wang, T.; Zhao, S.; Feng, K.; Li, L.; Wu, P. Molecular identification of phenylalanine ammonia lyase-encoding genes EfPALs and EfPAL2-interacting transcription factors in Euryale ferox. Front. Plant Sci. 2023, 14, 1114345. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A. Las antocianinas como colorantes naturales y compuestos bioactivos: Revisión. Acta Biológica Colomb. 2008, 13, 27–36. [Google Scholar]
- Chatham, L.A.; Juvik, J.A. Linking anthocyanin diversity, hue, and genetics in purple corn. G3 Genes Genomes Genet. 2021, 11, jkaa062. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Lawson, D.; Xie, D.-Y. Metabolic engineering of anthocyanins in dark tobacco varieties. Physiol. Plant. 2017, 159, 2–12. [Google Scholar] [CrossRef]
- Shi, M.Z.; Xie, D.Y. Biosynthesis and metabolic engineering of anthocyanins in Arabidopsis thaliana. Recent. Pat. Biotechnol. 2014, 8, 47–60. [Google Scholar] [CrossRef]
- Nurkhasanah, A.; Fardad, T.; Carrera, C.; Setyaningsih, W.; Palma, M. Ultrasound-Assisted Anthocyanins Extraction from Pigmented Corn: Optimization Using Response Surface Methodology. Methods Protoc. 2023, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Paredes-Lopez, O. Natural Colorants for Food and Nutraceutical Uses; CRC Press: Boca Raton, Florida, USA, 2002. [Google Scholar] [CrossRef]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red cabbage—Stability to simulated gastrointestinal digestion. Phytochemistry 2007, 68, 1285–1294. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, L.; Deng, Y.; Chi, J.; Zhang, Y.; Wei, Z.; Zhang, M. Phenolic content and antioxidant activity of eight representative sweet corn varieties grown in South China. Int. J. Food Prop. 2017, 20, 3043–3055. [Google Scholar] [CrossRef]
- Sultan, S.M.; Dikshit, N.; Mohanty, C.S.; Rout, P.K.; Raina, S.K. Biochemical evaluation of dent corn (Zea mays L.) genotypes cultivated under rainfed conditions in the hills of north western Indian Himalayan state of Jammu and Kashmir. J. Appl. Nat. Sci. 2018, 10, 196–201. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Cadenas, E. Mitochondrial free radical production and cell signaling. Mol. Asp. Med. 2004, 25, 17–26. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Zolla, G.; Afaray-Carazas, A.; Vera-Vega, M.; Huanuqueño, H.; Begazo-Gutiérrez, H.; Chirinos, R.; Pedreschi, R.; Shetty, K. Integrated metabolite analysis and health-relevant in vitro functionality of white, red, and orange maize (Zea mays L.) from the Peruvian Andean race Cabanita at different maturity stages. Front. Nutr. 2023, 10, 1132228. [Google Scholar] [CrossRef]
- Urias-Lugo, D.A.; Heredia, J.B.; Muy-Rangel, M.D.; Valdez-Torres, J.B.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Anthocyanins and Phenolic Acids of Hybrid and Native Blue Maize (Zea mays L.) Extracts and Their Antiproliferative Activity in Mammary (MCF7), Liver (HepG2), Colon (Caco2 and HT29) and Prostate (PC3) Cancer Cells. Plant Foods Hum. Nutr. 2015, 70, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Duangpapeng, P.; Lertrat, K.; Lomthaisong, K.; Paul Scott, M.; Suriharn, B. Variability in Anthocyanins, Phenolic Compounds and Antioxidant Capacity in the Tassels of Collected Waxy Corn Germplasm. Agronomy 2019, 9, 158. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Huamán-Alvino, C.; Flores-Báez, O.; Aquino-Méndez, E.M.; Chirinos, R.; Campos, D.; Sevilla, R.; Fuentealba, C.; Pedreschi, R.; Sarkar, D.; et al. Evaluation of phenolic antioxidant-linked in vitro bioactivity of Peruvian corn (Zea mays L.) diversity targeting for potential management of hyperglycemia and obesity. J. Food Sci. Technol. 2019, 56, 2909–2924. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vital, D.A.; Gonzalez de Mejia, E. Anthocyanins from purple corn activate free fatty acid-receptor 1 and glucokinase enhancing in vitro insulin secretion and hepatic glucose uptake. PLoS ONE 2018, 13, e0200449. [Google Scholar] [CrossRef] [PubMed]
- Zaa, C.A.; Marcelo, Á.J.; An, Z.; Medina-Franco, J.L.; Velasco-Velázquez, M.A. Anthocyanins: Molecular Aspects on Their Neuroprotective Activity. Biomolecules 2023, 13, 1598. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, J.; Wang, W.; Lyu, L.; Wu, W.; Li, W. The Extraction and High Antiproliferative Effect of Anthocyanin from Gardenblue Blueberry. Molecules 2023, 28, 2850. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and Antioxidant Properties of Anthocyanin Rich Extracts from Blueberry and Blackcurrant Juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. OCL 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Melo, R.B.; de Barros Silva, P.G.; Oriá, R.B.; Melo, J.U.d.S.; da Silva Martins, C.; Cunha, A.M.; Vasconcelos, P.R.L. Anti-inflammatory effect of a fatty acid mixture with high ω-9:ω-6 ratio and low ω-6:ω-3 ratio on rats submitted to dental extraction. Arch. Oral. Biol. 2017, 74, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Andjelkovic, V.; Vukadinović, J.; Srebric, M.; Mladenović-Drinić, S. Pigmented maize—A potential source of β-carotene and α-tocopherol. J. Eng. Process. Manag. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Hossain, A.; Jayadeep, A. Determination of tocopherol and tocotrienol contents in maize by in vitro digestion and chemical methods. J. Cereal Sci. 2018, 83, 90–95. [Google Scholar] [CrossRef]
- Zhai, J.; Zhu, Y.; Wu, Y.; Li, N.; Cao, Y.; Guo, Y.; Xu, L. Antioxidant Effect of Tyr-Ala Extracted from Zein on INS-1 Cells and Type 2 Diabetes High-Fat-Diet-Induced Mice. Antioxidants 2022, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Zein and zein -based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Salguero, M.V.; Al-Obaide, M.A.I.; Singh, R.; Siepmann, T.; Vasylyeva, T.L. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp. Ther. Med. 2019, 18, 3461–3469. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.; Shin, S.J.; Park, Y.H.; Nam, Y.; Kim, C.W.; Lee, K.W.; Kim, S.M.; Jung, I.D.; Yang, H.D.; et al. Gram-negative bacteria and their lipopolysaccharides in Alzheimer’s disease: Pathologic roles and therapeutic implications. Transl. Neurodegener. 2021, 10, 49. [Google Scholar] [CrossRef]
- Haș, I.M.; Teleky, B.-E.; Szabo, K.; Simon, E.; Ranga, F.; Diaconeasa, Z.M.; Purza, A.L.; Vodnar, D.-C.; Tit, D.M.; Nițescu, M. Bioactive Potential of Elderberry (Sambucus nigra L.): Antioxidant, Antimicrobial Activity, Bioaccessibility and Prebiotic Potential. Molecules 2023, 28, 3099. [Google Scholar] [CrossRef]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhu, H.; Ma, N.; Ma, K.Y.; Chen, Z.-Y. Blueberry and cranberry anthocyanin extracts reduce bodyweight and modulate gut microbiota in C57BL/6 J mice fed with a high-fat diet. Eur. J. Nutr. 2021, 60, 2735–2746. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.; Sun, X.; Liu, X.; Choueiry, F.; Xu, R.; Shi, H.; Zhu, J. Black raspberry extract shifted gut microbe diversity and their metabolic landscape in a human colonic model. J. Chromatogr. B 2022, 1188, 123027. [Google Scholar] [CrossRef]
- Wu, T.; Chu, X.; Cheng, Y.; Tang, S.; Zogona, D.; Pan, S.; Xu, X. Modulation of Gut Microbiota by Lactobacillus casei Fermented Raspberry Juice In Vitro and In Vivo. Foods 2021, 10, 3055. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lu, H.F.; Chen, J.C.; Huang, H.C.; Chen, Y.H.; Su, Y.S.; Tung, C.Y.; Huang, C. Purple-leaf tea (Camellia sinensis L.) ameliorates high-fat diet induced obesity and metabolic disorder through the modulation of the gut microbiota in mice. BMC Complement. Med. Ther. 2020, 20, 376. [Google Scholar] [CrossRef]
- Morissette, A.; Kropp, C.; Songpadith, J.-P.; Moreira, R.J.; Costa, J.; Mariné-Casadó, R.; Pilon, G.; Varin, T.V.; Dudonné, S.; Boutekrabt, L.; et al. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E965–E980. [Google Scholar] [CrossRef]
- Loubet Filho, P.S.; Baseggio, A.M.; Vuolo, M.M.; Reguengo, L.M.; Telles Biasoto, A.C.; Correa, L.C.; Junior, S.B.; Alves Cagnon, V.H.; Betim Cazarin, C.B.; Maróstica Júnior, M.R. Gut microbiota modulation by jabuticaba peel and its effect on glucose metabolism via inflammatory signaling. Curr. Res. Food Sci. 2022, 5, 382–391. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Deng, R.; Chu, Q.; Zheng, X. Pomegranate peel anthocyanins prevent diet-induced obesity and insulin resistance in association with modulation of the gut microbiota in mice. Eur. J. Nutr. 2022, 61, 1837–1847. [Google Scholar] [CrossRef]
- Agrizzi Verediano, T.; Agarwal, N.; Stampini Duarte Martino, H.; Kolba, N.; Grancieri, M.; Dias Paes, M.C.; Tako, E. Effect of Black Corn Anthocyanin-Rich Extract (Zea mays L.) on Cecal Microbial Populations In Vivo (Gallus gallus). Nutrients 2022, 14, 4679. [Google Scholar] [CrossRef] [PubMed]
- Hester, S.N.; Mastaloudis, A.; Gray, R.; Antony, J.M.; Evans, M.; Wood, S.M. Efficacy of an Anthocyanin and Prebiotic Blend on Intestinal Environment in Obese Male and Female Subjects. J. Nutr. Metab. 2018, 2018, 7497260. [Google Scholar] [CrossRef]
- Gao, M.; Peng, X.; Tang, J.; Deng, J.; Wang, F.; Zhang, Y.; Zhao, P.; Kan, H.; Liu, Y. Anti-Inflammatory Effects of Camellia fascicularis Polyphenols via Attenuation of NF-κB and MAPK Pathways in LPS-Induced THP-1 Macrophages. J. Inflamm. Res. 2022, 15, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Cox, A.D.; Chang, H.; Kennett, M.; Rosa, C.; Chopra, S.; Li, S.; Reddivari, L. Maize near-isogenic lines with enhanced flavonoids alleviated dextran sodium sulfate-induced murine colitis via modulation of the gut microbiota. Food Funct. 2023, 14, 9606–9616. [Google Scholar] [CrossRef]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.E.; Mateus, N.; Calhau, C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, B.; Zhong, C.; Guo, J.; Zhang, L.; Mu, T.; Zhang, Q.; Bi, X. Chemoprevention of colorectal cancer by black raspberry anthocyanins involved the modulation of gut microbiota and SFRP2 demethylation. Carcinogenesis 2018, 39, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Torres-Maravilla, E.; Boucard, A.-S.; Al Azzaz, J.; Gontier, S.; Kulakauskas, S.; Langella, P.; Bermúdez-Humarán, L.G. Assessment of the safety of Levilactobacillus brevis CNCM I-5321, a probiotic candidate strain isolated from pulque with anti-proliferative activities. Benef. Microbes 2023, 14, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Modrackova, N.; Vlkova, E.; Tejnecky, V.; Schwab, C.; Neuzil-Bunesova, V. Bifidobacterium β-Glucosidase Activity and Fermentation of Dietary Plant Glucosides Is Species and Strain Specific. Microorganisms 2020, 8, 839. [Google Scholar] [CrossRef] [PubMed]
- Sáez, G.D.; Flomenbaum, L.; Zárate, G. Lactic Acid Bacteria from Argentinean Fermented Foods: Isolation and Characterization for their Potential Use as Starters for Fermentation of Vegetables. Food Technol. Biotechnol. 2018, 56, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Parmar, I.; Neir, S.V. Biotransformation of Cranberry Proanthocyanidins to Probiotic Metabolites by Lactobacillus rhamnosus Enhances Their Anticancer Activity in HepG2 Cells In Vitro. Oxidative Med. Cell. Longev. 2019, 2019, 4750795. [Google Scholar] [CrossRef]
- Rubio-Castillo, Á.E.; Zamora-Gasga, V.M.; Sánchez-Burgos, J.A.; Ruiz-Valdiviezo, V.M.; Montalvo-González, E.; Velázquez-Estrada, R.M.; González-Córdova, A.F.; Sáyago-Ayerdi, S.G. Gut metabolites produced during in vitro colonic fermentation of the indigestible fraction of a maize-based traditional Mexican fermented beverage, Tejuino. Food Chem. Mol. Sci. 2022, 5, 100150. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.; Ramos, C.L.; González-Avila, M.; Gschaedler, A.; Arrizon, J.; Schwan, R.F.; Dias, D.R. Probiotic properties of Weissella cibaria and Leuconostoc citreum isolated from tejuino—A typical Mexican beverage. LWT 2017, 86, 227–232. [Google Scholar] [CrossRef]
- Yañez-Ñeco, C.V.; Rodriguez-Colinas, B.; Amaya-Delgado, L.; Ballesteros, A.O.; Gschaedler, A.; Plou, F.J.; Arrizon, J. Galactooligosaccharide Production from Pantoea anthophila Strains Isolated from “Tejuino”, a Mexican Traditional Fermented Beverage. Catalysts 2017, 7, 242. [Google Scholar] [CrossRef]
- López-Sánchez, R.; Hernández-Oaxaca, D.; Escobar-Zepeda, A.; Ramos Cerrillo, B.; López-Munguía, A.; Segovia, L. Analysing the dynamics of the bacterial community in pozol, a Mexican fermented corn dough. Microbiology 2023, 169, 001355. [Google Scholar] [CrossRef]
- Rizo, J.; Guillén, D.; Díaz-Ruiz, G.; Wacher, C.; Encarnación, S.; Sánchez, S.; Rodríguez-Sanoja, R. Metaproteomic Insights Into the Microbial Community in Pozol. Front. Nutr. 2021, 8, 714814. [Google Scholar] [CrossRef]
- Méndez-Albores, J.A.; Arámbula-Villa, G.; Preciado-Ortíz, R.E.; Moreno-Martínez, E. Aflatoxins in pozol, a nixtamalized, maize-based food. Int. J. Food Microbiol. 2004, 94, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Escalante, A.; Wacher, C.; Farrés, A. Lactic acid bacterial diversity in the traditional Mexican fermented dough pozol as determined by 16S rDNA sequence analysis. Int. J. Food Microbiol. 2001, 64, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional fermented beverages in Mexico: Biotechnological, nutritional, and functional approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef] [PubMed]
- Wacher, C.; Cañas, A.; Cook, P.E.; Barzana, E.; Owens, J.D. Sources of microorganisms in pozol, a traditional Mexican fermented maize dough. World J. Microbiol. Biotechnol. 1993, 9, 269–274. [Google Scholar] [CrossRef]
- Phister, T.G.; O’Sullivan, D.J.; McKay, L.L. Identification of Bacilysin, Chlorotetaine, and Iturin A Produced by Bacillus sp. Strain CS93 Isolated from Pozol, a Mexican Fermented Maize Dough. Appl. Environ. Microbiol. 2004, 70, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Ampe, F.; ben Omar, N.; Guyot, J.P. Culture-independent quantification of physiologically-active microbial groups in fermented foods using rRNA-targeted oligonucleotide probes: Application to pozol, a Mexican lactic acid fermented maize dough. J. Appl. Microbiol. 1999, 87, 131–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bañuelos-Pineda, J.; Gómez-Rodiles Carmen, C.; Cuéllar- José, R.; Aguirre López Luis, O. The Maize Contribution in the Human Health. In Corn; Amanullah, Fahad, S., Eds.; IntechOpen: Rijeka, Croatia, 2018; Chapter 3. [Google Scholar]
- Hesseltine, C.W.; Wang, H.L. Indigenous Fermented Food of Non-Western Origin (Mycologia Memoir); J. Cramer: Lincoln, UK, 1986; Volume 11, 351p. [Google Scholar]
- Väkeväinen, K.; Hernández, J.; Simontaival, A.-I.; Severiano-Pérez, P.; Díaz-Ruiz, G.; von Wright, A.; Wacher-Rodarte, C.; Plumed-Ferrer, C. Effect of different starter cultures on the sensory properties and microbiological quality of Atole agrio, a fermented maize product. Food Control 2020, 109, 106907. [Google Scholar] [CrossRef]
- Robledo-Márquez, K.; Ramírez, V.; González-Córdova, A.F.; Ramírez-Rodríguez, Y.; García-Ortega, L.; Trujillo, J. Research opportunities: Traditional fermented beverages in Mexico. Cultural, microbiological, chemical, and functional aspects. Food Res. Int. 2021, 147, 110482. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Salinas, P.A.; Zavala-García, F.; Urías-Orona, V.; Muy-Rangel, D.; Heredia, J.B.; Niño-Medina, G. Chromatic, Nutritional and Nutraceutical Properties of Pigmented Native Maize (Zea mays L.) Genotypes from the Northeast of Mexico. Arab. J. Sci. Eng. 2020, 45, 95–112. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almonaitytė, A.; Jensen, I.-J.; Kurek, M.; Lerfall, J. Alginate microbeads incorporated with anthocyanins from purple corn (Zea mays L.) using electrostatic extrusion: Microencapsulation optimization, characterization, and stability studies. Int. J. Biol. Macromol. 2023, 246, 125684. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Giusti, M.M. The effect of pigment matrix, temperature and amount of carrier on the yield and final color properties of spray dried purple corn (Zea mays L.) cob anthocyanin powders. Food Chem. 2017, 227, 376–382. [Google Scholar] [CrossRef]
- Haggard, S.; Luna-Vital, D.; West, L.; Juvik, J.A.; Chatham, L.; Paulsmeyer, M.; Gonzalez de Mejia, E. Comparison of chemical, color stability, and phenolic composition from pericarp of nine colored corn unique varieties in a beverage model. Food Res. Int. 2018, 105, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Chatham, L.A.; Howard, J.E.; Juvik, J.A. A natural colorant system from corn: Flavone-anthocyanin copigmentation for altered hues and improved shelf life. Food Chem. 2020, 310, 125734. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, X.; Sun, H.; Zhan, Q.; Zhong, L.; Hu, Q.; Zhao, L. The critical roles of α-amylase and amyloglucosidase in improving the quality of black waxy corn beverages: Special attentions to the color and flavor. J. Cereal Sci. 2023, 110, 103625. [Google Scholar] [CrossRef]
- Ren, S.; Giusti, M.M. The effect of whey protein concentration and preheating temperature on the color and stability of purple corn, grape and black carrot anthocyanins in the presence of ascorbic acid. Food Res. Int. 2021, 144, 110350. [Google Scholar] [CrossRef]
- Cortés, G.A.; Salinas, M.Y.; Martín-Martinez, E.S.; Martínez-Bustos, F. Stability of anthocyanins of blue maize (Zea mays L.) after nixtamalization of seperated pericarp-germ tip cap and endosperm fractions. J. Cereal Sci. 2006, 43, 57–62. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, X.; Huang, G.; Xin, X.; Attaribo, T.; Zhang, Y.; Zhang, N.; Gui, Z. Enhancement of Stability and Antioxidant Activity of Mulberry Anthocyanins Through Succinic Acid Acylation. Food Technol. Biotechnol. 2022, 60, 321–329. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z.; et al. A comprehensive review on innovative and advanced stabilization approaches of anthocyanin by modifying structure and controlling environmental factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef]
- Jokioja, J.; Yang, B.; Linderborg, K.M. Acylated anthocyanins: A review on their bioavailability and effects on postprandial carbohydrate metabolism and inflammation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5570–5615. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, S.; Zhao, G.; Ye, F. Destabilisation and stabilisation of anthocyanins in purple-fleshed sweet potatoes: A review. Trends Food Sci. Technol. 2021, 116, 1141–1154. [Google Scholar] [CrossRef]
- Wu, Y.; Han, Y.; Tao, Y.; Li, D.; Xie, G.; Show, P.L.; Lee, S.Y. In vitro gastrointestinal digestion and fecal fermentation reveal the effect of different encapsulation materials on the release, degradation and modulation of gut microbiota of blueberry anthocyanin extract. Food Res. Int. 2020, 132, 109098. [Google Scholar] [CrossRef] [PubMed]
- Thibado, S.P.; Thornthwaite, J.T.; Ballard, T.K.; Goodman, B.T. Anticancer effects of Bilberry anthocyanins compared with NutraNanoSphere encapsulated Bilberry anthocyanins. Mol. Clin. Oncol. 2018, 8, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J.; León, I.E.; Ponce, A.G.; Alvarez, V.A. Active and pH-Sensitive Nanopackaging Based on Polymeric Anthocyanin/Natural or Organo-Modified Montmorillonite Blends: Characterization and Assessment of Cytotoxicity. Polymers 2022, 14, 4881. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.-M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.; Râpeanu, G.; Silvi, S.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Xiao, Z.; Xia, J.; Zhao, Q.; Niu, Y.; Zhao, D. Maltodextrin as wall material for microcapsules: A review. Carbohydr. Polym. 2022, 298, 120113. [Google Scholar] [CrossRef] [PubMed]
- Ćujić Nikolić, N.; Žilić, S.; Simić, M.; Nikolić, V.; Živković, J.; Marković, S.; Šavikin, K. Microencapsulates of Blue Maize Polyphenolics as a Promising Ingredient in the Food and Pharmaceutical Industry: Characterization, Antioxidant Properties, and In Vitro-Simulated Digestion. Foods 2023, 12, 1870. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Fuentes, B.; de Jesús-José, E.; Cabrera-Hidalgo, A.d.J.; Sandoval-Castilla, O.; Espinosa-Solares, T.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Liceaga, A.M.; Aguilar-Toalá, J.E. Plant-Based Fermented Beverages: Nutritional Composition, Sensory Properties, and Health Benefits. Foods 2024, 13, 844. [Google Scholar] [CrossRef]
- Penha, C.B.; Santos, V.D.P.; Speranza, P.; Kurozawa, L.E. Plant-based beverages: Ecofriendly technologies in the production process. Innov. Food Sci. Emerg. Technol. 2021, 72, 102760. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Pulsed electric field assisted extraction of natural food pigments and colorings from plant matrices. Food Chem. X 2022, 15, 100398. [Google Scholar] [CrossRef] [PubMed]
- Daji, G.A.; Green, E.; Abrahams, A.; Oyedeji, A.B.; Masenya, K.; Kondiah, K.; Adebo, O.A. Physicochemical Properties and Bacterial Community Profiling of Optimal Mahewu (A Fermented Food Product) Prepared Using White and Yellow Maize with Different Inocula. Foods 2022, 11, 3171. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Yana, D.; Aguilar-Morón, B.; Pezo-Torres, N.; Shetty, K.; Ranilla, L.G. Ancestral Peruvian ethnic fermented beverage “Chicha” based on purple corn (Zea mays L.): Unraveling the health-relevant functional benefits. J. Ethn. Foods 2020, 7, 35. [Google Scholar] [CrossRef]
- Shi, S.; Li, S.; Li, W.; Xu, H. Corn Silk Tea for Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2019, 2019, 2915498. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Linares, C.; Álvarez-Ríos, G.D.; Figueredo-Urbina, C.J.; Islas, L.A.; Lappe-Oliveras, P.; Nabhan, G.P.; Torres-García, I.; Vallejo, M.; Casas, A. Traditional Fermented Beverages of Mexico: A Biocultural Unseen Foodscape. Foods 2021, 10, 2390. [Google Scholar] [CrossRef] [PubMed]
- Wolfang-Engel, D.; Robert-Brocker, E. Orally Administered Preparations Containing Anthocyanins Made from Blue Maize. Spindles. Patent EP3417870A1, 26 December 2018. [Google Scholar]
- Neder-Suárez, D.; Lardizabal-Gutiérrez, D.; Zazueta-Morales, J.d.J.; Meléndez-Pizarro, C.O.; Delgado-Nieblas, C.I.; Ramírez Wong, B.; Gutiérrez-Méndez, N.; Hernández-Ochoa, L.R.; Quintero-Ramos, A. Anthocyanins and Functional Compounds Change in a Third-Generation Snacks Prepared Using Extruded Blue Maize, Black Bean, and Chard: An Optimization. Antioxidants 2021, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Chatham, L.A.; Paulsmeyer, M.; Juvik, J.A. Prospects for economical natural colorants: Insights from maize. Theor. Appl. Genet. 2019, 132, 2927–2946. [Google Scholar] [CrossRef] [PubMed]
- Paulsmeyer, M.N.; Juvik, J.A. R3-MYB repressor Mybr97 is a candidate gene associated with the Anthocyanin3 locus and enhanced anthocyanin accumulation in maize. Theor. Appl. Genet. 2023, 136, 55. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Rosales, A.; Molina-Macedo, A.; Leyva, M.; San Vicente, F.; Palacios-Rojas, N. Fresh/High-Zinc Maize: A Promising Solution for Alleviating Zinc Deficiency through Significant Micronutrient Accumulation. Foods 2023, 12, 2757. [Google Scholar] [CrossRef]
- Aluru, M.; Xu, Y.; Guo, R.; Wang, Z.; Li, S.; White, W.; Wang, K.; Rodermel, S. Generation of transgenic maize with enhanced provitamin A content. J. Exp. Bot. 2008, 59, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Putterill, J.; Dare, A.P.; Plunkett, B.J.; Cooney, J.; Peng, Y.; Souleyre, E.J.F.; Albert, N.W.; Espley, R.V.; Günther, C.S. Two genes, ANS and UFGT2, from Vaccinium spp. are key steps for modulating anthocyanin production. Front. Plant Sci. 2023, 14, 1082246. [Google Scholar] [CrossRef] [PubMed]
- Balcerowicz, M. Phytochrome-interacting factors at the interface of light and temperature signalling. Physiol. Plant 2020, 169, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Abna, F.; Avin, F.; Haron, N. Expression Level of Sh2 and Bt2 Genes in Some Advanced Corn Lines under Tropical Environment. J. Anim. Plant Sci. 2020, 30, 493–501. [Google Scholar] [CrossRef]
- Awe, S.; Aransiola, D.M.; Irondi, E.A. Microbial succession and anthocyanin concentration during sorghum fermentation. Meas. Food 2023, 12, 100109. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.; Kansal, S.K.; Garg, M.; Krishania, M. Production and characterization of anthocyanin-rich beer from black wheat by an efficient isolate Saccharomyces cerevisiae CMS12. Sci. Rep. 2023, 13, 5863. [Google Scholar] [CrossRef] [PubMed]
- Kellingray, L.; Le Gall, G.; Doleman, J.F.; Narbad, A.; Mithen, R.F. Effects of in vitro metabolism of a broccoli leachate, glucosinolates and S-methylcysteine sulphoxide on the human faecal microbiome. Eur. J. Nutr. 2021, 60, 2141–2154. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.-l.; Farzi, A.; Zhu, W.-y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
| Product | Product Presentation | Ingredients | Brand |
|---|---|---|---|
| Atole | Powder | Corn starch, flavors identical to natural, iodized salt, vitamin and mineral mixture (vitamin C, iron, niacin, zinc, vitamin B6, thiamine, vitamin A, folic acid, and vitamin B12), cinnamon, natural dyes (beta carotene, class IV caramel), and calcium carbonate | Maizena (Univeler, México) |
| Hanan chicha purple drink | Tea bags | Purple corn, pineapple, apple, quince, clove, cinnamon, and lemon | Hanan (Hanan Peruvian secrets, Perú) |
| Tascalate | Powder | Maize, cocoa, achiote, and cinnamon | Taabal (Chiapas, México), Tía Leti (Chiapas, México), Tatik Adelino (Chiapas, México) |
| Atole | Powder | Red corn (Zea mays indentata), amaranth (Amaranthus), and mesquite pod (Prosopis glandulosa) | Cañada de la Vírgen (San Miguel de Allende, México) |
| Gofio | Powder | Corn (100%), from organic farming | Biosano (Las palmas de gran Canaria, España) |
| Tejuino | Liquid concentrate | Not reported | El rayo (Colima, México) |
| Tejuino | Powder | Corn flour, piloncillo, salt | Xalisquero (Jalisco, México) |
| Pinole | Powder | Roasted and ground white corn, added sugars, and cinnamon | Especias Aries (Sonora, México) |
| Pozol | Powder | Nixtamalized corn, added sugars, toasted cocoa, and cinnamon | Tía Chalvi (Chiapas, México) |
| Tascalate | Powder | Toasted corn, toasted cocoa, added sugars, achiote, and cinnamon | Tía Chalvi (Chiapas, México) |
| Compounds | Method | Result | Reference |
|---|---|---|---|
| Anthocyanin-rich purple maize pericarp water extract | Complexation | The mix of Zn/alginate interacted with anthocyanins from purple corn, slowing their chemical degradation in a beverage model. | [49] |
| Anthocyanin water extracts from purple corn cob | Spray dried | Maltodextrin (5%) at 150 °C gave the highest pigment yield (90%) with good solubility and the least color loss. | [99] |
| Aqueous extract of corn pericarp | None | The high presence of condensed forms with C3G in the pigments from corn pericarp could potentially contribute to the color parameters and stability. | [100] |
| Aqueous extract of purple maize | Co-pigmentation (C-glycosyl flavones to anthocyanins) | Hyperchromic and bathochromic shifts are produced by C-glycosyl flavors, which are used to influence the color and have a significant protective impact on the anthocyanidin glucosides. | [101] |
| Dry black waxy corn beverage | Enzymatic treatment (α-amylase and amyloglucosidase) | Amylase and amyloglucosidase treatments led to an improvement in the quality of black waxy corn beverage by modifying the anthocyanin profile related to color and volatile compounds contributing to flavor properties. The volatile profile was increased, and the unpleasant flavors minimized. | [102] |
| Aqueous extract of purple maize | Encapsulation | The anthocyanin-loaded microbeads can maintain their color for 4 weeks when stored at 4 °C and 8 °C. | [96] |
| Purple corn anthocyanin powder | Whey protein (WP) addition | The anthocyanin half-life for purple corn increased two times when native WP (10 mg/mL) was added. | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Pavón, D.; Soto-Sigala, K.S.; Cano-Sampedro, E.; Méndez-Trujillo, V.; Navarro-Ibarra, M.J.; Pérez-Pasten-Borja, R.; Olvera-Sandoval, C.; Torres-Maravilla, E. Pigmented Native Maize: Unlocking the Potential of Anthocyanins and Bioactive Compounds from Traditional to Functional Beverages. Beverages 2024, 10, 69. https://doi.org/10.3390/beverages10030069
Reyes-Pavón D, Soto-Sigala KS, Cano-Sampedro E, Méndez-Trujillo V, Navarro-Ibarra MJ, Pérez-Pasten-Borja R, Olvera-Sandoval C, Torres-Maravilla E. Pigmented Native Maize: Unlocking the Potential of Anthocyanins and Bioactive Compounds from Traditional to Functional Beverages. Beverages. 2024; 10(3):69. https://doi.org/10.3390/beverages10030069
Chicago/Turabian StyleReyes-Pavón, Diana, Kathleen Stephany Soto-Sigala, Edén Cano-Sampedro, Vianey Méndez-Trujillo, María Josse Navarro-Ibarra, Ricardo Pérez-Pasten-Borja, Carlos Olvera-Sandoval, and Edgar Torres-Maravilla. 2024. "Pigmented Native Maize: Unlocking the Potential of Anthocyanins and Bioactive Compounds from Traditional to Functional Beverages" Beverages 10, no. 3: 69. https://doi.org/10.3390/beverages10030069
APA StyleReyes-Pavón, D., Soto-Sigala, K. S., Cano-Sampedro, E., Méndez-Trujillo, V., Navarro-Ibarra, M. J., Pérez-Pasten-Borja, R., Olvera-Sandoval, C., & Torres-Maravilla, E. (2024). Pigmented Native Maize: Unlocking the Potential of Anthocyanins and Bioactive Compounds from Traditional to Functional Beverages. Beverages, 10(3), 69. https://doi.org/10.3390/beverages10030069







