Chemical Characterization of Cider Produced in Hardanger—From Juice to Finished Cider
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. HPLC-UV/RI Analysis of Sugars, Acids, Ethanol, as Well as Titratable Acidity
2.3. Enzymatic Determination of YAN
2.4. GC-MS Analyses of Volatile Compounds
2.5. HPLC-UV and HPLC-MS Analysis of Phenolic Compounds and Total Phenol Using Folin–Ciocâlteu Method
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Apple Juices and End Fermented Cider of Different Apple Fruit Cultivars
3.1.1. Acids, Sugars, Ethanol, and YAN
3.1.2. Total Phenols and Individual Polyphenols
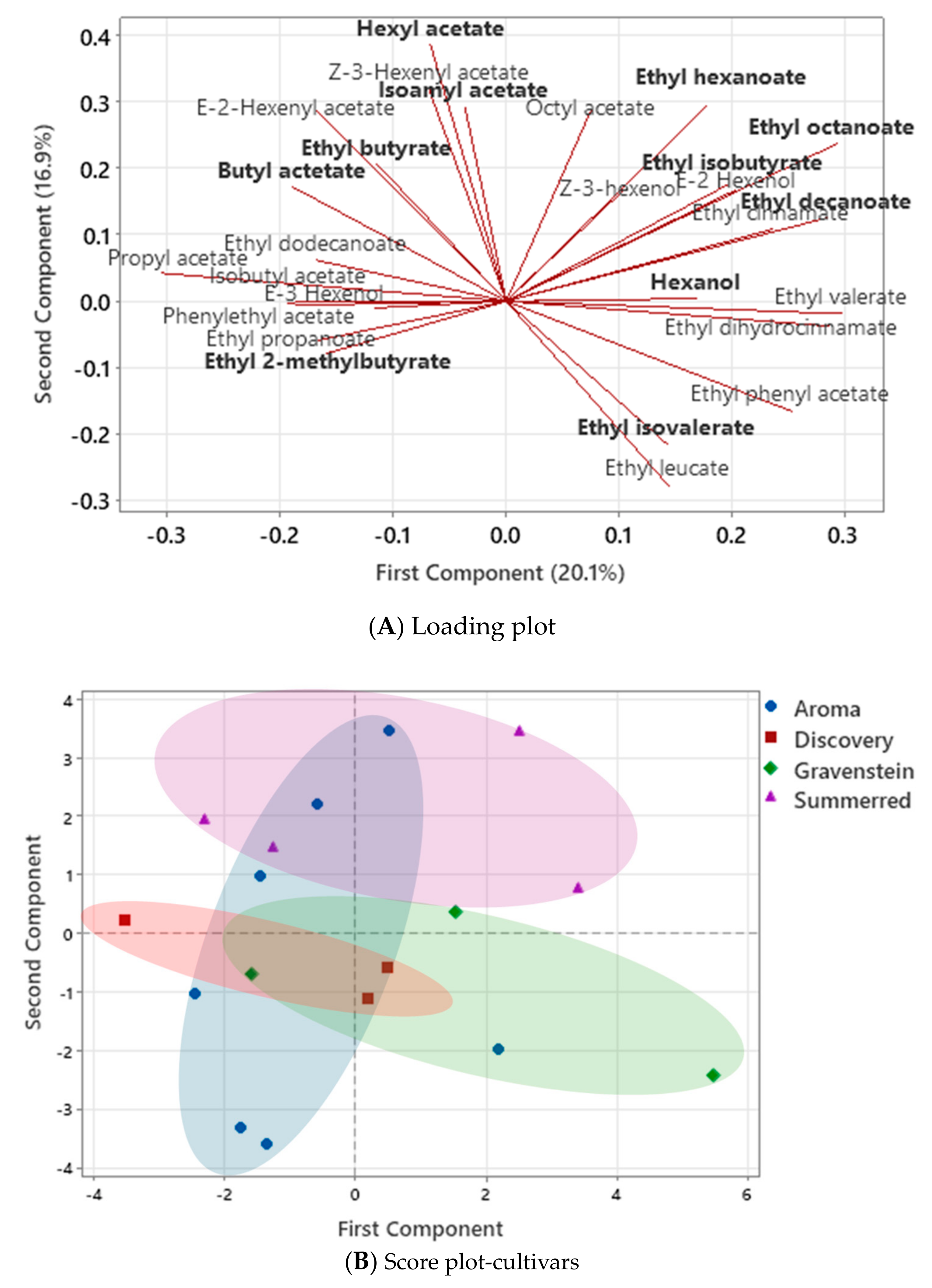
3.2. Volatile Compounds in Apple Juice and in Cider
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nogueira, A.; Guyot, S.; Marnet, N.; Lequéré, J.M. Effect of Alcoholic Fermentation in the Content of Phenolic Compounds in Cider Processing. Braz. Arch. Biol. Technol. 2008, 51, 1025–1032. [Google Scholar] [CrossRef]
- Ferremi Leali, N.; Salvetti, E.; Luzzini, G.; Salini, A.; Slaghenaufi, D.; Fusco, S.; Ugliano, M.; Torriani, S.; Binati, R.L. Differences in the Volatile Profile of Apple Cider Fermented with Schizosaccharomyces pombe and Schizosaccharomyces japonicus. Fermentation 2024, 10, 128. [Google Scholar] [CrossRef]
- Øvsthus, I.; Martelanc, M.; Radovanović Vukajlović, T.; Lesica, M.; Butinar, L.; Mozetič Vodopivec, B.; Antalick, G. Chemical composition of apple cider: A comparative study of Norwegian and French ciders. Acta Hortic. 2024, 1387, 303–310. [Google Scholar] [CrossRef]
- Česnik, U.; Martelanc, M.; Øvsthus, I.; Radovanović Vukajlović, T.; Hosseini, A.; Mozetič Vodopivec, B.; Butinar, L. Functional Characterization of Saccharomyces Yeasts from Cider Produced in Hardanger. Fermentation 2023, 9, 824. [Google Scholar] [CrossRef]
- Qin, Z.; Petersen, M.A.; Bredie, W.L.P. Flavor profiling of apple ciders from the UK and Scandinavian region. Food Res. Int. 2018, 105, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Vangdal, E.; Kvamm-Lichtenfeld, K. Ciders produced from Norwegian fresh consumption apple cultivars. Acta Hortic. 2018, 1205, 527–532. [Google Scholar] [CrossRef]
- Wicklund, T.; Skottheim, E.R.; Remberg, S.F. Various Factors Affect Product Properties in Apple Cider Production. Int. J. Food Stud. 2020, 9, a719. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Zagorac Dabić, D.; Gašić, U.; Tosti, T.; Natić, M.; Meland, M. Analysis of Apple Fruit (Malus × domestica Borkh.) Quality Attributes Obtained from Organic and Integrated Production Systems. Sustainability 2022, 14, 5300. [Google Scholar] [CrossRef]
- McGinley, M.; Nguyen, N.; Mott, J. Real-Time Response to Bacteria Infection of Bioethanol Fermentation Using a Short Rezex™ ROA Column. Appl. Noteb. 2008. [Google Scholar]
- Sadler, G.; Murphy, P. PH and Titratable Acidity. In Food Analysis; Springer: Boston, MA, USA, 2010; pp. 219–238. [Google Scholar] [CrossRef]
- Martelanc, M.; Antalick, A.; Radovanović Vukajlović, T.; Mozetič Vodopivec, B.; Sternad Lemut, M.; Hosseini, A.; Obradović, V.; Mesić, J.; Butinar, L. Aromatic Characterization of Graševina Wines from Slavonia and Podunavlje Sub-Regions. Beverages 2024, 10, 24. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Ivanović, M.; Albreht, A.; Krajnc, P.; Vovk, I.; Islamčević Razboršek, M. Sustainable ultrasound-assisted extraction of valuable phenolics from inflorescences of Helichrysum arenarium L. using natural deep eutectic solvents. Ind. Crops Prod. 2021, 160, 113102. [Google Scholar] [CrossRef]
- Whiting, G.C. Organic Acid Metabolism of Yeasts During Fermentation of Alcoholic Beverages—A Review. J. Inst. Brew. 1976, 82, 84–92. [Google Scholar] [CrossRef]
- Satora, P.; Sroka, P.; Duda-Chodak, A.; Tarko, T.; Tuszyński, T. The profile of volatile compounds and polyphenols in wines produced from dessert varieties of apples. Food Chem. 2008, 111, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Maslov Bandić, L.; Žulj Mihaljević, M.; Fruk, G.; Babojelić Skendrović, M.; Jemrić, T.; Jeromel, A. The profile of organic acids and polyphenols in apple wines fermented with different yeast strains. J. Food Sci. Technol. 2019, 56, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Peinado, R. Enological Chemistry, 1st ed.; Academic Press: London, UK; Waltham, MA, USA, 2012; ISBN 978-0-12-388438-1. [Google Scholar]
- Zhang, H.; Zhou, F.; Ji, B.; Nout, M.J.R.; Fang, Q.; Yang, Z. Determination of organic acids evolution during apple cider fermentation using an improved HPLC analysis method. Eur. Food Res. Technol. 2008, 227, 1183–1190. [Google Scholar] [CrossRef]
- Whiting, G.C.; Coggins, R.A. Organic acid metabolism in cider and perry fermentations. III.—Keto-acids in cider-apple juices and ciders. J. Sci. Food Agric. 1960, 11, 705–709. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Sanoner, P.; Drilleau, J.-F. Variability of the Polyphenolic Composition of Cider Apple (Malus domestica) Fruits and Juices. J. Agric. Food Chem. 2003, 51, 6240–6247. [Google Scholar] [CrossRef] [PubMed]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Chapman and Hall: Boca Raton, FL, USA, 1999. [Google Scholar]
- Reynolds, A.G. Managing Wine Quality, 2nd ed.; Woodhead Publishing: Duxford, UK, 2022; Volume 2: Oenology and Wine Quality, pp. 527–531. [Google Scholar]
- Waterhouse, A.; Sacks, G.L.; Jeffery, D.W. Flavan-3-ols and Condensed Tannin. Understanding Wine Chemistry; Wiley: Hoboken, NJ, USA, 2016; pp. 117–126. [Google Scholar] [CrossRef]
- Oszmianski, J.; Lee, C.Y. Inhibition of polyphenol oxidase activity and browning by honey. J. Agric. Food Chem. 1990, 38, 101892–101895. [Google Scholar] [CrossRef]
- Alberti, A.; Machado Dos Santos, T.P.; Ferreira Zielinski, A.A.; Eleutério Dos Santos, C.M.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on Chemical Profile in Apple Juice and Cider Made from Unripe, Ripe and Senescent Dessert Varieties. LWT Food Sci. Technol. 2016, 65, 436–443. [Google Scholar] [CrossRef]
- Wicklund, T.; Guyot, S.; Le Quéré, J.-M. Chemical Composition of Apples Cultivated in Norway. Crops 2021, 1, 8–19. [Google Scholar] [CrossRef]
- Kunicka-Styczyńska, A.; Pogorzelski, E. L-Malic Acid Effect on Organic Acid Profiles and Fermentation By-Products in Apple Wines. Czech J. Food Sci. 2009, 27, S228–S231. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Yang, R.; Xie, S.; Sockovie, E.; Khanizadeh, S. Which polyphenolic compounds contribute to the total antioxidant activities of apple? J. Agric. Food Chem. 2005, 53, 4989–4995. [Google Scholar] [CrossRef] [PubMed]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative Determination of the Odorants of Young Red Wines from Different Grape Varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Bingman, M.; Stellick, C.; Pelkey, J.; Scott, J.; Cole, C. Monitoring Cider Aroma Development throughout the Fermentation Process by Headspace Solid Phase Microextraction (HS-SPME) Gas Chromatography–Mass Spectrometry (GC-MS) Analysis. Beverages 2020, 6, 40. [Google Scholar] [CrossRef]
- Santos, C.; Pietrowski, G.; Braga, C.; Rossi, M.; Ninow, J.; Santos, T.; Wosiacki, G.; Jorge, R.; Nogueira, A. Apple Aminoacid Profile and Yeast Strains in the Formation of Fusel Alcohols and Esters in Cider Production. J. Food Sci. 2015, 80, C1170–C1177. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Espino-Díaz, M.; Sepulveda, D.R.; Aguilar, G.; Olivas, G. Biochemistry of Apple Aroma: A Review. Food Technol. Biotechnol. 2016, 54, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Li, F.; Cui, L.; Guo, Y. Effects of Fermentation Temperature on Key Aroma Compounds and Sensory Properties of Apple Wine. J. Food Sci. 2015, 80, S2937–S2943. [Google Scholar] [CrossRef] [PubMed]
- Antón, M.J.; Suárez Valles, B.; García Hevia, A.; Picinelli Lobo, A. Aromatic Profile of Ciders by Chemical Quantitative, Gas Chromatography-Olfactometry, and Sensory Analysis. J. Food Sci. 2014, 79, S92–S99. [Google Scholar] [CrossRef] [PubMed]
- Hinkley, J.L.; Bingman, M.T.; Lee, J.S.; Bradley, C.P.; Cole, C.A. Volatile Profile Survey of Five Apple Varieties Grown in Southwest Colorado from Juice to Finished, Dry-Hopped Cider. J. Am. Soc. Brew. Chem. 2023, 81, 131–140. [Google Scholar] [CrossRef]
- Sanz, C.; Olias, J.M.; Perez, A.G. Aroma biochemistry of fruits and vegetables. In Phytochemistry of Fruit and Vegetables: Proceedings of the Phytochemical Society of Europe; Tomás-Barberán, F.A., Robins, R.J., Eds.; Oxford University Press: Oxford, UK, 1997; pp. 125–156. [Google Scholar]
- Rosend, J.; Kuldjärv, R.; Rosenvald, S.; Paalme, T. The Effects of Apple Variety, Ripening Stage, and Yeast Strain on the Volatile Composition of Apple Cider. Heliyon 2019, 5, e01953. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.T.; Lee, P.-R.; Yu, B.; Liu, S.-Q. Cider Fermentation with Three Williopsis Saturnus Yeast Strains and Volatile Changes. Ann. Microbiol. 2015, 65, 921–928. [Google Scholar] [CrossRef]
- Sapers, G.M.; Abbott, J.; Massie, O.; Watada, A.; Finney, E.E. Volatile Composition of Mcintosh Apple Juice as A Function of Maturity and Ripeness Indices. J. Food Sci. 1977, 42, 44–47. [Google Scholar] [CrossRef]
| Producer | Variety | Year | Sample Type (Juice-J, Cider-C) | Production System and Production Volume (Litre of Cider) |
|---|---|---|---|---|
| Producer 1 | Summerred | 2019 | J and C | Hydropress, steel tank, production volume per year 10,000 L |
| Producer 2 | Discovery | 2019 | J and C | Steel tank, added yeast, production volume per year 5000 L |
| Gravenstein | 2019 | J and C | ||
| Aroma | 2021 | C | ||
| Producer 3 | Summerred | 2019 | J and C | Belt press, steel tank, added yeast, production volume per year 7000 L |
| Gravenstein | 2019 | J | ||
| Aroma | 2019 | J and C | ||
| Aroma | 2021 | C | ||
| Producer 4 | Discovery | 2019 | J and C | Belt press, steel tank, spontaneous fermentation, production volume per year 12,000 L |
| Aroma | 2021 | J and C | ||
| Gravenstein/ciderapple | 2019 | J and C | ||
| Gravenstein/Signe Tillisch | 2019 | J and C | ||
| Gul Gravenstein | 2021 | J and C | ||
| Producer 5 | Gravenstein/Elstar | 2021 | J and C | Belt press, steel tank, spontaneous fermentation, centrifugation, production volume per year 150,000 L |
| Producer 6 | Aroma | 2020 | J and C | Belt press, steel tank, added yeast and yeast nutrition, production volume per year 150,000 L |
| Summerred | 2021 | J and C | ||
| Discovery | 2021 | J and C | ||
| Gravenstein | 2021 | J and C | ||
| Rubinstep | 2021 | J and C | ||
| Producer 7 | Aroma | 2020 | J and C | Pack cider press, steel tank with floating lid, spontaneous fermentation, production volume per year 4000 L |
| Gravenstein | 2020 | J and C | ||
| Producer 8 | Summerred | 2020 | J and C | Belt press, steel tank, added yeast and nutrition, production volume per year 100,000 L |
| Producer 9 | Aroma | 2020 | J and C | Belt press, steel tank, added yeast and nutrition, production volume per year 130,000 L |
| Rubinstep | 2021 | J | ||
| Producer 10 | Gravenstein | 2020 | J | Belt press, steel tank, added yeast, production volume per year 60,000 L |
| Juice | Cider | ||||
|---|---|---|---|---|---|
| Parameter | Variety | N | Mean (Min–Max) | N | Mean (Min–Max) |
| Titratable acidity (g L−1 g of malic acid) | Aroma | 5 | 8.92 (6.54–12.8) | 7 | 7.07 (5.19–9.33) |
| Discovery | 3 | 5.95 (5.20–6.54) | 3 | 5.22 (3.89–6.73) | |
| Gravenstein | 5 | 7.43 (6.14–9.25) | 3 | 5.73 (5.47–5.90) | |
| Summerred | 4 | 7.99 (5.98–9.91) | 4 | 7.20 (7.79–9.05) | |
| Malic acid (g L−1) | Aroma | 5 | 13.0 (5.90–22.1) | 7 | 5.61 (0.60–10.7) |
| Discovery | 3 | 4.38 (2.50–7.15) | 3 | 2.96 (0.70–6.70) | |
| Gravenstein | 5 | 12.5 (6.46–21.6) | 3 | 3.52 (0.10–6.30) | |
| Summerred | 4 | 7.23 (3.72–14.6) | 4 | 6.57 (2.80–11.2) | |
| Lactic acid (g L−1) | Aroma | 5 | 0.16 (0.02–0.32) | 7 | 1.11 (0.10–4.81) |
| Discovery | 3 | 0.17 (0.06–0.36) | 3 | 1.06 (0.06–3.05) | |
| Gravenstein | 5 | 0.16 (0.09–0.32) | 3 | 0.49 (0.04–1.19) | |
| Summerred | 4 | 0.84 (0.04–3.03) | 4 | 0.77 (0.02–2.80) | |
| Citric acid (g L−1) | Aroma | 5 | 0.09 (ND–0.14) | 7 | 0.17 (0.05–0.60) |
| Discovery | 3 | 0.02 (ND–0.07) | 3 | 0.05 (ND–0.10) | |
| Gravenstein | 5 | 0.14 (0.09–0.20) | 3 | 0.20 (ND–0.50) | |
| Summerred | 4 | 0.04 (ND–0.10) | 4 | 0.07 (ND–0.10) | |
| Tartaric acid (g L−1) | Aroma | 5 | 0.17 (0.05–0.40) | 7 | 0.12 (0.07–0.21) |
| Discovery | 3 | 0.05 (0.03–0.09) | 3 | 0.10 (0.08–0.14) | |
| Gravenstein | 5 | 0.20 (0.10–0.38) | 3 | 0.16 (0.13–0.21) | |
| Summerred | 4 | 0.13 (0.05–0.27) | 4 | 0.13 (0.03–0.22) | |
| Acetic acid (g L−1) | Aroma | 5 | 0.02 (ND–0.10) | 7 | 0.01 (ND–0.10) |
| Discovery | 3 | 0.07 (ND–0.20) | 3 | ND | |
| Gravenstein | 5 | 0.08 (ND–0.30) | 3 | ND | |
| Summerred | 4 | 0.05 (ND–0.10) | 4 | 0.05 (ND–0.20) | |
| Fructose (g L−1) | Aroma | 5 | 79.0 (31.9–141) | 7 | 16.6 (1.30–38.9) |
| Discovery | 3 | 38.8 (22.1–67.9) | 3 | 7.90 (ND–18.2) | |
| Gravenstein | 5 | 90.2 (55.1–142) | 3 | 13.0 (ND–37.5) | |
| Summerred | 4 | 41.0 (22.8–63.4) | 4 | 6.38 (ND–18.1) | |
| Glucose (g L−1) | Aroma | 5 | 20.3 (8.20–31.3) | 7 | 9.24 (0.30–21.0) |
| Discovery | 3 | 12.6 (7.80–21.0) | 3 | 1.67 (0.70–3.10) | |
| Gravenstein | 5 | 26.3 (18.1–38.3) | 3 | 4.47 (1.4–9.1) | |
| Summerred | 4 | 9.82 (4.60–16.8) | 4 | 1.55 (0.40–2.80) | |
| Sucrose (g L−1) | Aroma | 5 | 38.7 (0.60–94.1) | 7 | 2.46 (ND–14.6) |
| Discovery | 3 | 9.90 (4.80–17.9) | 3 | ND | |
| Gravenstein | 5 | 27.6 (10.2–67) | 3 | ND | |
| Summerred | 4 | 33.5 (19.8–57.3) | 4 | 0.28 (ND–1.10) | |
| Fructose + Glucose (g L−1) | Aroma | 5 | 99.2 (40.0–172) | 7 | 25.8 (3.70–53.8) |
| Discovery | 3 | 51.5 (30.0–88.9) | 3 | 9.60 (1.20–21.4) | |
| Gravenstein | 5 | 117 (75.2–180) | 3 | 17.5 (2.90–46.5) | |
| Summerred | 4 | 50.8 (27.4–80.2) | 4 | 7.92 (1.10–20.9) | |
| Ethanol (vol %) | Aroma | 5 | ND | 7 | 8.45 (6.33–11.3) |
| Discovery | 3 | ND | 3 | 7.11 (5.33–8.12) | |
| Gravenstein | 5 | ND | 3 | 8.34 (6.33–10.3) | |
| Summerred | 4 | ND | 4 | 7.07 (2.77–11.4) | |
| YAN Total (mg L−1) | Aroma | 5 | 38.1 (17.7–62.3) | 7 | 9.37 (ND–22.5) |
| Discovery | 3 | 23.1 (0.80–35.5) | 3 | 12.9 (9.74–18.6) | |
| Gravenstein | 5 | 28.4 (3.81–51.7) | 3 | 17.0 (6.88–36.6) | |
| Summerred | 4 | 69.7 (49.9–101) | 4 | 12.5 (108–14.6) | |
| Juice (mg L−1) | Cider (mg L−1) | ||||
|---|---|---|---|---|---|
| Parameter | Variety | N | Mean (Min–Max) | N | Mean (Min–Max) |
| Total Phenol | Aroma | 5 | 483 (306–680) | 7 | 473 (334–685) |
| Discovery | 3 | 789 (746–836) | 3 | 496 (146–761) | |
| Gravenstein | 5 | 737 (572–933) | 3 | 306 (153–418) | |
| Summerred | 4 | 198 (160–217) | 4 | 215 (159–279) | |
| Gallic acid | Aroma | 5 | 0.25 (ND–1.00) | 7 | 1.00 (ND–3.00) |
| Discovery | 3 | 0.67 (ND–2.00) | 3 | 0.67 (ND–2.00) | |
| Gravenstein | 5 | ND | 3 | ND | |
| Summerred | 4 | 0.67 (ND–2.00) | 4 | 1.25 (ND–5.00) | |
| Protocatechuic acid | Aroma | 5 | ND | 7 | ND |
| Discovery | 3 | ND | 3 | ND | |
| Gravenstein | 5 | ND | 3 | ND | |
| Summerred | 4 | ND | 4 | ND | |
| Procyanidin B1 | Aroma | 5 | 8.48 (ND–15.5) | 7 | 12.3 (ND–31.0) |
| Discovery | 3 | 12.0 (ND–18.0) | 3 | 21.7 (12.0–39.0) | |
| Gravenstein | 5 | 10.3 (ND–21.0) | 3 | 18.7 (15.2–22.0) | |
| Summerred | 4 | 5.47 (ND–16.4) | 4 | 2.8.0 (ND–10.0) | |
| Catechin | Aroma | 5 | 8.87 (ND–18.3) | 7 | 8.20 (ND–12.7) |
| Discovery | 3 | 12.7 (8.00–15.0) | 3 | 12.0 (10.0–14.0) | |
| Gravenstein | 5 | 7.90 (ND–14.0) | 3 | 15.0 (12.0–16.9) | |
| Summerred | 4 | 1.17 (ND–3.5) | 4 | 0.18 (ND–0.70) | |
| Caffeic acid | Aroma | 5 | 3.30 (0.50–6.70) | 7 | 7.63 (0.90–16.0) |
| Discovery | 3 | 3.67 (ND–7.00) | 3 | 22.3 (4.00–32.0) | |
| Gravenstein | 5 | 4.55 (2.00–10.0) | 3 | 6.40 (3.00–12.0) | |
| Summerred | 4 | 1.93 (ND–5.00) | 4 | 3.15 (0.60–10.0) | |
| Chlorogenic acid | Aroma | 5 | 85.6 (56.0–135) | 7 | 52.6 (25.0–101) |
| Discovery | 3 | 177 (151–214) | 3 | 146 (75.0–231) | |
| Gravenstein | 5 | 100 (46.6–142) | 3 | 139 (122–171) | |
| Summerred | 4 | 47.4 (22.3–97.0) | 4 | 62.5 (10.0–150) | |
| Procyanidin B2 | Aroma | 5 | 17.9 (ND–32.0) | 7 | 15.0 (ND–38.0) |
| Discovery | 3 | 31.3 (9.00–43.0) | 3 | 20.7 (12.0–31.0) | |
| Gravenstein | 5 | 15.5 (ND–33.0) | 3 | 24.9 (21.0–30.6) | |
| Summerred | 4 | 4.53 (ND–9.00) | 4 | 2.20 (ND–7.00) | |
| Epicatechin | Aroma | 5 | 26.9 (ND–42.1) | 7 | 34.2 (ND–47.8) |
| Discovery | 3 | 23.3 (ND–36.0) | 3 | 17.7 (ND–30.0) | |
| Gravenstein | 5 | 11.9 (ND–26.6) | 3 | 23.4 (23.0–24.2) | |
| Summerred | 4 | 4.50 (ND–13.5) | 4 | 0.58 (ND–2.30) | |
| Procyanidin C1 | Aroma | 5 | 0.75 (ND–3.00) | 7 | 2.00 (ND–5.00) |
| Discovery | 3 | ND | 3 | 1.00 (ND–3.00) | |
| Gravenstein | 5 | 1.25 (ND–4.00) | 3 | 1.67 (ND–5.00) | |
| Summerred | 4 | ND | 4 | ND | |
| p-Coumaric acid | Aroma | 5 | 0.50 (ND–1.60) | 7 | 1.07 (ND–3.00) |
| Discovery | 3 | 2.67 (ND–4.00) | 3 | 2.00 (ND–4.00) | |
| Gravenstein | 5 | 1.13 (ND–3.00) | 3 | 0.83 (ND–2.50) | |
| Summerred | 4 | ND | 4 | 0.08 (ND–0.30) | |
| Procyanidin A2 | Aroma | 5 | 0.33 (ND–1.30) | 7 | 0.64 (ND–2.30) |
| Discovery | 3 | ND | 3 | ND | |
| Gravenstein | 5 | ND | 3 | 0.97 (ND–2.90) | |
| Summerred | 4 | ND | 4 | ND | |
| Ferulic acid | Aroma | 5 | 0.75 (ND–3.00) | 7 | 0.43 (ND–3.00) |
| Discovery | 3 | ND | 3 | ND | |
| Gravenstein | 5 | ND | 3 | ND | |
| Summerred | 4 | ND | 4 | 0.23 (ND–0.90) | |
| Phloridzin | Aroma | 5 | 4.25 (ND–11.0) | 7 | 4.91 (ND–7.00) |
| Discovery | 3 | 3.67 (ND–11.0) | 3 | 4.00 (ND–12.0) | |
| Gravenstein | 5 | 4.10 (ND–12.0) | 3 | 8.03 (ND–14.0) | |
| Summerred | 4 | 3.33 (ND–10.0) | 4 | 4.00 (ND–11.0) | |
| Total Polyphenols | Aroma | 5 | 236 (68.0–401) | 7 | 186 (49.8–414) |
| Discovery | 3 | 269 (238–287) | 3 | 248 (179–360) | |
| Gravenstein | 5 | 174 (106–238) | 3 | 289 (209–382) | |
| Summerred | 4 | 77.0 (23.0–124) | 4 | 79.2 (11.0–193) | |
| Juice (mg L−1) | Cider (mg L−1) | ||||
|---|---|---|---|---|---|
| Parameter | Variety | N | Mean (Min–Max) | N | Mean (Min–Max) |
| Ethyl esters of aliphatic acids | |||||
| Ethyl propanoate | Aroma | 5 | 105 (39.6–253) | 7 | 270 (84.8–589) |
| Discovery | 3 | 141 (38.5–316) | 3 | 543 (338–763) | |
| Gravenstein | 5 | 65.7 (38.7–122.7) | 3 | 543 (291–897) | |
| Summerred | 4 | 45.1 (36.0–63.7) | 4 | 219 (121–357) | |
| Ethyl butyrate | Aroma | 5 | 709 (163–1346) | 7 | 648 (240–1149) |
| Discovery | 3 | 712 (42.0–1887) | 3 | 860 (304–1926) | |
| Gravenstein | 5 | 474 (63.0–859) | 3 | 796 (610–1149) | |
| Summerred | 4 | 195 (56.1–336) | 4 | 882 (409–1805) | |
| Ethyl hexanoate | Aroma | 5 | 47.4 (11.8–59.0) | 7 | 839 (311–2697) |
| Discovery | 3 | 53.5 (4.90–94.8) | 3 | 964 (798–1114) | |
| Gravenstein | 5 | 49.8 (9.90–66.4) | 3 | 1109 (687–1626) | |
| Summerred | 4 | 45.7 (12.1–68.5) | 4 | 2533 (630–6055) | |
| Ethyl octanoate | Aroma | 5 | 1.70 (ND–8.50) | 7 | 816 (179–2202) |
| Discovery | 3 | 6.17 (ND–18.5) | 3 | 841 (344–1639) | |
| Gravenstein | 5 | 2.32 (ND–11.6) | 3 | 1258 (514–1885) | |
| Summerred | 4 | 5.50 (ND–22.0) | 4 | 2171 (535–4523) | |
| Ethyl decanoate | Aroma | 5 | 30.7 (10.0–40.2) | 7 | 198 (8.60–608) |
| Discovery | 3 | 48.3 (40.8–62.2) | 3 | 400 (64.0–1005) | |
| Gravenstein | 5 | 40.6 (17.4–75.8) | 3 | 440 (175–910) | |
| Summerred | 4 | 38.4 (29.2–53.1) | 4 | 550 (148–866) | |
| Ethyl valerate | Aroma | 5 | 0.72 (0.40–1.5) | 7 | 5.69 (2.10–12.5) |
| Discovery | 3 | 1.7 (0.40–3.70) | 3 | 5.80 (5.20–6.50) | |
| Gravenstein | 5 | 1.36 (0.30–2.90) | 3 | 16.0 (7.20–23.3) | |
| Summerred | 4 | 1.00 (ND–2.5) | 4 | 6.62 (4.80–10.1) | |
| Ethyl dodecanoate | Aroma | 5 | 10.2 (4.10–16.7) | 7 | 61.0 (6.20–239) |
| Discovery | 3 | 16.9 (12.0–22.9) | 3 | 190 (23.1–302) | |
| Gravenstein | 5 | 19.1 (5.80–49.2) | 3 | 96.1 (13.6–248) | |
| Summerred | 4 | 9.63 (7.90–13.0) | 4 | 105 (23.3–264) | |
| Ethyl esters of branched acids | |||||
| Ethyl isobutyrate | Aroma | 5 | 1.88 (0.50–3.40) | 7 | 29.4 (5.30–58.4) |
| Discovery | 3 | 4.37 (ND–12.6) | 3 | 38.7 (34.3–42.0) | |
| Gravenstein | 5 | 2.24 (0.80–4.00) | 3 | 57.3 (41.5–68.0) | |
| Summerred | 4 | 0.63 (0.30–1.00) | 4 | 51.3 (31.1–64.1) | |
| Ethyl isovalerate | Aroma | 5 | 3.06 (1.50–8.60) | 7 | 9.34 (4.10–16.6) |
| Discovery | 3 | 3.87 (1.30–8.60) | 3 | 7.43 (4.90–9.30) | |
| Gravenstein | 5 | 3.66 (1.50–11.4) | 3 | 12.9 (9.80–16.0) | |
| Summerred | 4 | 3.45 (1.50–8.70) | 4 | 7.08 (4.80–9.50) | |
| Ethyl 2-methylbutyrate | Aroma | 5 | 75.9 (17.4–165) | 7 | 68.5 (24.9–125) |
| Discovery | 3 | 347 (20.0–986) | 3 | 153 (65.5–312) | |
| Gravenstein | 5 | 54.0 (17.4–164) | 3 | 56.1 (47.3–61.5) | |
| Summerred | 4 | 40.3 (16.8–101) | 4 | 52.9 (29.5–108) | |
| Ethyl leucate | Aroma | 5 | 4.70 (0.40–17.6) | 7 | 97.0 (25.6–340) |
| Discovery | 3 | 482 (2.00–1442) | 3 | 21.1 (11.4–26.4) | |
| Gravenstein | 5 | 2.56 (1.60–4.10) | 3 | 141 (28.1–320) | |
| Summerred | 4 | 1.58 (0.70–2.30) | 4 | 30.0 (16.9–40.1) | |
| Acetate esters | |||||
| propyl acetate | Aroma | 5 | 98.5 (23.3–170) | 7 | 86.5 (16.3–114) |
| Discovery | 3 | 635 (167–1150) | 3 | 152 (54.9–279) | |
| Gravenstein | 5 | 10.1 (6.90–18.2) | 3 | 49.0 (19.1–85.7) | |
| Summerred | 4 | 8.95 (5.10–16.3) | 4 | 74.0 (20.7–130) | |
| Isobutyl acetate | Aroma | 5 | 47.0 (16.6–98.4) | 7 | 124 (30.8–352) |
| Discovery | 3 | 64.0 (14.0–131) | 3 | 76.7 (55.3–88.1) | |
| Gravenstein | 5 | 5.80 (3.50–12.0) | 3 | 70.7 (30.8–107) | |
| Summerred | 4 | 7.18 (4.10–16.1) | 4 | 173 (47.3–297) | |
| Phenylethyl acetate | Aroma | 5 | 29.8 (9.2–69.2) | 7 | 401 (100–1402) |
| Discovery | 3 | 45 (18.2–97.7) | 3 | 282 (228–330) | |
| Gravenstein | 5 | 18.56 (9.1–32.6) | 3 | 419 (246–570) | |
| Summerred | 4 | 13.25 (8.1–26.1) | 4 | 532 (221–1120) | |
| Butyl acetate | Aroma | 5 | 2249 (261–6480) | 7 | 1573 (144–2821) |
| Discovery | 3 | 3286 (1366–4996) | 3 | 924 (308–1948) | |
| Gravenstein | 5 | 82.3 (28.9–128.7) | 3 | 231 (108–342) | |
| Summerred | 4 | 46.02 (26.5–55.1) | 4 | 315 (121–623) | |
| Isoamyl acetate | Aroma | 5 | 150 (47.6–386) | 7 | 1250 (314–2980) |
| Discovery | 3 | 537 (324–938) | 3 | 1128 (769–1525) | |
| Gravenstein | 5 | 6.56 (2.90–12.9) | 3 | 1962 (347–3381) | |
| Summerred | 4 | 5.2 (1.70–14.3) | 4 | 4138 (2590–6243) | |
| Hexyl acetate | Aroma | 5 | 796 (ND–2772) | 7 | 517 (ND–1171) |
| Discovery | 3 | 1147 (ND–2650) | 3 | 290 (164–382) | |
| Gravenstein | 5 | 53.7 (ND–89.2) | 3 | 307 (78.0–615) | |
| Summerred | 4 | 27.5 (ND–40.3) | 4 | 1241 (715–1817) | |
| z-3-hexenyl acetate | Aroma | 5 | 3.40 (1.20–8.80) | 7 | 3.73 (0.90–8.30) |
| Discovery | 3 | 2.97 (0.90–5.40) | 3 | 1.67 (0.90–2.20) | |
| Gravenstein | 5 | 0.92 (0.40–1.30) | 3 | 1.80 (1.00–3.00) | |
| Summerred | 4 | 0.65 (0.30–0.80) | 4 | 2.63 (1.60–4.30) | |
| E-2-hexenyl acetate | Aroma | 5 | 3.96 (1.10–9.30) | 7 | 0.97 (0.50–1.70) |
| Discovery | 3 | 6.50 (3.00–11.5) | 3 | 0.83 (0.60–1.10) | |
| Gravenstein | 5 | 1.36 (0.50–2.30) | 3 | 0.87 (0.60–1.00) | |
| Summerred | 4 | 0.68 (0.50–1.00) | 4 | 1.05 (0.60–1.70) | |
| Ethyl phenyl acetate | Aroma | 5 | 0.44 (0.30–0.60) | 7 | 2.56 (1.00–4.60) |
| Discovery | 3 | 0.40 (0.20–0.70) | 3 | 4.30 (2.80–6.20) | |
| Gravenstein | 5 | 0.42 (0.20–0.90) | 3 | 6.53 (2.20–13.5) | |
| Summerred | 4 | 0.30 (0.20–0.40) | 4 | 2.15 (1.70–2.60) | |
| Octyl acetate | Aroma | 5 | 5.06 (0.30–12.2) | 7 | 1.04 (0.30–2.20) |
| Discovery | 3 | 6.80 (2.60–15.2) | 3 | 1.40 (0.80–2.30) | |
| Gravenstein | 5 | 0.64 (0.10–1.10) | 3 | 1.27 (0.90–1.90) | |
| Summerred | 4 | 0.48 (0.20–0.60) | 4 | 1.25 (1.00–1.70) | |
| Ethyl Esters of hydroxycinnamic acid | |||||
| Ethyl cinnamate | Aroma | 5 | 0.76 (0.40–1.50) | 7 | 0.84 (0.40–1.60) |
| Discovery | 3 | 0.73 (0.40–1.30) | 3 | 0.63 (0.40–0.80) | |
| Gravenstein | 5 | 0.60 (0.10–1.10) | 3 | 1.03 (0.30–2.10) | |
| Summerred | 4 | 0.50 (0.40–0.60) | 4 | 4.47 (0.30–11.6) | |
| Ethyl dihydrocinnamate | Aroma | 5 | 0.22 (0.20–0.30) | 7 | 0.37 (ND–1.10) |
| Discovery | 3 | 0.20 (ND–0.40) | 3 | 0.10 (0.10–0.10) | |
| Gravenstein | 5 | 0.16 (ND–0.40) | 3 | 0.43 (0.10–0.90) | |
| Summerred | 4 | 0.08 (ND–0.30) | 4 | 0.60 (0.10–1.80) | |
| C6-Alcohols | |||||
| Hexanol | Aroma | 5 | 15,778 (2895–33,297) | 7 | 7799 (4602–12,850) |
| Discovery | 3 | 13,441 (2556–27,675) | 3 | 10,022 (6958–11,849) | |
| Gravenstein | 5 | 15,380 (6166–28,187) | 3 | 11,655 (6824–20,464) | |
| Summerred | 4 | 9101 (1752–24,950) | 4 | 8183 (6963–9490) | |
| Z-3-hexenol | Aroma | 5 | 142 (7.60–272) | 7 | 80.8 (10.2–187) |
| Discovery | 3 | 29.5 (11.6–42.2) | 3 | 31.9 (17.8–48.3) | |
| Gravenstein | 5 | 78.4 (11.7–164) | 3 | 58.6 (15.4–106) | |
| Summerred | 4 | 32.9 (5.60–97.1) | 4 | 34.9 (26.9–39.1) | |
| E-2 hexenol | Aroma | 5 | 648 (84.0–1839) | 7 | 6.57 (ND–14.5) |
| Discovery | 3 | 1267 (67.0–2059) | 3 | 13.4 (5.60–21.6) | |
| Gravenstein | 5 | 634 (75.0–1717) | 3 | 13.4 (8.70–17.8) | |
| Summerred | 4 | 552 (188–1207) | 4 | 10.4 (7.70–14.7) | |
| E-3 hexenol | Aroma | 5 | 19.8 (ND–37.6) | 7 | 23.1 (6.80–56.8) |
| Discovery | 3 | 25.8 (4.80–62.5) | 3 | 35.6 (21.8–51.8) | |
| Gravenstein | 5 | 18.1 (4.80–32.1) | 3 | 36.0 (11.5–50.4) | |
| Summerred | 4 | 11.5 (1.90–26.6) | 4 | 37.4 (28.4–57.8) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Øvsthus, I.; Martelanc, M.; Albreht, A.; Radovanović Vukajlović, T.; Česnik, U.; Mozetič Vodopivec, B. Chemical Characterization of Cider Produced in Hardanger—From Juice to Finished Cider. Beverages 2024, 10, 73. https://doi.org/10.3390/beverages10030073
Øvsthus I, Martelanc M, Albreht A, Radovanović Vukajlović T, Česnik U, Mozetič Vodopivec B. Chemical Characterization of Cider Produced in Hardanger—From Juice to Finished Cider. Beverages. 2024; 10(3):73. https://doi.org/10.3390/beverages10030073
Chicago/Turabian StyleØvsthus, Ingunn, Mitja Martelanc, Alen Albreht, Tatjana Radovanović Vukajlović, Urban Česnik, and Branka Mozetič Vodopivec. 2024. "Chemical Characterization of Cider Produced in Hardanger—From Juice to Finished Cider" Beverages 10, no. 3: 73. https://doi.org/10.3390/beverages10030073
APA StyleØvsthus, I., Martelanc, M., Albreht, A., Radovanović Vukajlović, T., Česnik, U., & Mozetič Vodopivec, B. (2024). Chemical Characterization of Cider Produced in Hardanger—From Juice to Finished Cider. Beverages, 10(3), 73. https://doi.org/10.3390/beverages10030073









