Synergetic Effects of Coffea liberica and Curcuma zanthorrhiza: Study of Sensory Profile, Proximate, and Chemical Compound
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Sample Preparation
2.3. Cupping Test
2.4. Sample Extraction
2.5. Total of Phenolics Content (TPC) and Total of Flavonoids (TFC)
2.6. Total Caffeine and Chlorogenic Acids
2.7. Antioxidant Activities
2.8. Proximate Analysis
2.9. Data Analysis
3. Results
3.1. Sensory Profile
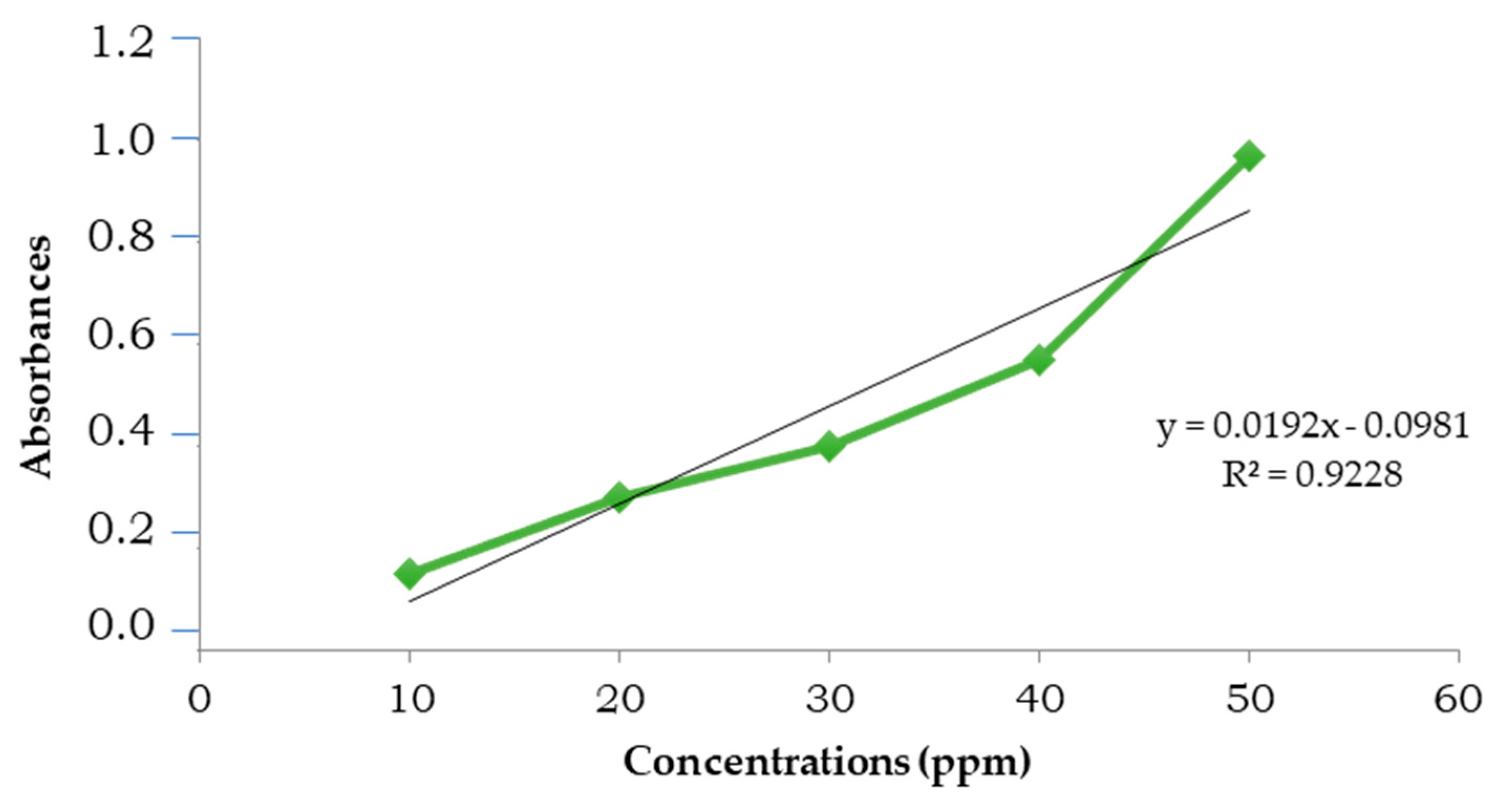
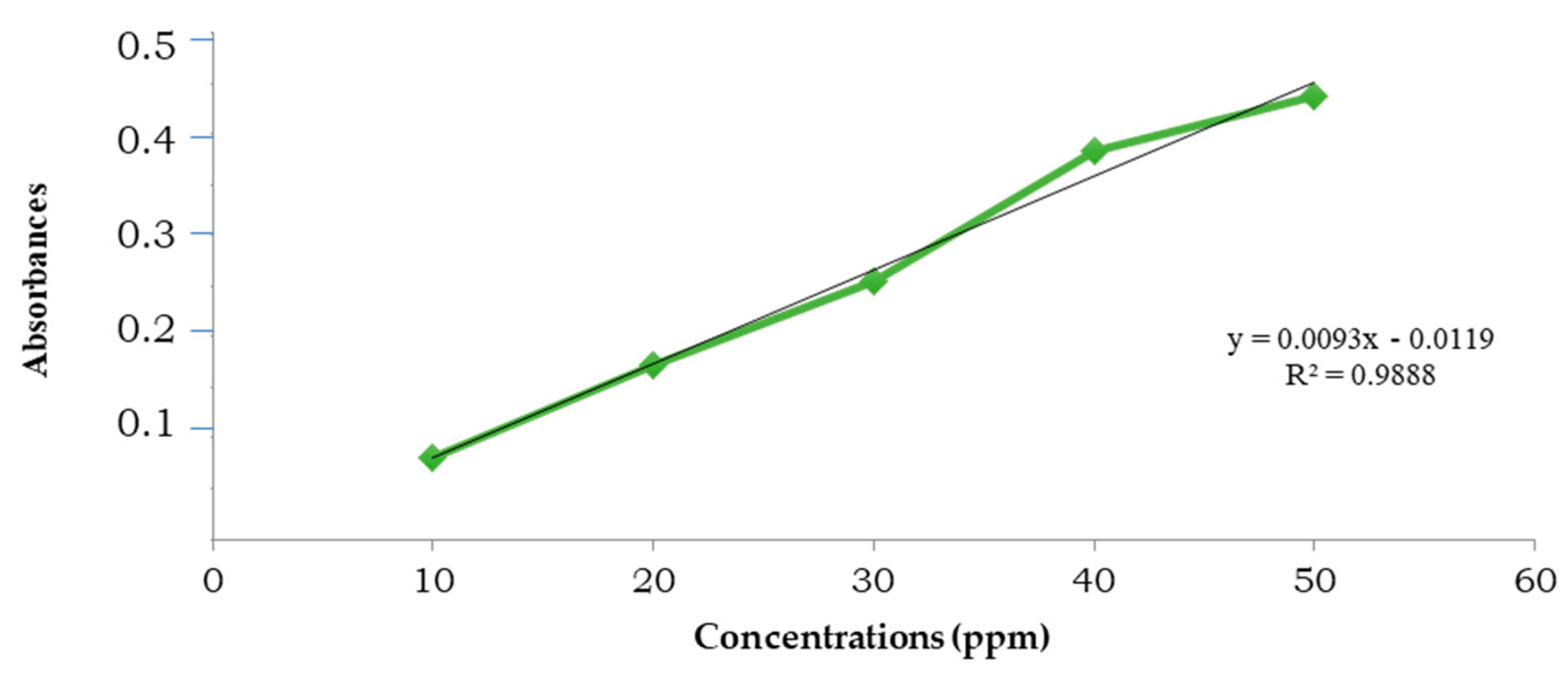
3.2. Total Phenolic and Flavonoid
3.3. Caffeine and Chlorogenic Acid Content
3.4. Antioxidant Activity
3.5. Physical–Chemical Analysis
3.6. Volatile Compound Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darma, R.; Salman, D. Problems identification of Arabica coffee commodities on traditional farming in Indonesia: A review. IOP Conf. Ser. Earth Environ. Sci. 2021, 886, 012069. [Google Scholar] [CrossRef]
- Umiyati, E.; Zulfanetti, Z.; Marselina, E. Sustainability Strategy of Small and Medium Micro Business During the Covid19 Pandemic in Jambi Province. In Proceedings of the 3rd Progress in Social Science, Humanities and Education Research Symposium (PSSHERS 2021), West Sumatra, Indonesia, 3 November 2021; Atlantis Press SARL: Paris, France, 2023; pp. 256–266. [Google Scholar] [CrossRef]
- Anshori, N.I.; Zainol, M.K.; Zin, Z.M. Antioxidant Activities of Different Varieties of Spent Coffee Ground (Scg) Extracted Using Ultrasonic-Ethanol Assisted Extraction Method. Univ. Malays. Teren. J. Undergrad. Res. 2021, 3, 33–42. [Google Scholar] [CrossRef]
- Saidi, B.B.; Suryani, E. Evaluasi Kesesuaian Lahan Untuk Pengembangan Kopi Liberika Di Kabupaten Tanjung Jabung Timur Jambi. J. Ilm. Ilmu. Terap. Univ. Jambi 2021, 5, 1–15. [Google Scholar]
- Mawardhi, A.D.; Setiadi, D. Strategi Pemanfaatan Lahan Gambut melalui Pengembangan Agroforestri Kopi Liberika (Coffea liberica) Strategy on Peatland Utilisaton through Development of Coffea Liberica Agroforestry. In Proceedings of the Seminar Nasional Lahan Suboptimal, Palembang, Indonesia, 18–19 October 2018; pp. 43–51. [Google Scholar]
- Mubarok, F.; Suwasono, S.; Palupi, N.W. Perubahan Kadar Kafein Biji Kopi Arabika Hasil Pengolahan Semi Basah Dengan Perlakuan Variasi Jenis Wadah Dan Lama Fermentasi. Berk. Imliah Pertan. 2014, 1–7. [Google Scholar]
- Artha, B.A.P.; Wulandari, Y.W.; Suhartatik, N. Aktivitas Antioksidan Kopi Rempah dengna Penambahan Kapulaga (Amomum compactum) dan Kayu Manis (Cinnamomum verum). JITIPARI J. Ilm. Teknol. Dan. Ind. Pangan. UNISRI 2020, 5, 48–58. [Google Scholar] [CrossRef]
- Mardhatilah, D. Pengaruh Penambahan Konsentrasi Jahe dan Rempah Pada Pembuatan Sirup Kopi. Agroteknose 2015, 6, 55–61. [Google Scholar]
- Sripoo, A.R.; Maheswari, U.T.N.; Rajeshkumar, S. Preparation of oregano, coffee and black cumin aqueous formulation and its anti inflammatory activity. Int. J. Health Sci. Qassim 2022, 6, 288–295. [Google Scholar] [CrossRef]
- Syamsudin, R.A.M.R.; Farid, P.; Farly, S.M.; Rina, A.P.A.; Cahyani, N.D.; Aprilya, S.; Yanti, R.; Khendri, F. Temulawak Plant (Curcuma xanthorrhiza Roxb) as a Traditional Medicine. J. Ilm. Farm. Bahari 2019, 10, 51–65. [Google Scholar] [CrossRef]
- Fathir, A.; Haikal, M.; Wahyudi, D. Ethnobotanical study of medicinal plants used for maintaining stamina in madura ethnic, East Java, Indonesia. Biodiversitas 2021, 22, 386–392. [Google Scholar] [CrossRef]
- Rosidi, A.; Khomsan, A.; Setiawan, B.; Riyadi, H.; Briawan, D. Antioxidant potential of temulawak (Curcuma xanthorrhiza roxb). Pak. J. Nutr. 2016, 15, 556–560. [Google Scholar] [CrossRef][Green Version]
- Widyastuti, I.; Luthfah, H.Z.; Hartono, Y.I.; Islamadina, R.; Can, A.T.; Rohman, A. Antioxidant Activity of Temulawak (Curcuma xanthorrhiza Roxb.) and its Classification with Chemometrics. Indones. J. Chemom. Pharm. Anal. 2021, 1, 28–41. [Google Scholar] [CrossRef]
- Sumardi, S.; Rasdiansyah, R. Kualitas Fisik dan Kimia Kopi Celup Arabika Rasa Kayu Manis pada Tingkat Penyangraian yang Berbeda. J. Ilm. Mhs. Pertan. 2022, 7, 323–329. [Google Scholar] [CrossRef]
- F Fatmawati, F.; Muhammad, M.; Fokaya, R. Feasibility Analysis of Spice Coffee Processing Business in Home Industries in Tabahawa Village, Ternate City. Agrikan J. Agribisnis Perikan. 2020, 13, 344–351. [Google Scholar] [CrossRef]
- Tarigan, I.L.; Munawaroh, S.; Sutrisno; Yusnaidar Latief, M. Liberica coffee enriched with Cinnamon (Cinnamomum verum): Synergetic study of sensory, antioxidant activity, and chemical components. Coffee Sci. 2023, 18, e182149. [Google Scholar] [CrossRef]
- Perdana, B.M.; Manihuruk, R.; Ashyar, R.; Heriyanti; Sutrisno. Evaluation of the effect of roasting process on the energy transition and the crystalline structures of Arabica, Robusta, and Liberica coffee from Jambi Indonesia Evaluation of the effect of roasting process on the energy transition and the crystalline. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345, 012021. [Google Scholar] [CrossRef]
- Isnidayu, A.V.; Sukartiko, A.C.; Ainuri, M. Indicator of Sensory Attributes of Speciality Coffee Originated from West Java Based on Biochemical Component. J. Tanam. Ind. Dan. Penyegar. 2020, 7, 1. [Google Scholar] [CrossRef]
- Somporn, C.; Kamtuo, A.; Theerakulpisut, P.; Siriamornpun, S. Effects of roasting degree on radical scavenging activity, phenolics and volatile compounds of Arabica coffee beans (Coffea arabica L. cv. Catimor). Int. J. Food Sci. Technol. 2011, 46, 2287–2296. [Google Scholar] [CrossRef]
- Ahmad, A.R.; Juwita, J.; Ratulangi, S.A.D.; Malik, A. Penetapan Kadar Fenolik dan Flavonoid Total Ekstrak Metanol Buah dan Daun Patikala (Etlingera elatior (Jack) R.M.SM). Pharm. Sci. Res. 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Antioxidant activity, total polyphenol, flavonoid and tannin contents of fermented green coffee beans with selected yeasts. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef]
- Arwangga, A.F.; Asih, I.A.R.A.; Sudiarta, I.W. Analisis Kandungan Kafein Pada Kopi di Desa Sesaot Narmada Menggunakan Spektrofotometri UV-Vis. J. Kim. 2016, 10, 110–114. [Google Scholar] [CrossRef]
- Furqan, M.; Nurman, S. Ekstrak Polar Kopi Hijau Arabika (Coffea arabica L.) sebagai Antihiperglikemi pada Mencit (Mus musculus). J. Healthc. Technol. Med. 2020, 6, 1323–1331. [Google Scholar]
- Selvi, A.T.; Joseph, G.S.; Jayaprakasha, G.K. Inhibition of growth and aflatoxin production in Aspergillus flavus by Garcinia indica extract and its antioxidant activity. Food Microbiol. 2003, 20, 455–460. [Google Scholar] [CrossRef]
- Suseno, H.; Haryanto, Galih, N.R.P.; Hidayati, N.; Alonto, C.; Irfan, M. Panduan Penerapan Dan Sertifikasi SNI Produk Kopi Bubuk; Badan Standarisasi Nasional: Jakarta, Indonesia, 2020. [Google Scholar]
- Maizura, M.; Aminah, A.; Aida, W.M.W. Total phenolic content and antioxidant activity of kesum (Polygonum minus), ginger (Zingiber officinale) and turmeric (Curcuma longa) extract. Int. Food Res. J. 2011, 18, 529–534. [Google Scholar]
- Cepeda, G.N.; Lisangan, M.M.; Silamba, I. Antibacterial Activity of Akway Bark Essential Oil (Drimys piperita Hook. f.) at Several Levels of Concentration, Acidity (pH) and Salt Content. J. Apl. Teknol. Pangan. 2019, 8, 149. [Google Scholar] [CrossRef]
- Górecki, M.; Hallmann, E. The antioxidant content of coffee and its in vitro activity as an effect of its production method and roasting and brewing time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Tarhan, O.; Rezaei, A.; Capanoglu, E.; Boostani, S.; Khoshnoudi-Nia, S.; Samborska, K.; Garavand, F.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Addition of milk to coffee beverages; the effect on functional, nutritional, and sensorial properties. Crit. Rev. Food Sci. Nutr. 2022, 62, 6132–6152. [Google Scholar] [CrossRef]
- Husniati, H.; Oktiani, D. Chlorogenic Acid Isolation from Coffee as Affected by the Homogeneity of Cherry Maturity. Pelita Perkeb Coffee Cocoa Res J. 2019, 35, 119–124. [Google Scholar] [CrossRef]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suarez-Quiroz, M.L.; Gonzalez-Amaro, R.M.; Hernandez-Estrada, Z.J.; Rayas-Duarte, P. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef]
- Yang, X.W.; Wang, N.; Li, W.; Xu, W.; Wu, S. Biotransformation of 4,5-O-dicaffeoylquinic acid methyl ester by human intestinal flora and evaluation on their inhibition of NO production and antioxidant activity of the products. Food Chem. Toxicol. 2013, 55, 297–303. [Google Scholar] [CrossRef]
- Mills, C.E.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. The effect of processing on chlorogenic acid content of commercially available coffee. Food Chem. 2013, 141, 3335–3340. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.H.; Doroudi, A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 2023, 13, 354–376. [Google Scholar] [CrossRef] [PubMed]
- Ahwan, A.; Suwarni, A.; Ariastuti, R.; Hafidz, R.; Mei Enjelina, S. Effect of Total Phenolic and Total Flavonoid Levels on the Antioxidant Power od Water Extract, Ethanol, and Chloroform of Green Tea Leaves (Camellia sinensis L). Med. Sains J. Ilm. Kefarmasian. 2024, 9, 17–28. [Google Scholar] [CrossRef]
- Seninde Chambers, E. Coffee flavor: A review. Beverages 2020, 6, 44. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Durak, A.; Gawlik-Dziki, U.; Pecio, Ł. Coffee with cinnamon—Impact of phytochemicals interactions on antioxidant and anti-inflammatory in vitro activity. Food Chem. 2014, 162, 81–88. [Google Scholar] [CrossRef]
- Yurasbe, N.Q.; Din, N.A.; Palaniveloo, K.; Manikam, S.; Nagappan, T. Phytochemical diversity and biological activities of Curcuma species from the East Coast of Peninsular Malaysia. Biodiversitas 2023, 24, 4243–4252. [Google Scholar] [CrossRef]
- Pinarli, B.; Simge Karliga, E.; Ozkan, G.; Capanoglu, E. Interaction of phenolics with food matrix: In vitro and in vivo approaches. Med. J. Nutr. Metab. 2020, 13, 63–74. [Google Scholar] [CrossRef]
- Neunert, G.; Górnaś, P.; Dwiecki, K.; Siger, A.; Polewski, K. Synergistic and antagonistic effects between alpha-tocopherol and phenolic acids in liposome system: Spectroscopic study. Eur. Food Res. Technol. 2015, 241, 749–757. [Google Scholar] [CrossRef]
- Durak, A.; Gawlik-Dziki, U.; Sugier, D. Coffee enriched with willow (Salix purpurea and Salix myrsinifolia) bark preparation—Interactions of antioxidative phytochemicals in a model system. J. Funct. Foods. 2015, 18, 1106–1116. [Google Scholar] [CrossRef]
- Erskine, E.; Gültekin Subaşl, B.; Vahapoglu, B.; Capanoglu, E. Coffee Phenolics and Their Interaction with Other Food Phenolics: Antagonistic and Synergistic Effects. ACS Omega 2022, 7, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Otálvaro, E.; Domínguez-Perles, R.; Mazo-Rivas, J.C.; García-Viguera, C. Bioavailability and radical scavenging power of phenolic compounds of cocoa and coffee mixtures. Food Sci. Technol. Int. 2022, 28, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Rodak, K.; Kokot, I.; Kratz, E.M. Caffeine as a factor influencing the functioning of the human body—Friend or foe? Nutrients 2021, 13, 3088. [Google Scholar] [CrossRef] [PubMed]
- Kuswardhani, N.; Mukti, N.P.; Sari, P. Antioxidant and sensory properties of ready to drink coffee-ginger made from decaffeinated and non-decaffeinated robusta coffee beans. IOP Conf. Ser. Earth Environ. Sci. 2021, 653, 012050. [Google Scholar] [CrossRef]
- Saifulloh, S.; Pamungkas, R.; Sari, D.A. Sensory Analysis of the Panelist’s Favorite Spiced Coffee Drinks. Gontor Agrotech. Sci. J. 2023, 8, 118–124. [Google Scholar] [CrossRef]
- Santosa, K.M.; Supriyadi; Anggrahini, S.; Rahmadian, Y. Sensory Analysis, Caffeine, Chlorogenic Acid and Non-Volatile Taste Compounds of Arabica Coffee (Coffea arabica) Fermented with Sugar Addition for Brew Taste. Indones. Food Nutr. Prog. 2021, 17, 37–44. [Google Scholar] [CrossRef]

| No. | Sample Codes | Information Ratio (%) |
|---|---|---|
| 1 | CB | C. zanthorrhiza (100%) |
| 2 | CO | Coffee Liberica (100%) |
| 3 | CH1 | Coffee: C. zanthorrhiza (99:1) |
| 4 | CH2 | Coffee: C. zanthorrhiza (97:3) |
| 5 | CH3 | Coffee: C. zanthorrhiza (95:5) |
| Parameters | Samples | |||
|---|---|---|---|---|
| CO ± SD | CH1 ± SD | CH2 ± SD | CH3 ± SD | |
| Aroma | 7.58 ± 0.02 | 7.13 ± 0.12 | 7.17 ± 0.12 | 7.17 ± 0.11 |
| Flavor | 7.67 ± 0.04 | 6.92 ± 0.01 | 7.08 ± 0.01 | 7.42 ± 0.01 |
| Aftertaste | 7.67 ± 0.15 | 6.92 ± 0.08 | 7.17 ± 0.03 | 7.25 ± 0.02 |
| Acidity | 7.77 ± 0.09 | 6.83 ± 0.08 | 7.08 ± 0.03 | 7.42 ± 0.05 |
| Body | 7.67 ± 0.09 | 6.58 ± 0.08 | 7.17 ± 0.04 | 7.08 ± 0.05 |
| Uniformity | 8.00 ± 0.00 | 10.00 ± 0.00 | 10.00 ± 0.03 | 10.00 ± 0.00 |
| Balance | 7.67 ± 0.08 | 6.58 ± 0.15 | 6.92 ± 0.05 | 7.33 ± 0.05 |
| Clean cup | 8.00 ± 0.00 | 10.00 ± 0.00 | 10.00 ± 0.00 | 10.00 ± 0.00 |
| Sweetness | 8.00 ± 0.00 | 10.00 ± 0.00 | 10.00 ± 0.00 | 10.00 ± 0.00 |
| Overall | 7.67 ± 0.08 | 6.25 ± 0.07 | 6.75 ± 0.07 | 7.08 ± 0.07 |
| Total score | 7.77 ± 0.073 | 7.72 ± 0.078 | 7.93 ± 0.051 | 8.08 ± 0.051 |
| Category | ** | ** | *** | *** |
| Final score | 77.7 ± 0.073 a | 77.2 ± 0.078 a | 79.3 ± 0.051 b | 80.8 ± 0.051 c |
| Group | Premium | Premium | Specialty | Specialty |
| Samples | TPC (mgGAE/g ± SD) | TFC (mgQE/g ± SD) |
|---|---|---|
| CB | 103.313 a ± 0.037 | 34.452 a ± 0.076 |
| CO | 42.271 b ± 0.037 | 8.43 b ± 0.001 |
| CH1 | 43.313 c ± 0.037 | 18.323 d ± 0.001 |
| CH2 | 46.542 d ± 0.037 | 20.903 e ± 0.009 |
| CH3 | 49.146 e ± 0.010 | 24.667 c ± 0.076 |
| Samples | Caffeine (%) ± SD | CGA (%) ± SD |
|---|---|---|
| CH3 | 0.559 a ± 0.041 | 2.611 a ± 0.002 |
| CH2 | 0.657 b ± 0.031 | 2.788 b ± 0.001 |
| CH1 | 0.761 c ± 0.044 | 2.893 c ± 0.001 |
| CO | 0.858 d ± 0.001 | 2.984 d ± 0.005 |
| CB | 0.000 d ± 0.000 | 0.000 d ± 0.000 |
| Samples | IC50 (ppm) ± SD | Antioxidant Activity |
|---|---|---|
| CH3 | 4.984 a ± 0.000 | Very Strong |
| CH2 | 8.39 c ± 0.188 | Very Strong |
| CH1 | 12.221 d ± 0.021 | Very Strong |
| Ascorbic Acid | 45.393 e ± 0.000 | Very Strong |
| CO | 72.122 b ± 0.004 | Strong |
| CB | 6.1408 f ± 0.051 | Very Strong |
| Samples | % Waters | % Ash | % Proteins | % Fats | % Carbohydrates |
|---|---|---|---|---|---|
| CO | 4.302 | 4.175 | 17.61 | 10.17 | 5.940 |
| CB | 8.205 | 3.209 | 1.52 | 1.35 | 5.185 |
| CH1 | 4.302 | 4.232 | 17.09 | 10.13 | 5.300 |
| CH2 | 4.412 | 4.244 | 16.80 | 9.75 | 4.742 |
| CH3 | 4.522 | 4.254 | 15.78 | 9.14 | 4.318 |
| Peak Compounds | R. Time (Minutes) | Area (%) | Molecular Formula | Compound Names |
|---|---|---|---|---|
| 1 | 22.307 | 7.98 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C27H52O5 | Dodecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | |||
| C57H10O6 | Glyceryl tridodecanoate | |||
| 2 | 22.447 | 30.97 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C27H52O5 | Dodecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | |||
| C57H10O6 | Glyceryl tridodecanoate | |||
| 3 | 22.622 | 10.30 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C49H94O6 | Octadecanoic acid, 2,3-bis[(1-oxotetradecyl)oxy]propyl ester | |||
| 4 | 22.800 | 7.16 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C35H66O6 | 2-Lauro-1,3-didecoin | |||
| 5 | 22.875 | 5.79 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C27H52O5 | Dodecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | |||
| 6 | 22.945 | 9.84 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C35H66O6 | 2-Lauro-1,3-didecoin | |||
| 7 | 23.091 | 15.82 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C26H48F3NO2 | Dodecanamide, N-Dodecyl-N-(Trifluoroacetyl) | |||
| 8 | 23.152 | 8.41 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C26H48F3NO2 | Dodecanamide, N-Dodecyl-N-(Trifluoroacetyl) | |||
| 9 | 23.285 | 2.35 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C47H90O6 | Hexadecanoic acid, 2-[(1-oxododecyl)oxy]-1,3-propanediyl ester | |||
| C55H106O6 | Eicosanoic acid, 2-[(1-oxohexadecyl)oxy]-1-[[(1-oxohexadecyl)oxy]methyl]ethyl ester | |||
| 10 | 23.444 | 1.03 | C35H66O6 | 2-Lauro-1,3-didecoin |
| C14H26O2 | Dodecanoic acid, ethenyl ester | |||
| C17H36 | 2,6,10-Trimethyltetradecane | |||
| 11 | 23.545 | 0.35 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C14H26O2 | Dodecanoic acid, ethenyl ester | |||
| C27H52O5 | Dodecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester |
| Peak Compounds | R. Time (Minute) | Area (%) | Molecular Formula | Compound Name |
|---|---|---|---|---|
| 1 | 15.563 | 0.40 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 2 | 16.533 | 0.64 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 3 | 17.123 | 0.98 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C49H94O6 | Octadecanoic acid, 2,3-bis[(1-oxotetradecyl)oxy]propyl ester | |||
| 4 | 17.758 | 1.91 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C14H26O2 | Dodecanoic acid, ethenyl ester | |||
| 5 | 17.865 | 3.05 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 6 | 18.602 | 0.93 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 7 | 18.732 | 0.65 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 8 | 19.249 | 3.15 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 9 | 19.367 | 2.21 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C27H52O5 | Dodecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | |||
| C49H98O4 | Lauric acid, 2-(hexadecyloxy)-3-(octadecyloxy)propyl ester | |||
| 10 | 19.414 | 2.87 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C27H52O5 | Dodecanoic acid, 1-(Hydroxymethyl)-1,2-Ethanediyl Ester | |||
| 11 | 19.696 | 15.20 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 12 | 19.833 | 1.51 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C27H52O5 | Dodecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | |||
| 13 | 19.925 | 1.41 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C49H94O | Octadecanoic acid, 2,3-bis[(1-oxotetradecyl)oxy]propyl ester | |||
| C21H36 | 14-β-H-Pregna | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxohexadecyl)oxy]-2-[(1-oxotetradecyl)oxy]propyl ester | |||
| 14 | 20.092 | 12.48 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 15 | 20.281 | 4.05 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 16 | 20.333 | 0.86 | C45H86O6 | Tetradecanoic acid, 1,2,3-propanetriyl ester |
| C36H72NO8P | L-Dimyristoyl lecithin | |||
| C49H94O6 | Octadecanoic acid, 2,3-bis[(1-oxotetradecyl)oxy]propyl ester | |||
| C45H86O6 | Tetradecanoic acid, 1,2,3-propanetriyl ester | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 17 | 20.425 | 4.72 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 18 | 20.463 | 4.03 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxohexadecyl)oxy]-2-[(1-oxotetradecyl)oxy]propyl ester | |||
| 19 | 20.747 | 8.35 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C47H90O6 | Hexadecanoic acid, 2-[(1-oxododecyl)oxy]-1,3-propanediyl ester | |||
| C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester | |||
| C57H10O6 | Glyceryl tridodecanoate | |||
| 20 | 20.828 | 9.46 | C47H90O6 | Hexadecanoic acid, 2-[(1-oxododecyl)oxy]-1,3-propanediyl ester |
| C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester | |||
| C35H66O6 | 2-Lauro-1,3-didecoin | |||
| C47H90O6 | Hexadecanoic acid, 2-[(1-oxododecyl)oxy]-1,3-propanediyl ester | |||
| 21 | 21.197 | 6.07 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 22 | 21.312 | 2.45 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C47H90O6 | Hexadecanoic acid, 2-[(1-oxododecyl)oxy]-1,3-propanediyl ester | |||
| C57H10O6 | Glyceryl tridodecanoate | |||
| 23 | 21.434 | 7.81 | C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester |
| C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester | |||
| 24 | 21.491 | 2.57 | C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester |
| C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester | |||
| 25 | 21.571 | 2.24 | C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester |
| C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester | |||
| C14H26O2 | Dodecanoic acid, ethenyl ester |
| Peak Compounds | R. Time (Minute) | Area (%) | Molecular Formula | Compound Name |
|---|---|---|---|---|
| 1 | 15.535 | 0.16 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C49H94O6 | Octadecanoic acid, 2,3-bis[(1-oxotetradecyl)oxy]propyl ester | |||
| 2 | 17.849 | 0.72 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 3 | 18.508 | 0.65 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C27H52O5 | Dodecanoic acid,1-(hydroxymethyl) | |||
| 4 | 18.603 | 1.13 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 5 | 18.724 | 0.44 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C27H52O5 | Dodecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | |||
| 6 | 19.049 | 1.03 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 7 | 19.247 | 2.10 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 8 | 19.367 | 2.92 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C27H52O5 | Dodecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | |||
| 9 | 19.411 | 3.84 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 10 | 19.592 | 2.76 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C27H52O5 | Dodecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | |||
| 11 | 19.647 | 3.96 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 12 | 20.070 | 7.74 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 13 | 20.276 | 3.54 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxohexadecyl)oxy]-2-[(1-oxotetradecyl)oxy]propyl ester | |||
| 14 | 20.333 | 1.10 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C49H94O6 | Octadecanoic acid, 2,3-bis[(1-oxotetradecyl)oxy]propyl ester | |||
| 15 | 20.455 | 9.43 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester. | |||
| 16 | 20.721 | 12.90 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 17 | 20.815 | 8.81 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C57H10O6 | Glyceryl tridodecanoate | |||
| C49H94O6 | Octadecanoic acid, 2,3-bis[(1-oxotetradecyl)oxy]propyl ester | |||
| 18 | 20.950 | 2.38 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C47H90O6 | Hexadecanoic acid, 2-[(1-oxododecyl)oxy]-1,3-propanediyl ester | |||
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 19 | 21.087 | 8.62 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C47H90O6 | Hexadecanoic acid, 2-[(1-oxododecyl)oxy]-1,3-propanediyl ester | |||
| C57H10O6 | Glyceryl tridodecanoate | |||
| 20 | 21.315 | 1.13 | C47H90O6 | Hexadecanoic acid, 2-[(1-oxododecyl)oxy]-1,3-propanediyl ester |
| C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester | |||
| C45H86O6 | Tetradecanoic acid, 1,2,3-propanetriyl ester | |||
| 21 | 21.408 | 2.18 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C51H98O6 | Octadecanoic acid, 3-[(1-oxohexadecyl)oxy]-2-[(1-oxotetradecyl)oxy]propyl ester | |||
| C20H38O2 | Octadecanoic acid, ethenyl ester | |||
| C19H40 | n-Nonadecane | |||
| C32H66 | Dotriacontane | |||
| 22 | 21.489 | 1.46 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester | |||
| 23 | 21.582 | 2.65 | C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester |
| C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester | |||
| 24 | 21.695 | 5.40 | C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester |
| C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester | |||
| 25 | 21.784 | 3.43 | C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester |
| C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester | |||
| 26 | 21.918 | 3.27 | C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester |
| 27 | 22.014 | 3.98 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C47H90O6 | Hexadecanoic acid, 2-[(1-oxododecyl)oxy]-1,3-propanediyl ester | |||
| C57H10O6 | Glyceryl tridodecanoate | |||
| 28 | 22.216 | 1.77 | C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester |
| C47H90O6 | Hexadecanoic acid, 2-[(1-oxododecyl)oxy]-1,3-propanediyl ester | |||
| C57H10O6 | Glyceryl tridodecanoate | |||
| 29 | 22.514 | 0.50 | C51H98O6 | Octadecanoic acid, 3-[(1-oxododecyl)oxy]-1,2-propanediyl ester |
| C39H74O6 | Dodecanoic acid, 1,2,3-propanetriyl ester | |||
| C47H90O6 | Hexadecanoic acid, 2-[(1-oxododecyl)oxy]-1,3-propanediyl ester |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latief, M.; Muntasir, R.W.; Wijaya, D.E.; Tarigan, I.L.; Sutrisno, S. Synergetic Effects of Coffea liberica and Curcuma zanthorrhiza: Study of Sensory Profile, Proximate, and Chemical Compound. Beverages 2025, 11, 9. https://doi.org/10.3390/beverages11010009
Latief M, Muntasir RW, Wijaya DE, Tarigan IL, Sutrisno S. Synergetic Effects of Coffea liberica and Curcuma zanthorrhiza: Study of Sensory Profile, Proximate, and Chemical Compound. Beverages. 2025; 11(1):9. https://doi.org/10.3390/beverages11010009
Chicago/Turabian StyleLatief, Madyawati, Retno Widya Muntasir, Dhian Eka Wijaya, Indra Lasmana Tarigan, and Sutrisno Sutrisno. 2025. "Synergetic Effects of Coffea liberica and Curcuma zanthorrhiza: Study of Sensory Profile, Proximate, and Chemical Compound" Beverages 11, no. 1: 9. https://doi.org/10.3390/beverages11010009
APA StyleLatief, M., Muntasir, R. W., Wijaya, D. E., Tarigan, I. L., & Sutrisno, S. (2025). Synergetic Effects of Coffea liberica and Curcuma zanthorrhiza: Study of Sensory Profile, Proximate, and Chemical Compound. Beverages, 11(1), 9. https://doi.org/10.3390/beverages11010009







