Water Quality in Selected Small Drinking Water Systems of Missouri Rural Communities
Abstract
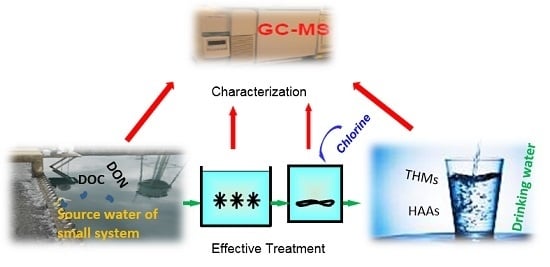
:1. Introduction
Objective and Scope
2. Materials and Methods
2.1. Description of the Small Drinking Water Systems
2.2. Water Sample Collection
2.3. Chemical Analysis
3. Results and Discussion
3.1. System I
3.2. System II
3.3. System III
3.4. Disscusion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DBP | Disinfection Byproduct |
| DOC | Dissolved Organic Carbon |
| EPA | Environmental Protection Agency |
| N-DBP | Nitrogen Containing Disinfection Byproduct |
| NOM | Natural Organic Matter |
| PAC | Powdered Activated Carbon |
| SUVA | Specific UV Absorbance |
| THM | Trihalomethane |
| THMFP | Trihalomethane Formation Potential |
| TTHM | Total Trihalomethanes |
| US | the United States |
| UV254 | Absorbance of Ultraviolet Light at Wavelength 254 nm |
References
- Krasner, S.W.; McGuire, M.J.; Jacangelo, J.G.; Patania, N.L.; Reagan, K.M.; Aieta, E.M. The occurrence of disinfection by-products in United States drinking water. J. Am. Water Works Assoc. 1989, 81, 41–53. [Google Scholar]
- Edzwald, J. Water Quality & Treatment, 6th ed.; American Water Works Association, McGraw Hill: New York, NY, USA, 2011. [Google Scholar]
- Hua, G.; Reckhow, D.A. Characterization of disinfection byproduct precursors based on hydrophobicity and molecular size. Environ. Sci. Technol. 2007, 41, 3309–3315. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, L.; Lu, Y.; Jiang, Y. Factors affecting formation of disinfection by-products during chlorination of Cyciops. J. Water Supply Res. Tech. AQUA 2013, 62, 169–175. [Google Scholar] [CrossRef]
- Roccaro, P.; Vagliasindi, F.; Korshin, G. Relationships between trihalomethanes, haloacetic acids, and haloacetonitriles formed by the chlorination of raw, treated, and fractionated surface waters. J. Water Supply Res. Tech. AQUA 2014, 63, 21–30. [Google Scholar] [CrossRef]
- Woods, G.C.; Dickenson, E.R.V. Evaluation of the final UCMR2 database: Nationwide trends in NDMA. J. Am. Water Works Assoc. 2015, 107, E58–E68. [Google Scholar] [CrossRef]
- Richardson, S.D.; Thruston, A.D., Jr.; Krasner, S.W.; Weinberg, H.S.; Miltner, R.J.; Schenck, K.M.; Narotsky, M.G.; McKague, A.B.; Simmons, J.E. Integrated disinfection byproducts mixtures research: Comprehensive characterization of water concentrates prepared from chlorinated and ozonated/postchlorinated drinking water. J. Toxicol. Environ. Health Part A 2008, 71, 1165–1186. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Rodrigues, M.J.; Sadiq, R. Disinfection byproducts in Canadian provinces: Associated cancer risks and medical expenses. J. Hazard. Mater. 2011, 187, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Guo, W.; Mo, J.; He, Y.; Liu, Y.; Liu, W.; Liang, Y.; Yang, X. The occurrence of disinfection by-products in municipal drinking water in China's Pearl River Delta and a multipathway cancer risk assessment. Sci. Total Environ. 2013, 447, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Grellier, J.; Rushton, L.; Briggs, D.J.; Nieuwenhuijsen, M.J. Assessing the human health impacts of exposure to disinfection by-products—A critical review of concepts and methods. Environ. Int. 2015, 78, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Bellar, T.A.; Lichtenberg, J.J.; Kroner, R.C. The occurrence of organohalides in chlorinated drinking waters. J. Am. Water Works Assoc. 1974, 66, 703–706. [Google Scholar]
- Urbansky, E.T.; Magnuson, M.L. Analyzing drinking water for disinfection byproducts. Anal. Chem. 2002, 74, 260A–267A. [Google Scholar] [CrossRef] [PubMed]
- Stage 1 and Stage 2 Disinfectants and Disinfection Byproducts Rules. Available online: http://water.epa.gov/lawsregs/rulesregs/sdwa/stage2/regs_factsheet.cfm (accessed on 8 April 2016).
- APHA; AWWA; WPCE. Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]
- EPA Method 5710B. Trihalomethane Formation Potential (THMFP). In The Standard Methods for the Examination of Water & Wastewater, 21st ed.; Eaton, A.; Clesceri, L.; Rice, E.; Greenberg, A. (Eds.) APHA, AWWA, WEF: Baltimore, MD, USA, 2005.
- Shi, H.; Adams, C. Occurrence and formation of trihalomethane in marine aquaria studied using solid phase microextraction gas chromatography-mass spectrometry. Water Environ. Res. 2012, 84, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.T.; Gao, S.; Dahlgren, R.A. Physical and chemical fractionation of dissolved organic matter and trihalomethane precursors: A review. J. Water Supply Res. Tech. AQUA 2005, 54, 475–507. [Google Scholar]
- Arslan-Alaton, I.; Aynur-Koyunluoglu, S. Toxicity and biodegradability behavior of xenobiotic chemicals before and after ozonation: A case study with commercial textile tannins. Ozone Sci. Eng. 2007, 29, 443–450. [Google Scholar] [CrossRef]
- He, Y.Y.; Wang, X.C. Adsorption of a typical polycyclic aromatic hydrocarbon by humic substances in water and the effect of coexisting metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2011, 379, 93–101. [Google Scholar] [CrossRef]
- Matilainen, A.; Gjessing, E.T.; Lahtinen, T.; Hed, L.; Bhatnagar, A.; Sillanpää, M. An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 2011, 83, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.J.; Schlenger, P.; García-Valverde, M. Monitoring changes in the structure and properties of humic substances following ozonation using UV-Vis, FTIR and 1H NMR techniques. Sci. Total Environ. 2016, 541, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Cohn, C.A.; Borda, M.J.; Schoonen, M.A. RNA decomposition by pyrite-induced radicals and possible role of lipids during the emergence of life. Earth Planet. Sci. Lett. 2004, 225, 271–278. [Google Scholar] [CrossRef]
- Che, H.; Bae, S.; Lee, W. Degradation of trichloroethylene by Fenton reaction in pyrite suspension. J. Hazard. Mater. 2011, 185, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.; Doyle, F.; Sedlak, D. Inhibitory effect of dissolved silica on H2O2 decomposition by iron(III) and manganese(IV) oxides: Implication for H2O2 based in situ chemical oxidation. Environ. Sci. Techol. 2012, 46, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qu, J.; Liu, H.; Zhao, X. Characterization of isolated fractions of dissolved organic matter from sewage treatment plant and the related disinfection by-products formation potential. J. Hazard. Mater. 2009, 164, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.; Lou, J.; Han, J. A study of removing SUVA and trihalomethanes by biological activated carbon. Int. J. Environ. Chem. Ecol. Geol. Geophys. Eng. 2013, 7, 434–437. [Google Scholar]
- Westerhoff, P.; Mash, H. Dissolved organic nitrogen in drinking water supplies: A review. J. Water Supply Res. Tech.—AQUA 2002, 51, 415–448. [Google Scholar]
- Krasner, S.W.; Mitch, W.A.; McCurry, D.L.; Hanigan, D.; Westerhoff, P. Formation, precursors, control, and occurrence of nitrosamines in drinking water: A review. Water Res. 2013, 47, 4433–4450. [Google Scholar] [CrossRef] [PubMed]
- McCurry, D.L.; Krasner, S.W.; von Gunten, U.; Mitch, W.A. Determinants of disinfectant pretreatment efficacy for nitrosamine control in chloraminated drinking water. Water Res. 2015, 84, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xian, Q.; Yang, G.; Gong, T.; Li, A.; Feng, J. Formation potential of N-nitrosamines from soluble microbial products (SMPs) exposed to chlorine, chloramine and ozone. RSC Adv. 2015, 5, 83682–83688. [Google Scholar] [CrossRef]
- Table of Regulated Drinking Water Contaminants. Available online: https://www.epa.gov/your-drinking-water/table-regulated-drinking-water-contaminants#Disinfectants (accessed on 23 March 2016).
- Drinking Water Disinfection: A Review of Disinfection Practices and Issues. Available online: http://www.waterandhealth.org/drinkingwater/dwwp.pdf (accessed on 8 April 2016).
- Edwards, J.K. Water Quality & Treatment: A Handbook on Drinking Water, 6th ed.; American Water Works Association: Denver, CO, USA, 2010. [Google Scholar]
- Spuhler, D.; Rengifo-Herrera, J.A.; Cesar Pulgarin, C. The effect of Fe2+, Fe3+, H2O2 and the photo-Fenton reagent at near neutral pH on the solar disinfection (SODIS) at low temperatures of water containing Escherichia coli K12. Appl. Catal. B 2010, 96, 126–141. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Cheng, C.T.; Chau, K.W.; Li., G. Multiple criteria data envelopment analysis for full ranking units associated to environment impact assessment. Inter. J. Environ. Pollut. 2006, 28, 448–464. [Google Scholar] [CrossRef]
- Wu, C.L.; Chau, K.W.; Li, Y.S. Methods to improve neural network performance in daily flows prediction. J. Hydrol. 2009, 372, 80–93. [Google Scholar] [CrossRef]
- Wang, W.C.; Xu, D.M.; Chau, K.W.; Lei, G.J. Assessment of river water quality based on theory of variable fuzzy sets and fuzzy binary comparison method. Water Resour. Manag. 2014, 28, 4183–4200. [Google Scholar] [CrossRef]
- Chowdhury, S.; Champagne, P.; Mclellan, P.J. Models for predicting disinfection byproduct (DBP) formation in drinking water: A chronological review. Sci. Total Environ. 2009, 407, 4189–4206. [Google Scholar] [CrossRef] [PubMed]

| Facility | Location | Population Served (As of 2011) | Yield (MGPD) | Source Water |
|---|---|---|---|---|
| System I | West of Missouri | ~5300 | 0.58 | Ground |
| System II | North of Missouri | ~3900 | 0.22 | Reservoir |
| System III | Center of Missouri | ~8400 | 1.13 | Missouri River |
| Sample ID | UV254 | DOC (mg/L) | SUVA (UV254/DOC) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2nd | 3rd | 4th | 2nd | 3rd | 4th | 2nd | 3rd | 4th | |
| 1-1 | 0.105 | 0.207 | 0.308 | 2.8 | 3.3 | 2.6 | 0.038 | 0.063 | 0.118 |
| 1-2 | 0.076 | 0.082 | 0.074 | 3.4 | 3.5 | 3.9 | 0.022 | 0.023 | 0.019 |
| 1-3 | 0.081 | 0.058 | 0.049 | 2. 7 | 2.9 | 2.8 | 0.030 | 0.020 | 0.018 |
| 1-4 | 0.060 | 0.053 | 0.038 | 2.6 | 3.0 | 3.1 | 0.023 | 0.018 | 0.012 |
| 1-5 | 0.054 | 0.052 | 0.046 | 2.8 | 3.0 | 2.8 | 0.020 | 0.017 | 0.016 |
| 1-6 | 0.051 | 0.054 | 0.045 | 2.7 | 3.0 | 2.8 | 0.019 | 0.018 | 0.016 |
| 1-7 | 0.052 | 0.059 | 0.046 | 3.1 | 3.1 | 3.2 | 0.017 | 0.019 | 0.014 |
| Sample ID | THM Concentrations (µg/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHCl3 | CHBrCl2 | CHBr2Cl | THMFP | |||||||||
| 2nd | 3rd | 4th | 2nd | 3rd | 4th | 2nd | 3rd | 4th | 2nd | 3rd | 4th | |
| 1-1 | 66.7 | 111.7 | 54.0 | 33.4 | 79.3 | 36.5 | 16.2 | 50.5 | 20.6 | 117.5 | 247.0 | 112.4 |
| 1-2 | 77.0 | - | - | 35.2 | - | - | 18.5 | - | - | 132.0 | - | - |
| 1-7 | 74.6 | 161.8 | 54.6 | 22.8 | 83.9 | 36.8 | 17.3 | 58.3 | 28.5 | 122.2 | 314.4 | 127.9 |
| Sample ID | UV254 | DOC (mg/L) | SUVA (UV254:DOC) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | |
| 2-1 | 0.297 | 0.154 | 0.142 | 0.092 | 8.9 | 7.6 | 7.9 | 6.8 | 0.033 | 0.020 | 0.018 | 0.013 |
| 2-2 | 0.055 | 0.055 | 0.052 | 0.040 | 5.0 | 3.7 | 4.5 | 5.0 | 0.011 | 0.015 | 0.012 | 0.008 |
| 2-3 | 0.055 | 0.041 | 0.035 | 0.037 | 3.4 | 4.0 | 4.5 | 5.2 | 0.016 | 0.010 | 0.008 | 0.007 |
| 2-4 | 0.038 | 0.038 | 0.035 | 0.035 | 2.7 | 3.9 | 4.7 | 5.0 | 0.014 | 0.010 | 0.007 | 0.007 |
| 2-5 | 0.037 | 0.038 | 0.034 | 0.036 | 3.2 | 4.0 | 4.3 | 5.0 | 0.012 | 0.010 | 0.008 | 0.007 |
| 2-6 | 0.030 | 0.033 | 0.035 | 0.034 | - | 3.8 | 4.6 | 5.0 | - | 0.009 | 0.008 | 0.007 |
| Sample ID | THM Concentrations (µ/L) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHCl3 | CHBrCl2 | CHBr2Cl | THMFP | |||||||||||||
| 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | |
| 2-1 | 191.3 | 233.9 | 230.7 | 133.1 | 10.4 | 21.1 | 39.2 | 42.8 | 0.1 | 3.9 | 5.6 | 9.3 | 201.9 | 252.0 | 275.5 | 186.2 |
| 2-2 | 103.7 | 89.3 | - | 9.4 | 17.9 | - | 0.8 | 5.2 | - | 113.9 | 105.3 | - | ||||
| 2-3 | 125.8 | - | - | 10.4 | - | - | 0.8 | - | 136.9 | - | - | |||||
| 2-4 | 129.4 | - | - | 9.4 | - | - | 0.8 | - | 139.6 | - | - | |||||
| 2-5 | 129.7 | - | 77.9 | 9.6 | - | 19.7 | 0.8 | 7.9 | 140.1 | - | 105.9 | |||||
| 2-6 | 90.1 | 99.4 | - | 71.6 | 5.4 | 12.6 | - | 24.1 | 0.6 | 5.2 | - | 12.0 | 96.1 | 111.6 | - | 109.0 |
| Sample ID | UV254 | DOC(ppm) | SUVA (UV254:DOC) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2nd | 3rd | 4th | 2nd | 3rd | 4th | 2nd | 3rd | 4th | |
| 3-1 | 0.091 | 0.082 | 0.072 | 4.0 | 4.1 | 4.9 | 0.022 | 0.020 | 0.015 |
| 3-2 | 0.073 | 0.082 | N/A | 3.5 | 4.2 | N/A | 0.021 | 0.020 | N/A |
| 3-3 | 0.007 | 0.066 | 0.060 | 3.6 | 4.2 | 4.1 | 0.002 | 0.016 | 0.015 |
| 3-4 | 0.061 | 0.056 | 0.053 | 3.4 | 3.7 | 3.6 | 0.018 | 0.015 | 0.015 |
| 3-5 | 0.060 | 0.056 | 0.052 | 3.6 | 4.2 | 4.4 | 0.017 | 0.013 | 0.012 |
| 3-6 | 0.045 | 0.045 | 0.042 | 3.6 | 3.6 | 3.5 | 0.012 | 0.012 | 0.012 |
| 3-7 | 0.042 | 0.059 | 0.057 | 3.5 | 3.7 | 3.4 | 0.012 | 0.016 | 0.017 |
| 3-8 | 0.041 | 0.039 | 0.050 | 3.6 | 3.5 | 3.2 | 0.011 | 0.011 | 0.016 |
| Sample ID | THM Concentrations (ppb) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHCl3 | CHBrCl2 | CHBr2Cl | THMFP | |||||||||||||
| 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | |
| 3-1 | 166.8 | 74.49 | 136.9 | 103.3 | 25.1 | 48.47 | 22.4 | 30.5 | 2.7 | 29.30 | 4.7 | 6.9 | 194.6 | 156.9 | 163.6 | 141.3 |
| 3-2 | 118.4 | - | - | - | 22.9 | - | - | - | 4.0 | - | 145.3 | - | - | - | ||
| 3-3 | 134.6 | 73.51 | - | - | 22.4 | 51.12 | - | - | 2.9 | 29.91 | 159.9 | 160.0 | - | - | ||
| 3-4 | 120.5 | - | - | - | 20.7 | - | - | - | 2.9 | - | 144.1 | - | - | - | ||
| 3-5 | 120.1 | 60.49 | - | - | 20.5 | 47.36 | - | - | 2.7 | 33.38 | 143.3 | 148.3 | - | - | ||
| 3-6 | 116.1 | - | - | - | 14.7 | - | - | - | 2.7 | - | 133.5 | - | - | - | ||
| 3-7 | 135.1 | - | - | - | 13.7 | - | - | - | 2.6 | - | 151.4 | - | - | - | ||
| 3-8 | 79.5 | 33.34 | 76.9 | 82.2 | 14.5 | 14.91 | 17.5 | 22.0 | 3.5 | 15.60 | 5.1 | 7.5 | 97.5 | 71.1 | 99.6 | 102.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, B.; Mu, R.; Shi, H.; Inniss, E.; Yang, J. Water Quality in Selected Small Drinking Water Systems of Missouri Rural Communities. Beverages 2016, 2, 10. https://doi.org/10.3390/beverages2020010
Hua B, Mu R, Shi H, Inniss E, Yang J. Water Quality in Selected Small Drinking Water Systems of Missouri Rural Communities. Beverages. 2016; 2(2):10. https://doi.org/10.3390/beverages2020010
Chicago/Turabian StyleHua, Bin, Ruipu Mu, Honglan Shi, Enos Inniss, and John Yang. 2016. "Water Quality in Selected Small Drinking Water Systems of Missouri Rural Communities" Beverages 2, no. 2: 10. https://doi.org/10.3390/beverages2020010
APA StyleHua, B., Mu, R., Shi, H., Inniss, E., & Yang, J. (2016). Water Quality in Selected Small Drinking Water Systems of Missouri Rural Communities. Beverages, 2(2), 10. https://doi.org/10.3390/beverages2020010