The Impact of Wine Style and Sugar Addition in liqueur d’expedition (dosage) Solutions on Traditional Method Sparkling Wine Composition
Abstract
:1. Introduction
2. Materials and Methods
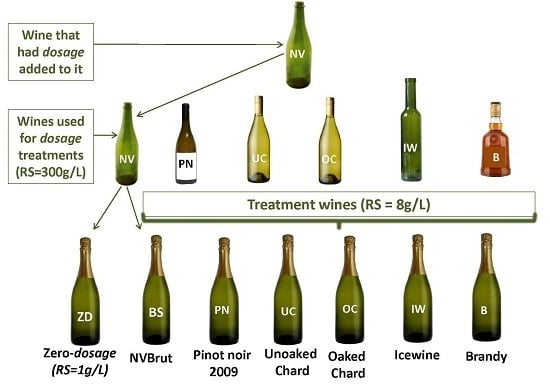
2.1. Sparkling Wine Treatments
2.2. Chemical Analyses
2.3. Reagents, Chemicals and Standards
2.4. Sample Preparation of Wines for Volatile Aroma Compound (VOC) Analyses
2.5. Preparation of Volatile Aroma Compound (VOC) Standards
2.6. Headspace Solid- Phase Micro-Extraction Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS)
2.7. Data Processing of Volatile Aroma Compounds (VOCs)
2.8. Foam Analyses
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analyses
3.1.1. Chemical Parameters of the Wines Used as Dosage Bases before Sugar Addition
3.1.2. Chemical Parameters of Wines at 15 Weeks after Dosage Addition
3.1.3. Dissolved Oxygen
3.2. Volatile Aroma Compounds (VOCs) in the Wines
3.3. Volatile Aroma Compounds (VOCs) in the Dosage Solutions
3.4. Volatile Aroma Compounds (VOCs) in Wines at 5, 10 and 15 Weeks Post-Disgorging
3.5. Five Weeks Post-Disgorging
3.6. Ten Weeks Post-Disgorging
3.7. Post-Disgorging at 15 Weeks
3.8. Odour Thresholds in Sparkling Wines
4. Foam Analyses
5. Sensory Difference Testing
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IS | Internal standard |
| NV | Non-vintage wine produced from a selection of base wines from a range of years |
| VOCs | Volatile aroma compounds |
References
- Kemp, B.; Alexandre, H.; Robillard, B.; Marchal, R. Review: Effect of production phase on bottle-fermented sparkling wine quality. J. Agric. Food Chem. 2015, 63, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Fusté, J.; Sartini, E.; Flores-Rubio, C.; Caixach, J.; Lopez-Tamames, E.; Buxaderas, S. Viability of total phenol index value as a quality marker of sparkling wines, “cavas”. Food Chem. 2009, 114, 782–790. [Google Scholar] [CrossRef]
- Jackson, R. Specific and Distinctive Wine Styles. In Wine Science: Principles and Practices, 4th ed.; Academic Press: San Diego, CA, USA, 2014; p. 714. [Google Scholar]
- Martin, N.; Minard, A.; Brun, O. Sweetness, sourness, and total taste intensity in Champagne wine. Am. J. Enol. Vitic. 2002, 53, 6–13. [Google Scholar]
- Pérez-Magariño, S.; Ortgea-Heras, M.; Bueno-Herrera, M.; Martỉnez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B. Grape variety, aging on lees and aging in bottle after disgorging influences on volatile composition and foamability of sparkling wines. LWT-Food Sci. Technol. 2015, 61, 47–55. [Google Scholar] [CrossRef]
- Buxaderas, S.; Lopez-Tamames, E. Managing the quality of sparkling wines. In Managing Wine Quality, 1st ed.; Reynolds, A., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2010; pp. 553–588. [Google Scholar]
- Iland, P.; Bruer, N.; Wilkes, E. Chemical Analysis of Grapes and Wine: Techniques and Concepts, 2nd ed.; Patrick Iland Wine Promotions: Adelaide, Australia, 2004. [Google Scholar]
- Nurgel, C.; Pickering, G.; Inglis, D. Sensory and chemical characteristics of Canadian Icewines. J. Sci. Food Agric. 2004, 84, 1675–1684. [Google Scholar] [CrossRef]
- Botezatu, A.; Kemp, B.; Pickering, G. Chemical and sensory evaluation of silicone and polylactic acid-based remedial treatments for elevated methoxypyrazine levels in wine. Molecules 2016, 21, 1238. [Google Scholar] [CrossRef] [PubMed]
- Etiévant, P.X. Wine. In Volatile Compounds in Food and Beverages. Food Science and Technology; Maarse, H., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1991; Chapter 14; pp. 483–546. [Google Scholar]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitive determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- San Juan, F.; Ferreira, V.; Cacho, J.; Escudero, A. Quality and aromatic sensory descriptors (mainly fresh and dried fruit character) of Spanish red wines can be predicted from their aroma-active chemical composition. J. Agric. Food Chem. 2011, 59, 7916–7924. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.M.; Bamford, C.W. Measurement and characterization of bubble nucleation in beer. J. Food Sci. 2002, 67, 2696–2701. [Google Scholar] [CrossRef]
- Curioni, A.; Vincenzi, S.; Bona, S. Some results on the evaluation of the foam behaviour for sparkling wines. In Proceedings of the 3rd International Symposium/33rd International CAVA Congress, 3rd Oenoviti International Symposium, Cava Challenges, Subirats, Spain, 20 May 2015.
- Jeonga, Y.N.; Kanga, B.A.; Jeonga, M.J.; Songa, M.J.; Hautusb, M.J.; Leea, H.S. Sensory discrimination by consumers of multiple stimuli from a reference: Stimulus configuration in A-Not AR and constant-ref. duo-trio superior to triangle and unspecified tetrad? Food Qual. Preference 2015, 47, 10–22. [Google Scholar] [CrossRef]
- Kim, M.-A.; Chae, J.-E.; van Hout, D.; Lee, H.-S. Discriminations of the A-Not A difference test improved when “A” was familiarized using a brand image. Food Qual. Preference 2012, 23, 3–12. [Google Scholar] [CrossRef]
- Van Hout, D.; Hautus, M.J.; Lee, H.S. Investigation of test performance over repeated sessions using signal detection theory: Comparison of three non-attribute-specified difference tests 2-AFCR, A-NOT A and 2-AFC. J. Sens. Stud. 2011, 26, 311–321. [Google Scholar] [CrossRef]
- Kemp, S.E.; Hollowood, T.; Hort, J. Sensory test methods. In Sensory Evaluation: A Practical Handbook; Wiley-Blackwell Publishing: Chichester, UK, 2009; pp. 80–83. [Google Scholar]
- Lee, H.-S.; van Hout, D.; Hautus, M.J. Comparison of performance in the A-Not A, 2-AFC, and same-different tests for the flavor discrimination of margarines: The effect of cognitive decision strategies. Food Qual. Preference 2007, 18, 920–928. [Google Scholar] [CrossRef]
- Stefenon, C.A.; de Martini-Bonesi, C.; Mazarotto, V.; Barabé, D.; Agostini, F.; Perin, J.; Serafini, L.L.; Vanerlinde, R. Sugar levels in Charmat sparkling wines can affect quality and resveratrol levels. Redox Rep. Commun. Free Radic. Res. 2010, 15, 1–6. [Google Scholar]
- American Society of Testing and Materials (ASTM). Standard Practice E679. Determination of Odor and Taste Thresholds by a Forced-Choice Ascending Concentration Series Method of Limits; American Society of Testing and Materials: Philadelphia, PA, USA, 1979. [Google Scholar]
- Ganss, S.; Kirsch, F.; Winterhalter, P.; Fischer, U.; Schmarr, H.G. Aroma changes due to second fermentation and glycosylated precursors in Chardonnay and Riesling sparkling wines. J. Agric. Food. Chem. 2011, 59, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- González-Marco, A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Concentration of volatile compounds in Chardonnay wine fermented in stainless steel tanks and oak barrels. Food Chem. 2008, 108, 213–219. [Google Scholar] [CrossRef]
- Tsakiris, A.; Kallithraka, S.; Kourkoutas, Y. Grape brandy production, composition and sensory evaluation. J. Sci. Food. Agric. 2014, 94, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Francioli, S.; Guerra, M.; Lopez-Tammames, E.; Guadayoi, J.M.; Caixach, J. Aroma of Sparkling Wines by Headspace/Solid Phase Microextraction and Gas Chromatography/Mass Spectrometry. Am. J. Enol. Vitic. 1999, 50, 404–408. [Google Scholar]
- Riu-Aumatell, M.; Bosch-Fusté, J.; Lopez-Tamames, E.; Buxaderas, S. Development of volatile compounds of cava (Spanish sparkling wine) during long ageing time in contact with lees. Food Chem. 2006, 95, 237–242. [Google Scholar] [CrossRef]
- Bosch-Fusté, J.; Riu-Aumatella, M.; Guadayol, J.M.; Caixach, J.; López-Tamames, E.; Buxaderas, S. Volatile profiles of sparkling wines obtained by three extraction methods and gas chromatography–mass spectrometry (GC–MS) analysis. Food Chem. 2007, 105, 428–435. [Google Scholar] [CrossRef]
- Coelho, E.; Coimbra, M.A.; Nogueria, J.M.F.; Rocha, S.M. Quantification approach for assessment of sparkling wine volatiles from different soils, ripeness stages, and varieties by stir bar sorptive extraction with liquid desorption. Anal. Chim. Acta 2009, 635, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Cliff, M.A.; Pickering, G.J. Determination of odour detection thresholds for acetic acid and ethyl acetate in ice wine. J. Wine Res. 2006, 17, 45–52. [Google Scholar] [CrossRef]
- Escudero, A.; Gogorza, B.; Melús, M.; Ortín, N.; Cacho, J.; Ferreira, V. Characterization of the aroma of a wine from Maccabeo. Key role played by compounds with low odour activity values. J. Agric. Food Chem. 2004, 52, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, B. Influence of CO2 in Organoleptic Qualities of Champagnes. Experiments and Methodology Developments. Ph.D. Thesis, University of Reims, Reims, France, 2007. [Google Scholar]
- McMahon, K.M. Sensory and Analytical Assessment of Sparkling Wines. Ph.D. Thesis, Washington State University, Washington, DC, USA, 2016. [Google Scholar]
- Liger-Belair, G.; Cilindre, C.; Gougeon, R.D.; Lucio, M.; Gebefügi, I.; Jeandet, P.; Schmitt-Kopplin, P. Unraveling different chemical fingerprints between a champagne wine and its aerosols. Proc. Natl. Acad. Sci. USA 2009, 106, 16545–16549. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Bayόn, M.A.; Santos, M.; Martin-Άlvarez, P.J.; Reineccius, G. Influence of carbonation on aroma release from liquid systems using an artificial throat and a proton transfer reaction-mass spectrophotometric technique (PTR-MS). Flavour Fragr. J. 2009, 24, 226–233. [Google Scholar] [CrossRef]
- Marchal, R.; Tabary, L.; Valade, M.; Moncomble, D.; Viaux, L.; Robillard, B.; Jeandot, P. Effects of Botrytis cinerea infection on Champagne wine foaming. J. Sci. Food Agric. 2001, 81, 1371–1378. [Google Scholar] [CrossRef]
- Herrero, P.; Sáenz-Navajas, P.; Culleré, L.; Ferreira, V.; Chatin, A.; Chaperon, V.; Litoux-Desrues, F.; Escudero, A. Chemosensory characterization of Chardonnay and Pinot Noir base wines of Champagne. Two very different varieties for a common product. Food Chem. 2016, 207, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Cilindre, C.; Liger-Belair, G.; Villaume, S.; Jeandot, P.; Marchal, R. Foaming properties of various Champagne wines depending on several parameters: Grape variety, aging, protein and CO2 content. Anal. Chim. Acta 2010, 660, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Condé, B.; Fuentes, S.; Caron, M.; Xiao, D.; Collmann, R.; Howell, K. Development of a robotic and computer vision method to assess foam quality in sparkling wines. Food Control 2017, 71, 383–392. [Google Scholar] [CrossRef]


| Aroma Compound | Aroma Descriptors | Odor Threshold (µg/L) | Purity (%) | CAS No. | Supplier |
|---|---|---|---|---|---|
| d11 Ethyl hexanoate ISTD | N/A | N/A | 98.7 | 2159-19-5 | CDN Isotopes, Pointe-Claire, QC, Canada |
| Ethyl ester: Linear fatty acid derivatives | |||||
| Ethyl octanoate | Fruity, apricot, pineapple | 580 a | >99 | 106-32-1 | Sigma Aldrich |
| Ethyl hexanoate | Apple, blackberry | 62 d | 99 | 123-66-0 | Sigma Aldrich |
| Ethyl butanoate | Acid fruit, candy, strawberry | 20 ᵇ and 125 d | 99 | 105-54-4 | Sigma Aldrich |
| Ethyl esters: Branched acid derivatives | |||||
| Ethyl isobutyrate | Apple, citrus, tropical fruit | 15 ᶜ | 99 | 97-62-1 | Sigma Aldrich |
| Ethyl isovalerate | Mint, fruit | 3 ᶜ | 98 | 108-64-5 | Sigma Aldrich |
| Ethyl-2-methylbutyrate | Sweet fruit | 18 ᶜ | 99 | 7452-79-1 | Sigma Aldrich |
| Alcohols | |||||
| 2-Phenylethanol | Roses | 14,000 ᶜ | 99 | 60-12-8 | Sigma Aldrich |
| 1-Hexanol | Herbal, green, grass | 8000 ᵇ | 99.5 | 111-27-3 | Sigma Aldrich |
| Volatile Aroma Compound | Retention Time (mins) | Target Ions (m/z) | Confirming Ions (m/z) | Standard Curve (R2) |
|---|---|---|---|---|
| Ethyl hexanoate-d11—IS | 26 | 91 | 50, 110 | - |
| Ethyl esters: Linear fatty acid derivatives | ||||
| Ethyl butanoate | 15.77 | 88 | 101, 60 | 0.9774 |
| Ethyl hexanoate | 26.8 | 88 | 115, 60 | 0.9746 |
| Ethyl octanoate | 41.31 | 88 | 101, 129 | 0.9936 |
| Ethyl esters: Branched acid derivatives | ||||
| Ethyl Isobutyrate | 13.57 | 11,243 | 71, 116 | 0.9954 |
| Ethyl isovalerate | 18.39 | 881.3 | 85, 130 | 0.9934 |
| Ethyl 2-methylbutyrate | 18.82 | 57,229 | 102, 130 | 0.9832 |
| Alcohols | ||||
| 1-Hexanol | 22.87 | 56 | 55, 84 | 0.9678 |
| 2-Phenylethanol | 50.62 | 92 | 88, 122 | 0.9294 |
| Chemical Analysis | NV Sparkling | Oaked Chardonnay | Unoaked Chardonnay | Pinot Noir 2009 | Vidal Icewine | Brandy |
|---|---|---|---|---|---|---|
| pH | 3.10 ± 0.01 e | 3.32 ± 0.01 d | 3.4 ± 0.01 c | 3.1±0.01 e | 3.6 ± 0.01 b | 3.9 ± 0.02 a |
| TA (g/L) | 8.40 ± 0.1 b | 4.50 ± 0.1 d | 6.5 ± 0.1 c | 9.2 ± 0.1 a | 8.2 ± 0.1 b | 0.2 ± 0.20 e |
| Residual sugar (g/L) | 0.40 ± 0.1 b | 26.9 ± 2.6 b | 1.0 ± 0.1 b | 0.4 ± 0.1 b | 223 ± 15.4 a | 4.2 ± 0.10 b |
| Alcohol (% v/v) | 12.30 ± 0.1 d | 12.8 ± 0.1 c | 13.1 ± 0.1 b | 12.3 ± 0.1 d | 10.5 ± 0.1 e | 38.8 ± 0.10 a |
| Free SO2 (ppm) | 8.00 ± 1.0 b | 864 ± 42 a | 41 ± 3 b | 5 ± 1 b | 60 ±1 b | 6 ± 1.00 b |
| Total SO2 (ppm) | 64.00 ± 2.1 d | 959 ± 14 a | 139 ± 6 c | 55 ± 2 d | 477 ± 25 b | 7 ± 1.00 e |
| Total phenolics (A.U.) | 4.40 ± 0.7 d | 12 ± 0.89 a | 5.3 ± 0.09 d | 7.4 ± 0.10 c | 8.4 ± 0.10 c | 10.5 ± 0.39 b |
| Chemical Analysis | BS | ZD | OC | UC | PN | IW | B |
|---|---|---|---|---|---|---|---|
| pH | 3.20 ± 0.02 a | 3.35 ± 0.02 b | 3.09 ± 0.01 c | 3.09 ± 0.01 c | 3.31 ± 0.01 a | 3.08 ± 0.01 c | 3.09 ± 0.01 c |
| TA (g/L) | 8.2 ± 0.1 a | 8.2 ± 0.1 a | 8.0 ± 0.1 a | 7.9 ± 0.1 a | 8.0 ± 0.1 a | 8.2 ± 0.2 a | 7.9 ± 0.1 a |
| Residual sugar (g/L) | 7.6 ± 0.1 a | 1.1 ± 0.1 d | 7.5 ± 0.2 a,b | 7.7 ± 0.3 a | 7.1 ± 0.1 a,b | 6.3 ± 0.1 c | 7.0 ± 0.3 b |
| Alcohol (% v/v) | 12.4 ± 0.1 b | 12.3 ± 0.1 b | 12.4 ± 0.1b | 12.3 ± 0.1 b | 12.3 ± 0.1 b | 12.3 ± 0.1 b | 12.9 ± 0.1 a |
| Free SO2 (ppm) | 5 ± 1 a,b | 4 ± 1 b | 5 ± 1 a,b | 3 ± 1 a | 4 ± 1 b | 4 ± 1 b | 4 ± 1 b |
| Total SO2 (ppm) | 54 ± 12 a,b | 49 ± 6 a,b | 59 ± 7 a | 52 ± 1 a,b | 48 ± 2 b | 46 ± 1 b | 53 ± 2 a,b |
| Total phenolics (A.U.) | 1.4 ± 0.2 a,b | 1.5 ± 0.2 b | 1.8 ± 0.1 a,b | 1.3 ± 0.1 b | 1.8 ± 0.3 a,b | 1.6 ± 0.1 a,b | 2.1 ± 0.1 a |
| Compound (μg/L) | ZD | BS | OC | UC | PN | IW | B |
|---|---|---|---|---|---|---|---|
| 5 weeks | |||||||
| Ethyl esters: Linear fatty acid derivatives | |||||||
| Ethyl hexanoate | 164 ± 7 F | 1177 ± 20 B | 522 ± 27 D | 905 ± 26 C | 132 ± 2 F | 352 ± 17 E | 1687 ± 89 A |
| Ethyl octanoate | 651 ± 3 B | 339 ± 10 C | 381 ± 10 B,C | 460 ± 42 B,C | 349 ± 5 B,C | 666 ± 125 B,C | 23,429 ± 157 A |
| Ethyl butanoate | 41 ± 3 A | 53 ± 4 A | 57 ± 7 A | 52 ± 3 A | 39 ± 1 A | 30 ± 2 A | 69 ± 15 A |
| Ethyl esters: Branched acid derivatives | |||||||
| Ethyl isovalerate | 33 ± 12 D | 117 ± 1 B | 76 ± 1 C | 13 ± 6 D | 154 ± 2 A | 61 ± 1 C | 59 ± 2 C |
| Ethyl isobutyrate | 69 ± 11 A,B | 56 ± 3 A,B | 52 ± 10 B | 41 ± 9 B | 86 ± 1 A | 38 ± 8 B | 62 ± 7 A,B |
| Ethyl-2-methylbutyrate | 12 ± 1 C | ND | 7 ± 1 D | 12 ± 1 B,C | 25 ± 2 A | 0.4 ± 1 E | 18 ± 2 B |
| Alcohols | |||||||
| 2-Phenylethanol | 24,527 ± 453 B | 12,901 ± 1281 C | 51,502 ± 6662 A | 13,448 ± 165 C | 14,263 ± 531 C | 14,124 ± 895 B,C | 5826 ± 23 C |
| 1-Hexanol | 736 ± 57 B | 556 ± 54 B,C | 433 ± 36 BC | 577 ± 50 B,C | 423 ± 6 B,C | 209 ± 10 C | 1458 ± 253 A |
| 10 weeks | |||||||
| Ethyl esters: Linear fatty acid derivatives | |||||||
| Ethyl hexanoate | 164 ± 6 D | 164 ± 7 D | 503 ± 28 B | 457 ± 6 B,C | 131 ± 6 D | 339 ± 17 C | 1750 ± 89 A |
| Ethyl octanoate | 651 ± 3 B | 650 ± 2 B | 374 ± 11 B | 619 ± 4 B | 353 ± 5 B | 577 ± 125 B | 23,540 ± 157 A |
| Ethyl butanoate | 41 ± A,B | 41 ± 3 A,B | 61 ± 6 A | 42 ± 3 A,B | 40 ± 2 A,B | 32 ± 2 B | 58 ± 15 A |
| Ethyl esters: Branched acid derivatives | |||||||
| Ethyl isovalerate | 117 ± 2 AB | 118 ± 1 A,B | 77 ± 1 A,B | 56 ± 0 B | 156 ± 2 A | 62 ± 1 A,B | 101 ± 63 A,B |
| Ethyl isobutyrate | 68 ± 11 AB | 68 ± 11 A,B | 45 ± 10 B | ND | 86 ± 1 A | 31 ± 11 C | 56 ± 7 A,B |
| Ethyl-2-methylbutyrate | 11 ± 0 C | 12 ± 1 C | 7 ± 1 D | ND | 27 ± 2 A | ND | 17 ± 2 B |
| Alcohols | |||||||
| 2-Phenylethanol | 23,321 ± 1251 C | 23,527 ± 354 C | 56,213 ± 6662 A | 37,182 ± 2958 B | 13,887 ± 532 C,D | 14,757 ± 895 C,D | 5842 ± 23 D |
| 1-Hexanol | 647 ± 68 B | 648 ± 67 B | 468 ± 50 B | 213 ± 2 B | 131 ± 2 B | 289 ± 109 B | 1278 ± 253 A |
| 15 weeks | |||||||
| Ethyl esters: Linear fatty acid derivatives | |||||||
| Ethyl hexanoate | 165 ± 6.5 D | 164 ± 7,D | 498 ± 27 B | 456 ± 5.5 B,C | 131 ± 1.6 D | 339 ± 17 C | 1750 ± 89 A |
| Ethyl octanoate | 648 ± 1 A | 651 ± 3 A | 374 ± 10 A | 619 ± 4 A | 353 ± 5 A | 577 ± 125 A | 23,441 ± 157 B |
| Ethyl butanoate | 41 ± 3 A | 41 ± 3 A | 51 ± 7 A | 51 ± 11 A | 39 ± 1 A | 32 ± 2 A | 58 ± 15 A |
| Ethyl esters: Branched acid derivatives | |||||||
| Ethyl isovalerate | 116 ± 2 B | 117 ± 1 B | 77 ± 1 C | 56 ± 11 B | 153 ± 2 A | 61 ± 1 D | 56 ± 0 B |
| Ethyl isobutyrate | 68 ± 12 A,B | 69 ± 11 A,B | 47 ± 10 B | ND | 87 ± 1 A | 44 ± 8 B | 56 ± 7 A,B |
| Ethyl-2-methylbutyrate | 12 ± 0 C | 12 ± 2 C | 7 ± 0 D | ND | 27 ± 1 A | ND | 17 ± 2 B |
| Alcohols | |||||||
| 2-Phenylethanol | 24,558 ± 453 B,C | 24,527 ± 3314 B,C | 56,213 ± 662 A | 32,747 ± 6662 B | 13,888 ± 895 C,D | 14,758 ± 531 C,D | 5859 ± 23 D |
| 1-Hexanol | 647 ± 68 B | 648 ± 68 B,C | 408 ± 35 B,C | 212 ± 1.2 C | 427 ± 5.7 B,C | 286 ± 109 B,C | 1278 ± 253 A |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemp, B.; Hogan, C.; Xu, S.; Dowling, L.; Inglis, D. The Impact of Wine Style and Sugar Addition in liqueur d’expedition (dosage) Solutions on Traditional Method Sparkling Wine Composition. Beverages 2017, 3, 7. https://doi.org/10.3390/beverages3010007
Kemp B, Hogan C, Xu S, Dowling L, Inglis D. The Impact of Wine Style and Sugar Addition in liqueur d’expedition (dosage) Solutions on Traditional Method Sparkling Wine Composition. Beverages. 2017; 3(1):7. https://doi.org/10.3390/beverages3010007
Chicago/Turabian StyleKemp, Belinda, Casey Hogan, Shufen Xu, Lisa Dowling, and Debbie Inglis. 2017. "The Impact of Wine Style and Sugar Addition in liqueur d’expedition (dosage) Solutions on Traditional Method Sparkling Wine Composition" Beverages 3, no. 1: 7. https://doi.org/10.3390/beverages3010007
APA StyleKemp, B., Hogan, C., Xu, S., Dowling, L., & Inglis, D. (2017). The Impact of Wine Style and Sugar Addition in liqueur d’expedition (dosage) Solutions on Traditional Method Sparkling Wine Composition. Beverages, 3(1), 7. https://doi.org/10.3390/beverages3010007