Occurrence of Ochratoxin A in Coffee: Threads and Solutions—A Mini-Review
Abstract
:1. Coffee as a Unique Beverage
2. Ochratoxin A as a Coffee Contaminant
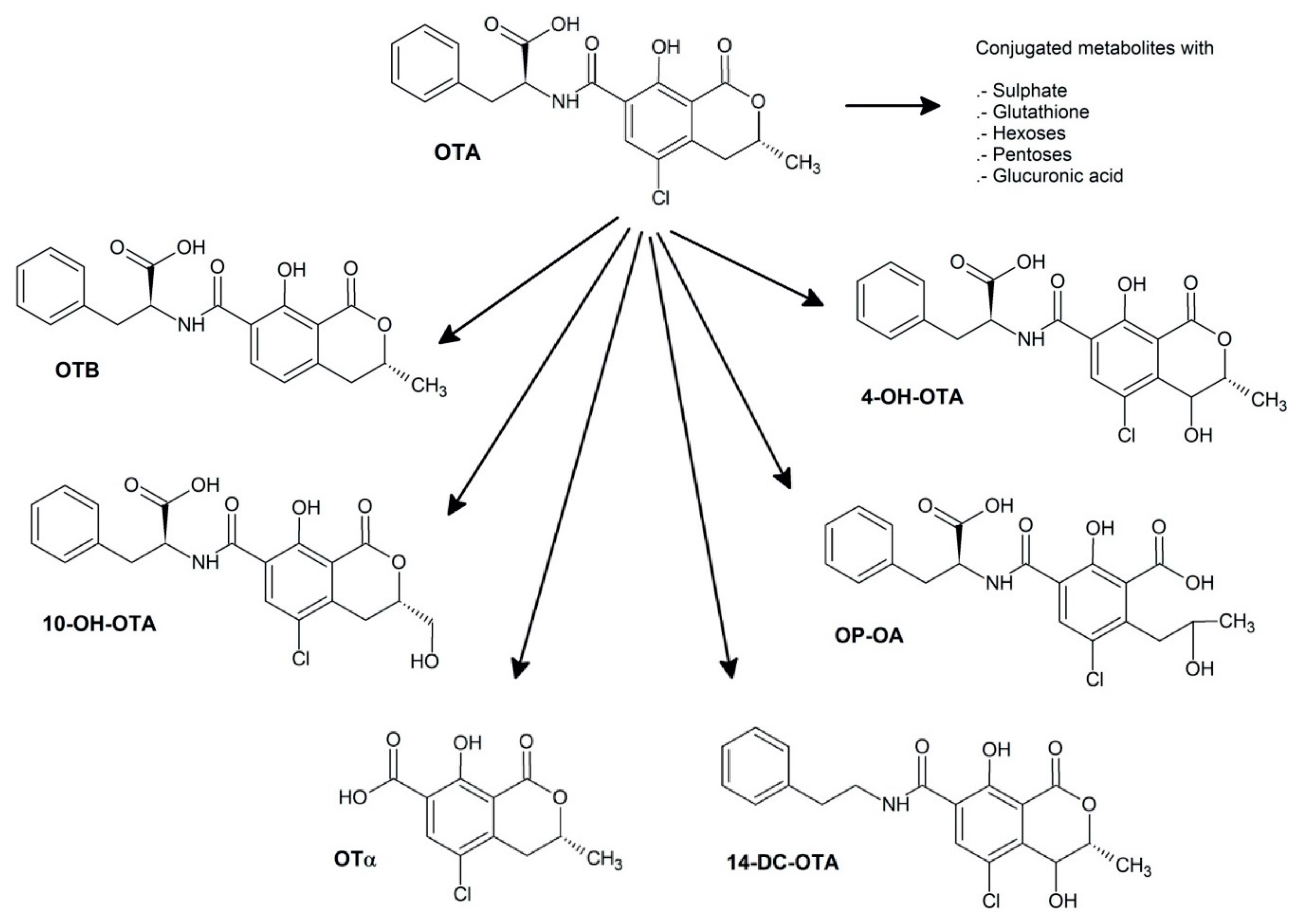
2.1. Ochratoxin A
2.2. Fungal Contamination and Coffee
3. Can Ochratoxin A Reduce Coffee Consumption?
4. Strategies to Reduce Ochratoxin A
4.1. Coffee Roasted
4.2. Coffee Composition: Caffeine
4.3. Physical Methods
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Organization, I.C. Annual Review 2016/2017; International Coffee Organization: London, UK, 2018; pp. 1–50. [Google Scholar]
- Dorea, J.G.; da Costa, T.H. Is coffee a functional food? Br. J. Nutr. 2005, 93, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galarce-Bustos, O.; Alvarado, M.; Vega, M.; Aranda, M. Occurence of ochratoxin A in roasted and instant coffees in Chilean. Food Control 2014, 46, 102–107. [Google Scholar] [CrossRef]
- Nishitsuji, K.; Watanabe, S.; Xiao, J.; Nagatomo, R.; Ogawa, H.; Tsunematsu, T.; Umemoto, H.; Morimoto, Y.; Akatsu, H.; Inoue, K.; et al. Effect of coffee or coffee components on gut microbiome and short-chain fatty acids in a mouse model of metabolic syndrome. Sci. Rep. 2018, 8, 16173. [Google Scholar] [CrossRef]
- Samoggia, A.; Riedel, B. Coffee consumption and purchasing behavior review: Insights for further research. Appetite 2018, 129, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Jeszka-Skowron, M.; Sentkowska, A.; Pryrzynska, K.; Peña, M.P. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
- Napolitano, A.; Fogliano, V.; Tafuri, A.; Ritieni, A. Natural occurrence of ochratoxin A and antioxidant activities of green and roasted coffees and corresponding byproducts. J. Agric. Food Chem. 2007, 55, 10499–10504. [Google Scholar] [CrossRef]
- Alves, R.C.; Casal, S.; Alves, M.R.; Oliveira, M.B. Discrimination between arabica and robusta coffee species on the basis of their tocopherol profiles. Food Chem. 2009, 114, 295–299. [Google Scholar] [CrossRef]
- Priftis, A.; Mitsiou, D.; Halabalaki, M.; Ntasi, G.; Stagos, D.; Skaltsounis, L.A.; Kouretas, D. Roasting has a distinct effect on the antimutagenic activity of coffee varieties. Mutat. Res. 2018, 829–830, 33–42. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T. Coffee and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2011, 51, 363–373. [Google Scholar] [CrossRef]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Adawi, M.; Amital, H.; Shoenfeld, Y. Coffee and autoimmunity: More than a mere hot beverage! Autoimmun. Rev. 2017, 16, 712–721. [Google Scholar] [CrossRef]
- Machado-Fragua, M.D.; Struijk, E.A.; Graciani, A.; Guallar-Castillon, P.; Rodriguez-Artalejo, F.; Lopez-Garcia, E. Coffee consumption and risk of physical function impairment, frailty and disability in older adults. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Pohland, A.E.; Schuller, P.L.; Steyn, P.S.; Van Egmond, H.P. Physico-chemical data for selected mycotoxins. Pure Appl. Chem. 1982, 54, 2219–2284. [Google Scholar] [CrossRef]
- Natori, S.; Sakaki, S.; Kurata, H.; Udagawa, S.; Ichinoe, M. Chemical and cytotoxicity survey on the production of ochratoxins and penicillic acid by Aspergillus ochraceus Wilhelm. Chem. Pharm Bull. (Tokyo) 1970, 18, 2259–2268. [Google Scholar] [CrossRef]
- van der Merwe, K.J.; Steyn, P.S.; Fourie, L.; Scott, D.B.; Theron, J.J. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature 1965, 205, 1112–1113. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Bayman, P.; Baker, J.L. Ochratoxins: A global perspective. Mycopathologia 2006, 162, 215–223. [Google Scholar] [CrossRef]
- el Khoury, A.; Atoui, A. Ochratoxin a: General overview and actual molecular status. Toxins (Basel) 2010, 2, 461–493. [Google Scholar] [CrossRef]
- Heussner, A.H.; Bingle, L.E. Comparative Ochratoxin Toxicity: A Review of the Available Data. Toxins (Basel) 2015, 7, 4253–4282. [Google Scholar] [CrossRef] [Green Version]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins (Basel) 2016, 8, 191. [Google Scholar] [CrossRef]
- Bittner, A.; Cramer, B.; Harrer, H.; Humpf, H.-U. Structure elucidation and in vitro cytotoxicity of ochratoxin α amide, a new degradation product of ochratoxin A. Mycotoxin Res. 2015, 31, 83–90. [Google Scholar] [CrossRef]
- Bittner, A.; Cramer, B.; Humpf, H.U. Matrix binding of ochratoxin A during roasting. J. Agric. Food Chem. 2013, 61, 12737–12743. [Google Scholar] [CrossRef]
- Cramer, B.; Konigs, M.; Humpf, H.U. Identification and in vitro cytotoxicity of ochratoxin A degradation products formed during coffee roasting. J. Agric. Food Chem. 2008, 56, 5673–5681. [Google Scholar] [CrossRef]
- Koszegi, T.; Poor, M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins (Basel) 2016, 8, 111. [Google Scholar] [CrossRef]
- Levi, C.P.; Trenk, H.L.; Mohr, H.K. Study of the occurrence of ochratoxin A in green coffee beans. J. Assoc. Off. Anal. Chem. 1974, 57, 866–870. [Google Scholar]
- Mata, A.T.; Ferreira, J.P.; Oliveira, B.R.; Batoreu, M.C.; Barreto Crespo, M.T.; Pereira, V.J.; Bronze, M.R. Bottled water: Analysis of mycotoxins by LC-MS/MS. Food Chem. 2015, 176, 455–464. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Piemontese, L.; Gambacorta, L.; Zivoli, R.; Longobardi, F. Food coloring agents and plant food supplements derived from Vitis vinifera: A new source of human exposure to ochratoxin A. J. Agric. Food Chem. 2015, 63, 3609–3614. [Google Scholar] [CrossRef]
- Hult, K.; Plestina, R.; Habazin-Novak, V.; Radic, B.; Ceovic, S. Ochratoxin A in Human Blood and Balkan Endemic Nephropathy. Arch. Toxicol 1982, 51, 313–321. [Google Scholar] [CrossRef]
- WHO. Ochratoxin A.; WHO: Geneva, Switzerland, 2008; pp. 356–429. [Google Scholar]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Novotna, E. Ochratoxin A: Developmental and reproductive toxicity-an overview. Birth Defects Res. B Dev. Reprod. Toxicol. 2013, 98, 493–502. [Google Scholar] [CrossRef]
- Coronel, M.B.; Sanchis, V.; Ramos, A.J.; Marin, S. Review. Ochratoxin A: Presence in human plasma and intake estimation. Food Sci. Technol. Int. 2010, 16, 5–18. [Google Scholar] [CrossRef]
- IARC. Some Naturally Occuring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; WHO/IARC, Ed.; IARC Press: Lyon, France, 1993; Volume 56, pp. 489–521. [Google Scholar]
- Gekle, M.; Sauvant, C.; Schwerdt, G. Ochratoxin A at nanomolar concentrations: A signal modulator in renal cells. Mol. Nutr. Food Res. 2005, 49, 118–130. [Google Scholar] [CrossRef]
- Schwerdt, G.; Holzinger, H.; Sauvant, C.; Konigs, M.; Humpf, H.U.; Gekle, M. Long-term effects of ochratoxin A on fibrosis and cell death in human proximal tubule or fibroblast cells in primary culture. Toxicology 2007, 232, 57–67. [Google Scholar] [CrossRef]
- Stefanovic, V.; Toncheva, D.; Polenakovic, M. Balkan nephropathy. Clin. Nephrol. 2015, 83, 64–69. [Google Scholar] [CrossRef]
- Maaroufi, K.; Pfohl-Leszkowicz, A.; Achour, A.; el May, M.; Grosse, Y.; Hammami, M.; Ellouz, F.; Creppy, E.E.; Bacha, H. Ochratoxin A genotoxicity, relation to renal tumors. Arch. Inst. Pasteur Tunis 1994, 71, 21–31. [Google Scholar]
- Cui, J.; Liu, J.; Wu, S.; Wang, Y.; Shen, H.; Xing, L.; Wang, J.; Yan, X.; Zhang, X. Oxidative DNA damage is involved in ochratoxin A-induced G2 arrest through ataxia telangiectasia-mutated (ATM) pathways in human gastric epithelium GES-1 cells in vitro. Arch. Toxicol. 2013, 87, 1829–1840. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Cui, J.; Xing, L.; Shen, H.; Wu, S.; Lian, H.; Wang, J.; Yan, X.; Zhang, X. Ochratoxin A induces oxidative DNA damage and G1 phase arrest in human peripheral blood mononuclear cells in vitro. Toxicol. Lett. 2012, 211, 164–171. [Google Scholar] [CrossRef]
- Woo, C.S.; El-Nezami, H. Maternal-Fetal Cancer Risk Assessment of Ochratoxin A during Pregnancy. Toxins (Basel) 2016, 8, 87. [Google Scholar] [CrossRef]
- Arlt, V.M.; Pfohl-Leszkowicz, A.; Cosyns, J.; Schmeiser, H.H. Analyses of DNA adducts formed by ochratoxin A and aristolochic acid in patients with Chinese herbs nephropathy. Mutat. Res. 2001, 494, 143–150. [Google Scholar] [CrossRef]
- Arbillaga, L.; Azqueta, A.; Ezpeleta, O.; Lopez de Cerain, A. Oxidative DNA damage induced by Ochratoxin A in the HK-2 human kidney cell line: Evidence of the relationship with cytotoxicity. Mutagenesis 2007, 22, 35–42. [Google Scholar] [CrossRef]
- Guerra, M.C.; Galvano, F.; Bonsi, L.; Speroni, E.; Costa, S.; Renzulli, C.; Cervellati, R. Cyanidin-3-O-beta-glucopyranoside, a natural free-radical scavenger against aflatoxin B1- and ochratoxin A-induced cell damage in a human hepatoma cell line (Hep G2) and a human colonic adenocarcinoma cell line (CaCo-2). Br. J. Nutr. 2005, 94, 211–220. [Google Scholar] [CrossRef]
- Schaaf, G.J.; Nijmeijer, S.M.; Maas, R.F.; Roestenberg, P.; de Groene, E.M.; Fink-Gremmels, J. The role of oxidative stress in the ochratoxin A-mediated toxicity in proximal tubular cells. Biochim. Biophys. Acta 2002, 1588, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Yu, T.; Qi, X.; Yang, B.; Shi, L.; Luo, H.; He, X.; Huang, K.; Xu, W. miR-122 plays an important role in ochratoxin A-induced hepatocyte apoptosis in vitro and in vivo. Toxicol. Res. 2016, 5, 160–167. [Google Scholar] [CrossRef]
- Hennemeier, I.; Humpf, H.U.; Gekle, M.; Schwerdt, G. Role of microRNA-29b in the ochratoxin A-induced enhanced collagen formation in human kidney cells. Toxicology 2014, 324, 116–122. [Google Scholar] [CrossRef]
- Mally, A. Ochratoxin a and mitotic disruption: Mode of action analysis of renal tumor formation by ochratoxin A. Toxicol. Sci. 2012, 127, 315–330. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. An update on direct genotoxicity as a molecular mechanism of ochratoxin a carcinogenicity. Chem. Res. Toxicol. 2012, 25, 252–262. [Google Scholar] [CrossRef]
- Castellanos-Onorio, O.; Gonzalez-Rios, O.; Guyot, B.; Fontana, T.A.; Guiraud, J.P.; Schorr-Galindo, S.; Durand, N.; Suárez-Quiroz, M. Effect of two different roasting techniques on the Ochratoxin A (OTA) reduction in coffee beans (Coffea arabica). Food Control 2011, 22, 1184–1188. [Google Scholar] [CrossRef]
- Alvindia, D.G.; de Guzman, M.F. Survey of Philippine coffee beans for the presence of ochratoxigenic fungi. Mycotoxin Res. 2016, 32, 61–67. [Google Scholar] [CrossRef]
- Vega, F.E.; Posada, F.; Peterson, S.W.; Gianfagna, T.J.; Chaves, F. Penicillium species endophytic in coffee plants and ochratoxin A production. Mycologia 2006, 98, 31–42. [Google Scholar] [CrossRef]
- Casas-Junco, P.P.; Ragazzo-Sanchez, J.A.; Ascencio-Valle, F.J.; Calderon-Santoyo, M. Determination of potentially mycotoxigenic fungi in coffee (Coffea arabica L.) from Nayarit. Food Sci. Biotechnol. 2018, 27, 891–898. [Google Scholar] [CrossRef]
- EU. Assessment of Dietary Intake of Ochratoxin A by the Population of EU Member States; Reports on Tasks for Scientific Cooperation; Directorate-General Health and Consumer, Ed.; Scientific Cooperation: Brussels, Belgium, 2002; p. 153. [Google Scholar]
- Batista, L.R.; Chalfoun, S.M.; Prado, G.; Schwan, R.F.; Wheals, A.E. Toxigenic fungi associated with processed (green) coffee beans (Coffea arabica L.). Int. J. Food Microbiol. 2003, 85, 293–300. [Google Scholar] [CrossRef]
- Bucheli, P.; Taniwaki, M.H. Research on the origin, and on the impact of post-harvest handling and manufacturing on the presence of ochratoxin A in coffee. Food Addit. Contam. 2002, 19, 655–665. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. How will climate change affect mycotoxins in food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef] [Green Version]
- Taniwaki, M.H.; Teixeira, A.A.; Teixeira, A.R.R.; Copetti, M.V.; Iamanaka, B.T. Ochratoxigenic fungi and ochratoxin A in defective coffee beans. Food Res. Int. 2014, 61, 161–166. [Google Scholar] [CrossRef]
- Trivedi, A.B.; Doi, E.; Kitabatake, N. Detoxification of Ochratoxin A on Heating under Acidic and Alkaline Conditions. Biosci. Biotechnol. Biochem. 1992, 56, 741–745. [Google Scholar] [CrossRef] [Green Version]
- Commission, E. Commission regulation (EC) N° 1881/2006 of 19 December 2006, setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L 364, 5–24. [Google Scholar]
- EFSA. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to ochratoxin A in food. EFSA J. 2006, 365, 1–56. [Google Scholar]
- Otteneder, H.; Majerus, P. Ochratoxin A (OTA) in coffee: Nation-wide evaluation of data collected by German Food Control 1995-1999. Food Addit. Contam. 2001, 18, 431–435. [Google Scholar] [CrossRef]
- Bandeira, R.D.C.C.; Uekane, T.M.; Cunha, C.P.; Geaquinto, L.R.O.; Cunha, V.S.; Caixeiro, J.M.R.; Godoy, R.L.O.; Cruz, M.H.C. Development and validation of a method for the analysis of ochratoxin A in roasted coffee by liquid chromatography/electrospray-mass spectrometry in tandem (LC/ESI-MS/MS). Quim. Nova 2012, 35, 66–71. [Google Scholar] [CrossRef]
- Benites, A.J.; Fernandes, M.; Boleto, A.R.; Azevedo, S.; Silva, S.; Leitão, A.L. Occurrence of ochratoxin A in roasted coffee samples commercialized in Portugal. Food Control 2017, 73, 1223–1228. [Google Scholar] [CrossRef]
- Drunday, V.; Pacin, A. Occurrence of Ochratoxin A in coffee beans, ground roasted coffee and soluble coffee and method validation. Food Control 2013, 30, 675–678. [Google Scholar]
- Leoni, L.A.; Soares, L.M.; Oliveira, P.L. Ochratoxin A in Brazilian roasted and instant coffees. Food Addit. Contam. 2000, 17, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Tozlovanu, M.; Pfohl-Leszkowicz, A. Ochratoxin A in roasted coffee from French supermarkets and transfer in coffee beverages: Comparison of analysis methods. Toxins (Basel) 2010, 2, 1928–1942. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Toman, J.; Bazin, I.; Roubal, T. Transfer of ochratoxin A into tea and coffee beverages. Toxins (Basel) 2014, 6, 3438–3453. [Google Scholar] [CrossRef]
- Micco, C.; Grossi, M.; Miraglia, M.; Brera, C. A study of the contamination by ochratoxin A of green and roasted coffee beans. Food Addit. Contam. 1989, 6, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Perez De Obanos, A.; Gonzalez-Penas, E.; Lopez De Cerain, A. Influence of roasting and brew preparation on the ochratoxin A content in coffee infusion. Food Addit. Contam. 2005, 22, 463–471. [Google Scholar] [CrossRef]
- Studer-Rohr, I.; Dietrich, D.R.; Schlatter, J.; Schlatter, C. The occurrence of ochratoxin A in coffee. Food Chem. Toxicol. 1995, 33, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Tsubouchi, H.; Yamamoto, K.; Hisada, K.; Sakabe, Y.; Udagawa, S. Effect of roasting on ochratoxin A level in green coffee beans inoculated with Aspergillus ochraceus. Mycopathologia 1987, 97, 111–115. [Google Scholar] [CrossRef]
- Lombaert, G.A.; Pellaers, P.; Chettiar, M.; Lavalee, D.; Scott, P.M.; Lau, B.P. Survey of Canadian retail coffees for ochratoxin A. Food Addit. Contam. 2002, 19, 869–877. [Google Scholar] [CrossRef]
- Fazekas, B.; Tar, A.K.; Zomborszky-Kovacs, M. Ochratoxin a contamination of cereal grains and coffee in Hungary in the year 2001. Acta Vet. Hung. 2002, 50, 177–188. [Google Scholar] [CrossRef]
- Prelle, A.; Spadaro, D.; Denca, A.; Garibaldi, A.; Gullino, M.L. Comparison of clean-up methods for ochratoxin A on wine, beer, roasted coffee and chili commercialized in Italy. Toxins (Basel) 2013, 5, 1827–1844. [Google Scholar] [CrossRef]
- Coronel, M.B.; Marin, S.; Cano, G.; Ramos, A.J.; Sanchis, V. Ochratoxin A in Spanish retail ground-roasted coffee: Occurence and assessment of the exposure in Catalonia. Food Control 2011, 23, 414–419. [Google Scholar] [CrossRef]
- Patel, S.; Hazel, C.M.; Winterton, A.G.; Gleadle, A.E. Survey of ochratoxin A in UK retail coffees. Food Addit. Contam. 1997, 14, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Uekane, T.M.; Bandeira, R.D.C.C.; Silva, M.C. Comparação de métodos de análise para ocratoxina A no café: Uma revisão. Prespectivas da Ciência e Tecnologia 2010, 2, 44–54. [Google Scholar]
- Cramer, B.; Osteresch, B.; Munoz, K.A.; Hillmann, H.; Sibrowski, W.; Humpf, H.U. Biomonitoring using dried blood spots: Detection of ochratoxin A and its degradation product 2’R-ochratoxin A in blood from coffee drinkers. Mol. Nutr. Food Res. 2015, 59, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Blanc, M.; Pittet, A.; Munoz-Box, R.; Viani, R. Behavior of Ochratoxin A during Green Coffee Roasting and Soluble Coffee Manufacture. J. Agric. Food Chem. 1998, 46, 673–675. [Google Scholar] [CrossRef]
- Romani, S.; Pinnavaia, G.G.; Dalla Rosa, M. Influence of roasting levels on ochratoxin a content in coffee. J. Agric. Food Chem. 2003, 51, 5168–5171. [Google Scholar] [CrossRef] [PubMed]
- Tsubouchi, H.; Terada, H.; Yamamoto, K.; Sakabe, Y. Ochratoxin A found in commercial roast coffee. J. Agric. Food Chem. 1988, 36, 540–542. [Google Scholar] [CrossRef]
- van der Stegen, G.H.; Essens, P.J.; van der Lijn, J. Effect of roasting conditions on reduction of ochratoxin a in coffee. J. Agric. Food Chem. 2001, 49, 4713–4715. [Google Scholar] [CrossRef]
- Nehad, E.A.; Farag, M.M.; Kawther, M.S.; Abdel-Samed, A.K.; Naguib, K. Stability of ochratoxin A (OTA) during processing and decaffeination in commercial roasted coffee beans. Food Addit. Contam. 2005, 22, 761–767. [Google Scholar] [CrossRef]
- Kumar, S.; Kunwar, A.; Gautam, S.; Sharma, A. Inactivation of A. ochraceus spores and detoxification of ochratoxin A in coffee beans by gamma irradiation. J. Food Sci. 2012, 77, T44–T51. [Google Scholar] [CrossRef]
- Chen, B.Y.; Huang, H.W.; Cheng, M.C.; Wang, C.Y. Influence of high-pressure processing on the generation of gamma-aminobutyric acid and microbiological safety in coffee beans. J. Sci. Food Agric. 2018, 98, 5625–5631. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Pan, A.; Malik, V.S.; Manson, J.E.; Willett, W.C.; van Dam, R.M.; Hu, F.B. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 155–166. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Y.; Tang, L. Caffeine induces sustained apoptosis of human gastric cancer cells by activating the caspase9/caspase3 signalling pathway. Mol. Med. Rep. 2017, 16, 2445–2454. [Google Scholar] [CrossRef]
- Prediger, R.D. Effects of caffeine in Parkinson’s disease: From neuroprotection to the management of motor and non-motor symptoms. J. Alzheimers Dis. 2010, 20 (Suppl. 1), S205–S220. [Google Scholar] [CrossRef]
- Tiwari, K.K.; Chu, C.; Couroucli, X.; Moorthy, B.; Lingappan, K. Differential concentration-specific effects of caffeine on cell viability, oxidative stress, and cell cycle in pulmonary oxygen toxicity in vitro. Biochem. Biophys. Res. Commun. 2014, 450, 1345–1350. [Google Scholar] [CrossRef] [Green Version]
- Akbar, A.; Medina, A.; Magan, N. Efficacy of different caffeine concentrations on growth and ochratoxin A production by Aspergillus species. Lett. Appl. Microbiol. 2016, 63, 25–29. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Tice, G.; Marino, D. Caffeine inhibition of ochratoxin A production. J. Food Sci. 1982, 47, 319–321. [Google Scholar] [CrossRef]
- Tsubouchi, H.; Terada, H.; Yamamoto, K.; Hisada, K.; Sakabe, Y. Caffeine degradation and increased ochratoxin A production by toxigenic strains of Aspergillus ochraceus isolated from green coffee beans. Mycopathologia 1985, 90, 181–186. [Google Scholar] [CrossRef]
- Cheng, B.; Furtado, A.; Henry, R.J. The coffee bean transcriptome explains the accumulation of the major bean components through ripening. Sci. Rep. 2018, 8, 11414. [Google Scholar] [CrossRef]
- Di Stefano, V.; Pitonzo, R.; Cicero, N.; D’Oca, M.C. Mycotoxin contamination of animal feedingstuff: Detoxification by gamma-irradiation and reduction of aflatoxins and ochratoxin A concentrations. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 2034–2039. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef]
- Nascimento, L.C.; Lima, L.C.O.; Picolli, R.H.; Fiorini, J.E.; Duarte, S.M.S.; Silva, J.M.S.F.; Oliveira, N.M.S.; Veiga, S.M.O.M. Ozone and ultrasound: Alternative processes in the treatment of fermented coffee. Food Sci. Technol. 2008, 28, 282–294. [Google Scholar] [CrossRef]

| Reference | ||
|---|---|---|
| Name | N-[(5-chloro-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl)carbonyl]-l-phenylalanine | --- |
| Chemical structure |  | |
| Formula | C20H18ClNO6 | [13] |
| Molecular weight | 403.8 g·mol−1 | [13] |
| Melting point | 168–173 °C (drying for 1 hour at 60 °C) 159 °C (recrystallized from benzene-hexane) 169 °C (recrystallized from xylene) | [14] [15] [16] |
| [α]21D | −46.8° (c = 2.65 mmol/L in chloroform) | [14] |
| [α]20D | −118° (c = 1.1 mmol/L in chloroform) | [16] |
| λmax | 214 nm (ε = 37.2 × 10−3 L·mol−1·cm−1) 282 nm (ε = 0.89 × 10−3 L·mol−1·cm−1) 332 nm (ε = 6.33 × 10−3 L·mo−1·cm−1) (c = 0.0281 mmol/L in methanol) | [14] |
| pKa | 4.2–4.4 and 7.0–7.3 | [17] |
| Country | n | % Positive | Average (µg/kg) | Range of OTA (µg/kg) | Reference |
|---|---|---|---|---|---|
| Argentina | 24 | 54 | 1.00 | 0.11–5.78 | [64] |
| Brazil | 34 | 68 | 0.9 | 0.3–6.5 | [65] |
| Brazil | 16 | 31 | 0.09–9 | [62] | |
| Canada | 71 | 59 | 0.6 | 0.1–2.3 | [72] |
| Chile | 24 | 100 | 0.47 | tr–0.84 | [3] |
| France | 30 | 100 | tr–11.9 | [66] | |
| Germany | 490 | 44 | 1.48 | <0.15–12.1 | [61] |
| Hungary | 38 | 58 | 0.5 | 0.17–0.91 | [73] |
| Italy | 30 | 27 | 0.27 | tr–<5.0 | [74] |
| Portugal | 11 | 27 | 1.66 | 0.71–10.31 | [63] |
| Spain | 72 | 49 | 1.05 | 1.21–4.21 | [75] |
| UK | 20 | 85 | 0.6 | 0.2–2.1 | [76] |
| Method | % Reduction | Remarks | Reference |
|---|---|---|---|
| Roasting | 67 | Temp = 470 °C; Time = 2.5 min; batch size = 5 kg | [82] |
| 63 | Temp = 490 °C; Time = 2.5 min; batch size = 5 kg | ||
| 74 | Temp = 490 °C; Time = 4 min; batch size = 10 kg | ||
| 53 | Temp = 400 °C; Time = 10 min; batch size = 15 kg | ||
| 84 | Temp = 425 °C; Time = 10 min; batch size = 15 kg | ||
| [OTA]initial = 4.9 µg/kg; (Gothot RN 100 pilot size roaster) | |||
| Roasting | 77.9a; 64.1b | Temp = 450 °C; Time = 6 min; batch size = 3 kg | [80] |
| 90.5a; 88.7b | Temp = 450 °C; Time = 7 min; batch size = 3 kg | ||
| 96.8a; 95.1b | Temp = 450 °C; Time = 9 min; batch size = 3 kg | ||
| a[OTA]initial = 29.30 µg/kg; b[OTA]initial = 9.64 µg/kg; (Laboratory roaster, model 500, STA) | |||
| Roasting | 31.1 | Temp = 180 °C; Time = 10 min; [OTA]initial = 29.36 µg/kg (Normal coffee roaster) | [83] |
| Roasting | 79.1 | Temp = 260 °C; Time = 5 min; batch size = 0.5 kg; [OTA]initial = 2.54 µg/kg; (Precision Coffee Roaster model, Hearthware Home Products) | [69] |
| Roasting | 25.1a; 15.1b | Temp = 230 °C; Time = 6 min; batch size = 0.2 kg | [49] |
| 59.5a; 67.9b | Temp = 230 °C; Time = 9 min; batch size = 0.2 kg | ||
| 87.0a; 89.6b | Temp = 230 °C; Time = 12 min; batch size = 0.2 kg | ||
| 97.2a; 95.1b | Temp = 230 °C; Time = 15 min; batch size = 0.2 kg | ||
| (Rotating cylinder- Probat-Werne type RE1 pilot roaster) | |||
| 7.4a; 9.3b | Temp = 230 °C; Time = 0.9 min; batch size = 0.2 kg | ||
| 36.0a; 30.9b | Temp = 230 °C; Time = 1.7 min; batch size = 0.2 kg | ||
| 72.4a; 64.3b | Temp = 230 °C; Time = 2.6 min; batch size = 0.2 kg | ||
| 77.6a; 73.4b | Temp = 230 °C; Time = 3.5 min; batch size = 0.2 kg | ||
| (Fluidized bed - Neuhaus Neotec pilot roaster) a[OTA]initial = 5.3 µg/kg; b[OTA]initial = 57.2 µg/kg | |||
| Gamma radiation | 5 | aw = 0.59; Dried green coffee beans; | [84] |
| 9 | aw = 0.65; Incubated at RH 76 for 72 h; | ||
| 20 | aw = 0.71; Incubated at RH 99 for 72 h | ||
| 90 | aw = 0.82; Steamed for 1 h | ||
| ≈100 | aw = 0.93; Soaked for 20 h | ||
| [OTA]initial = 50 ppb; radiation = 10 kGy (Gamma Chamber 5000) | |||
| High-pressure | 0 | P = 50 MPa; Time storage = 10 days; [OTA]initial = 4.4 µg/kg | [85] |
| 54.6 | P = 200 MPa; Time storage = 10 days; [OTA]initial = 3.9 µg/kg | ||
| 93.2 | P = 400 MPa; Time storage = 10 days; [OTA]initial = 4.4 µg/kg | ||
| 96.2 | P = 600 MPa; Time storage = 10 days; [OTA]initial = 4.5 µg/kg | ||
| 14.6 | P = 50 MPa; Time storage = 20 days; [OTA]initial = 4.4 µg/kg | ||
| 43.6 | P = 200 MPa; Time storage = 20 days; [OTA]initial = 3.9 µg/kg | ||
| 93.2 | P = 400 MPa; Time storage = 20 days; [OTA]initial = 4.4 µg/kg | ||
| 96.0 | P = 600 MPa; Time storage = 20 days; [OTA]initial = 4.5 µg/kg | ||
| 19.1 | P = 50 MPa; Time storage = 30 days; [OTA]initial = 4.4 µg/kg | ||
| 45.7 | P = 200 MPa; Time storage = 30 days; [OTA]initial = 3.9 µg/kg | ||
| 91.9 | P = 400 MPa; Time storage = 30 days; [OTA]initial = 4.4 µg/kg | ||
| 95.5 | P = 600 MPa; Time storage = 30 days; [OTA]initial = 4.5 µg/kg | ||
| 26.9 | P = 50 MPa; Time storage = 50 days; [OTA]initial = 4.4 µg/kg | ||
| 36.3 | P = 200 MPa; Time storage = 50 days; [OTA]initial = 3.9 µg/kg | ||
| 90.8 | P = 400 MPa; Time storage = 50 days; [OTA]initial = 4.4 µg/kg | ||
| 94.4 | P = 600 MPa; Time storage = 50 days; [OTA]initial = 4.5 µg/kg | ||
| (BaoTou KeFa High Pressure Technology Co.) |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitão, A.L. Occurrence of Ochratoxin A in Coffee: Threads and Solutions—A Mini-Review. Beverages 2019, 5, 36. https://doi.org/10.3390/beverages5020036
Leitão AL. Occurrence of Ochratoxin A in Coffee: Threads and Solutions—A Mini-Review. Beverages. 2019; 5(2):36. https://doi.org/10.3390/beverages5020036
Chicago/Turabian StyleLeitão, Ana Lúcia. 2019. "Occurrence of Ochratoxin A in Coffee: Threads and Solutions—A Mini-Review" Beverages 5, no. 2: 36. https://doi.org/10.3390/beverages5020036
APA StyleLeitão, A. L. (2019). Occurrence of Ochratoxin A in Coffee: Threads and Solutions—A Mini-Review. Beverages, 5(2), 36. https://doi.org/10.3390/beverages5020036