Perception of Bitter Taste through Time-Intensity Measurements as Influenced by Taste Modulation Compounds in Steviol Glycoside Sweetened Beverages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials
2.3. Temporal Evaluation
2.4. Statistical Analysis
3. Results
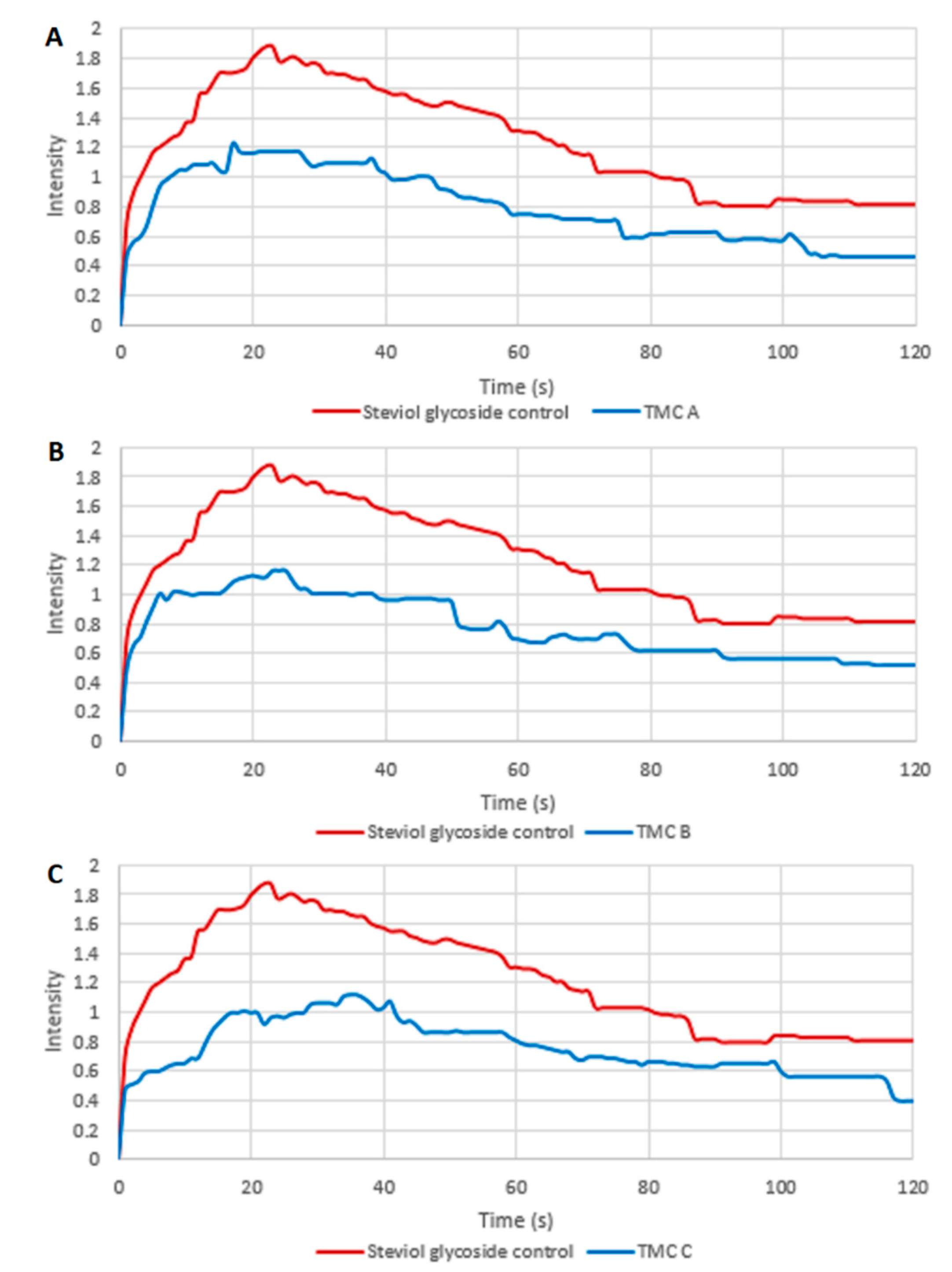
3.1. Comparison of Raw Average TI Curves among Control and Variant Samples with TMCs
3.2. Comparison of Raw Average TI Curve Parameters among Control and Variant Samples with TMCs
3.3. Comparison of Average Bitter Difference TI Curves among Control and Variant Samples with TMCs
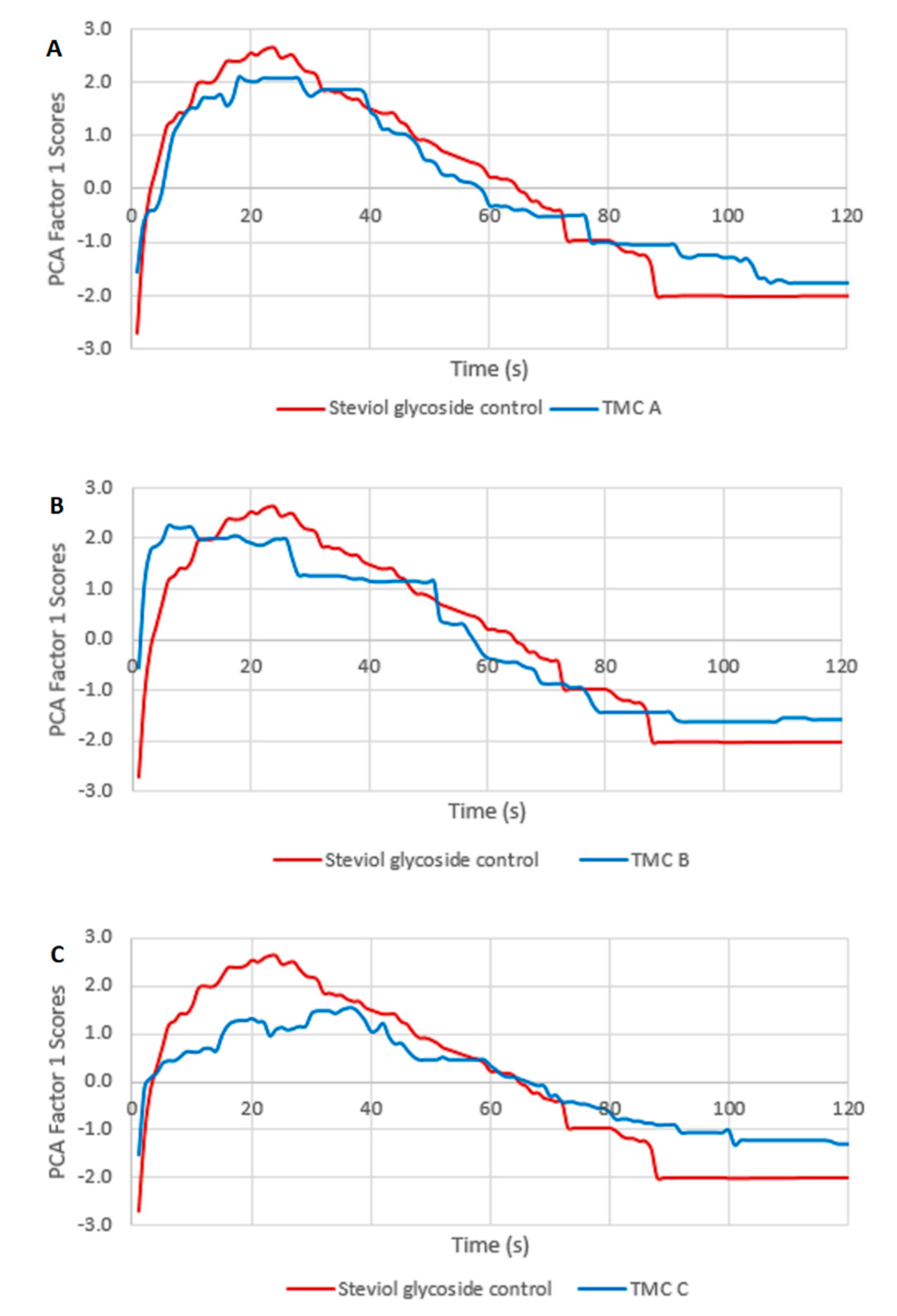
3.4. Comparison of PCA-Generated Average TI Curves among Control and Variant Samples with TMCs
4. Discussion
4.1. TI Parameters Show Significant Differences between the Steviol Glycoside Control and Steviol Glycosides with TMCs
4.2. Raw Average Bitterness Scores Were Reduced with Added TMCs
4.3. Bitter Difference Scores Shows Minimal Effects of TMCs
4.4. PCA-Generated Average Curves Show Minimal Differences among Samples
4.5. Multimodal Taste Modulation
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Despres, J.-P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes. Diabetes Care 2010, 33, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. Sweeteners and risk of obesity and type 2 diabetes: The role of sugar-sweetened beverages. Curr. Diabetes Rep. 2012, 12, 195–203. [Google Scholar] [CrossRef] [PubMed]
- The Food and Drug Administration. United States Department of Health and Human Services. High-Intensity Sweeteners. 2014. Available online: https://www.fda.gov/food/food-additives-petitions/high-intensity-sweeteners (accessed on 25 April 2019).
- Perrier, J.D.; Mihalov, J.J.; Carlson, S.J. FDA regulatory approach to steviol glycosides. Food Chem. Toxicol. 2018, 122, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E. Sales of Plant-Based Steviol Glycosides Skyrocket as Consumers Seek Natural Solutions to Cut Back Sugar. Available online: https://www.foodnavigator-usa.com/Article/2018/04/17/Sales-of-plant-based-Steviol glycosides-skyrocket-as-consumers-seek-natural-solutions-to-cut-back-sugar# (accessed on 10 January 2019).
- Prakash, I.; Markosyan, A.; Bunders, C. Development of Next Generation Stevia Sweetener: Rebaudioside M. Foods 2014, 3, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Saniah, K.; Samsiah, M.S. The application of Stevia as sugar substitute in carbonated drinks using Response Surface Methodology. J. Trop. Agric. Food Sci. 2012, 40, 23–34. [Google Scholar]
- DuBois, G.E.; Prakash, I. Non-Caloric Sweeteners, Sweetness Modulators, and Sweetener Enhancers. Annu. Rev. Food Sci. Technol. 2012, 3, 353–380. [Google Scholar] [CrossRef]
- Reis, R.C.; Minim, V.P.R.; Bolini, H.M.A.; Dias, B.R.P.; Minim, L.A.; Ceresino, E.B. Sweetness equivalence of different sweeteners in strawberry-flavored yogurt. J. Food Qual. 2011, 34, 163–170. [Google Scholar] [CrossRef]
- Purkayastha, S.; Pugh, G.; Lynch, B.; Roberts, A.; Kwok, D.; Tarka, S.M. In vitro metabolism of rebaudioside B, D, and M under anaerobic conditions: Comparison with rebaudioside A. Regul. Toxicol. Pharmacol. 2013, 68, 259–268. [Google Scholar] [CrossRef]
- Prakash, I.; Campbell, M.; Chaturvedula, V.S.P. Catalytic hydrogenation of the sweet principles of Stevia rebaudiana, rebaudioside B, rebaudioside C, and rebaudioside D and sensory evaluation of their reduced derivatives. Int. J. Mol. Sci. 2012, 13, 15126–15136. [Google Scholar] [CrossRef]
- Marcinek, K.; Krejpcio, Z. Stevia Rebaudiana bertoni? chemical composition and functional properties. ACTA 2015, 14, 145–152. [Google Scholar]
- Nikiforov, A.I.; Rihner, M.O.; Eapen, A.K.; Thomas, J.A. Metabolism and toxicity studies supporting the safety of rebaudioside D. Int. J. Toxicol. 2013, 32, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Soldo, T.; Ottinger, H. Structural and functional characterization of a multimodal taste enhancer in beef bouillon. In Natural Flavors and Fragrances; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2005; pp. 173–188. [Google Scholar]
- Kawai, M.; Okiyama, A.; Ueda, Y. Taste enhancements between various amino acids and IMP. Chem. Senses 2002, 27, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Ottinger, H.; Soldo, T.; Hofmann, T. Discovery and structure determination of a novel Maillard-derived sweetness enhancer by application of the comparative taste dilution analysis (cTDA). J. Agric. Food Chem. 2003, 51, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Servant, G.; Tachdjian, C.; Tang, X.-Q.; Werner, S.; Zhang, F.; Kamdar, P.; Petrovic, G.; Ditschun, T.; Brust, P.; Brune, N.; et al. Positive allosteric modulators of the human sweet taste receptor enhance sweet taste. Proc. Natl. Acad. Sci. USA 2010, 107, 4746–4751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servant, G.; Tachdjian, C.; Li, X.; Karanewsky, D.S. The sweet taste of true synergy: Positive allosteric modulation of the human sweet taste receptor. Trends Pharmacol Sci. 2011, 32, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Soldo, T.; Blank, I.; Hofmann, T. (+)-(S)-alapyridaine—A general taste enhancer? Chem. Senses 2003, 28, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Klebansky, B.; Fine, R.M. Molecular mechanism of the sweet taste enhancers. Proc. Natl. Acad. Sci. USA 2010, 107, 4752–4757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villard, R.; Robert, F.; Blank, I.; Bernardinelli, G.; Soldo, T.; Hofmann, T. Racemic and Enantiopure Synthesis and Physicochemical Characterization of the Novel Taste Enhancer N-(1-Carboxyethyl)-6-(hydroxymethyl) pyridinium-3-ol Inner Salt. J. Agric. Food Chem. 2003, 51, 4040–4045. [Google Scholar] [CrossRef]
- Katsuragi, Y.; Sugiura, Y.; Lee, C.; Otsuji, K.; Kurihara, K. Selective inhibition of bitter taste by lipoprotein. Pharm. Res. 1995, 12, 658–662. [Google Scholar] [CrossRef]
- Ley, J.P. Masking bitter taste by Molecules. Chemosens. Percept. 2008, 1, 58–77. [Google Scholar] [CrossRef]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillmann, H.; Mattes, J.; Brockhoff, A.; Dunkel, A.; Meyerhof, W.; Hofmann, T. Sensomics analysis of taste compounds in balsamic vinegar and discovery of 5-acetoxymethyl-2-furaldehyde as a novel sweet taste modulator. J. Agric. Food Chem. 2012, 60, 9974–9990. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, K.V.; Peter, R.; Paetz, S. Characterization of flavor modulating effects in complex mixtures via high temperature liquid chromatography. J. Agric. Food Chem. 2010, 58, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Lawless, H.T. Evidence for Neural Inhibition in Bittersweet Taste Mixtures. J. Comp. Physiol. Psychol. 1979, 93, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, M.I.; Vincken, J.P.; Sanders, M.; Castro, C.; Stieger, M.; Agosin, E. Identification, quantification, and sensory characterization of steviol glycosides from differently processed stevia rebaudiana commercial extracts. J. Agric. Food Chem. 2014, 62, 11797–11804. [Google Scholar] [CrossRef] [PubMed]
- Olabi, A.; Lawless, H.T. Persistence of context effects after training and with intensity references. J. Food Sci. 2008, 73, 185–189. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2009, 35, 157–170. [Google Scholar] [CrossRef]
- Hellfritsch, C.; Brockhoff, A.; Stähler, F.; Meyerhof, W.; Hofmann, T. Human psychometric and taste receptor responses to steviol glycosides. J. Agric. Food Chem. 2012, 60, 6782–6793. [Google Scholar] [CrossRef]
- Van Buuren, S. Analyzing time-intensity responses in sensory evaluation. Food Technol. 1992, 46, 101–104. [Google Scholar]
- Dijksterhuis, G.B. Principal Component Analysis of Time-Intensity Bitterness Curves1. J. Sens. Stud. 1993, 8, 317–328. [Google Scholar] [CrossRef]
- Azevedo, B.M.; Ferreira, J.M.M.; Luccas, V.; Bolini, H.M.A. The Influence of the Rebaudioside A Content of Stevia (Stevia rebaudiana Bertoni) on the Determination of Sweetness Equivalence in Bittersweet Chocolates, Using the Time-Intensity Analysis. J. Food Sci. 2016, 81, S3006–S3014. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, B.M.; Morais-Ferreira, J.M.; Luccas, V.; Bolini, H.M.A. Bittersweet chocolates containing prebiotic and sweetened with stevia (Stevia rebaudiana Bertoni) with different Rebaudioside A contents: Multiple time–intensity analysis and physicochemical characteristics. Int. J. Food Sci. Technol. 2017, 52, 1731–1738. [Google Scholar] [CrossRef]
- Cliff, M.; Heymann, H. Development and use of time-intensity methodology for sensory evaluation: A review. Food Res. Int. 1993, 26, 375–385. [Google Scholar] [CrossRef]
- De Melo, L.L.M.M.; Bolini, H.M.A.; Efraim, P. Equisweet milk chocolates with intense sweeteners using time-intensity method. J. Food Qual. 2007, 30, 1056–1067. [Google Scholar] [CrossRef]
- De Souza, V.R.; Pereira, P.A.P.; Pinheiro, A.C.M.; Bolini, H.M.A.; Borges, S.V.; Queiroz, F. Analysis of various sweeteners in low-sugar mixed fruit jam: Equivalent sweetness, time-intensity analysis and acceptance test. Int. J. Food Sci. Technol. 2013, 48, 1541–1548. [Google Scholar] [CrossRef]
- Morais Ferreira, J.M.; Azevedo, B.M.; Silva, F.G.D.; Luccas, V.; Bolini, H.M.A. Isosweetness concentrations of sucrose and high-intensity sweeteners and antioxidant activity in white chocolate with functional properties. Int. J. Food Sci. Technol. 2016, 51, 2114–2122. [Google Scholar] [CrossRef]
- Morais, E.C.; Pinheiro, A.C.M.; Nunes, C.A.; Bolini, H.M.A. Multiple Time-Intensity Analysis and Temporal Dominance of Sensations of Chocolate Dairy Dessert Using Prebiotic and Different High-Intensity Sweeteners. J. Sens. Stud. 2014, 29, 339–350. [Google Scholar] [CrossRef]
- Palazzo, A.B.; Carvalho, M.A.R.; Efraim, P.; Bolini, H.M.A. The determination of isosweetness concentrations of sucralose, rebaudioside and neotame as sucrose substitutes in new diet chocolate formulations using the time-intensity analysis. J. Sens. Stud. 2011, 26, 291–297. [Google Scholar] [CrossRef]
- Ovejero-López, I.; Bro, R.; Bredie, W.L.P. Univariate and multivariate modelling of flavour release in chewing gum using time-intensity: A comparison of data analytical methods. Food Qual. Prefer. 2005, 16, 327–343. [Google Scholar] [CrossRef]
- Piggott, J.R.; Hunter, E.A.; Margomenou, L. Comparison of methods of analysis of time-intensity data: Application to Scotch malt whisky. Food Chem. 2000, 71, 319–326. [Google Scholar] [CrossRef]
- Calvino, A.; Garrido, D. Single Value and Time Intensity Measurements. J. Sens. Stud. 1998, 15, 47–64. [Google Scholar] [CrossRef]
- Cliff, M.; Noble, A.C. Time-Intensity Evaluation of Sweetness and Fruitiness and Their Interaction in a Model Solution. J. Food Sci. 1990, 55, 450–454. [Google Scholar] [CrossRef]
- Zhao, L.; Tepper, B.J. Perception and acceptance of selected high-intensity sweeteners and blends in model soft drinks by propylthiouracil (PROP) non-tasters and super-tasters. Food Qual. Prefer. 2007, 18, 531–540. [Google Scholar] [CrossRef]
- Dubois, G.E. Replication of Sugar Taste Enabled by Taste Modulators & Enhancers. In Proceedings of the Global Food Forum’s Meeting of Sweetener Systems Conference, Oak Brook, IL, USA, 23 October 2018. [Google Scholar]
- Slack, J.P.; Brockhoff, A.; Batram, C.; Menzel, S.; Sonnabend, C.; Born, S.; Behrens, M.; Furrer, S.; Meyerhof, W.; Ungureanu, L.; et al. Modulation of bitter taste perception by a small molecule hTAS2R antagonist. Curr. Biol. 2010, 20, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Harmen, C.L.; Hallagan, J.B. The FEMA Science Committee Sensory Data Task Force. Sensory Testing for Flavorings with Modifying Properties. Food Technol. 2013, 67, 44. [Google Scholar]
- Ennis, J.M.; Rousseau, B.; Ennis, D.M. Sensory difference tests as measurement instruments: A review of recent advances. J. Sens. Stud. 2014, 29, 89–102. [Google Scholar] [CrossRef]

| Samples | Usage Level (%) 1 |
|---|---|
| Rebaudioside A 97% (Control) | 0.0275 |
| Rebaudioside A 97% + TMC A 2 | 0.0275 + 0.001 |
| Rebaudioside A 97% + TMC B 3 | 0.0275 + 0.001 |
| Rebaudioside A 97% + TMC C 4 | 0.0275 + 0.001 |
| Sample | Imax 1 | Tmax 2 | AUC 3 |
|---|---|---|---|
| Rebaudioside A 97% (Control) | 2.03 a ± 0.41 | 19.89 a ± 4.97 | 147.52 a ± 40.56 |
| Rebaudioside A 97% + TMC A | 1.43 b ± 0.34 | 21.89 a ± 9.00 | 94.23 b ± 26.43 |
| Rebaudioside A 97% + TMC B | 1.48 b ± 0.30 | 22.44 a ± 9.73 | 92.52 b ± 25.00 |
| Rebaudioside A 97% + TMC C | 1.32 b ± 0.30 | 30.00 a ± 9.24 | 90.97 b ± 26.73 |
| p-value | p = 0.049 | p = 0.534 | p = 0.066 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierce-Feldmeyer, A.M.; Josephson, D.; Johnson, A.; Wieland, R. Perception of Bitter Taste through Time-Intensity Measurements as Influenced by Taste Modulation Compounds in Steviol Glycoside Sweetened Beverages. Beverages 2019, 5, 52. https://doi.org/10.3390/beverages5030052
Pierce-Feldmeyer AM, Josephson D, Johnson A, Wieland R. Perception of Bitter Taste through Time-Intensity Measurements as Influenced by Taste Modulation Compounds in Steviol Glycoside Sweetened Beverages. Beverages. 2019; 5(3):52. https://doi.org/10.3390/beverages5030052
Chicago/Turabian StylePierce-Feldmeyer, Alex M., Dave Josephson, Adrianne Johnson, and Robert Wieland. 2019. "Perception of Bitter Taste through Time-Intensity Measurements as Influenced by Taste Modulation Compounds in Steviol Glycoside Sweetened Beverages" Beverages 5, no. 3: 52. https://doi.org/10.3390/beverages5030052
APA StylePierce-Feldmeyer, A. M., Josephson, D., Johnson, A., & Wieland, R. (2019). Perception of Bitter Taste through Time-Intensity Measurements as Influenced by Taste Modulation Compounds in Steviol Glycoside Sweetened Beverages. Beverages, 5(3), 52. https://doi.org/10.3390/beverages5030052




