Amelioration of Smoke Taint in Cabernet Sauvignon Wine via Post-Harvest Ozonation of Grapes
Abstract
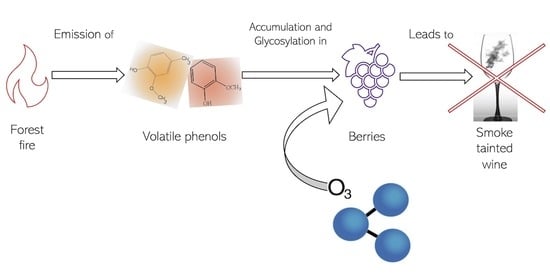
:1. Introduction
2. Materials and Methods
2.1. Smoke Exposure of Grapevines
2.2. Post-Harvest Ozonation of Grapes
2.3. Small-Scale Winemaking
2.4. Chemical Analysis of Wine
2.5. Sensory Analysis of Wine
2.6. Statistical Analysis
3. Results and Discussion
3.1. Influence of Post-Harvest Ozonation on Wine Composition
3.2. Influence of Post-Harvest Ozonation on Wine Sensory Profiles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moritz, M.A.; Parisien, M.-A.; Batllori, E.; Krawchuk, M.A.; Van Dorn, J.; Ganz, D.J.; Hayhoe, K. Climate change and disruptions to global fire activity. Ecosphere 2012, 3, 1–22. [Google Scholar] [CrossRef]
- Krstic, M.P.; Johnson, D.L.; Herderich, M.J. Review of smoke taint in wine: Smoke-derived volatile phenols and their glycosidic metabolites in grapes and vines as biomarkers for smoke exposure and their role in the sensory perception of smoke taint. Aust. J. Grape Wine Res. 2015, 21, 537–553. [Google Scholar] [CrossRef]
- Mirabelli-Montan, Y.A.; Marangon, M.; Graça, A.; Marangon, C.M.; Wilkinson, K.L. Techniques for mitigating the effects of smoke taint while maintaining quality in wine production. Molecules 2021, 26, 1672. [Google Scholar] [CrossRef]
- Kennison, K.R.; Gibberd, M.R.; Pollnitz, A.P.; Wilkinson, K.L. Smoke-derived taint in wine: The release of smoke-derived volatile phenols during fermentation of Merlot juice following grapevine exposure to smoke. J. Agric. Food Chem. 2008, 56, 7379–7383. [Google Scholar] [CrossRef]
- Sheppard, S.I.; Dhesi, M.K.; Eggers, N.J. Effect of pre- and post-veraison smoke exposure on guaiacol and 4-methylguaiacol concentration in mature grapes. Am. J. Enol. Vitic. 2009, 60, 98–103. [Google Scholar]
- Hayasaka, Y.; Baldock, G.A.; Parker, M.; Pardon, K.H.; Black, C.A.; Herderich, M.J.; Jeffery, D.W. Glycosylation of smoke derived volatile phenols in grapes as a consequence of grapevine exposure to bushfire smoke. J. Agric. Food Chem. 2010, 58, 10989–10998. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.L.; Ristic, R.; Pinchbeck, K.A.; Fudge, A.L.; Singh, D.P.; Pitt, K.M.; Downey, M.O.; Baldock, G.A.; Hayasaka, Y.; Parker, M.; et al. Comparison of methods for the analysis of smoke related phenols and their conjugates in grapes and wine. Aust. J. Grape Wine Res. 2011, 17, S22–S28. [Google Scholar] [CrossRef]
- Ristic, R.; Fudge, A.L.; Pinchbeck, K.A.; De Bei, R.; Fuentes, S.; Hayasaka, Y.; Tyerman, S.D.; Wilkinson, K.L. Impact of grapevine exposure to smoke on vine physiology and the composition and sensory properties of wine. Theor. Exp. Plant Phys. 2016, 28, 67–83. [Google Scholar] [CrossRef]
- Noestheden, M.; Dennis, E.G.; Zandberg, W. Quantitating volatile phenols in Cabernet Franc berries and wine after on-vine exposure to smoke from a simulated forest fire. J. Agric. Food Chem. 2018, 66, 695–703. [Google Scholar] [CrossRef]
- Parker, M.; Osidacz, P.; Baldock, G.A.; Hayasaka, Y.; Black, C.A.; Pardon, K.H.; Jeffery, D.W.; Geue, J.P.; Herderich, M.J.; Francis, I.L. Contribution of several volatile phenols and their glycoconjugates to smoke-related sensory properties of red wine. J. Agric. Food Chem. 2012, 60, 2629–2637. [Google Scholar] [CrossRef]
- Kennison, K.R.; Wilkinson, K.L.; Pollnitz, A.P.; Williams, H.G.; Gibberd, M.R. Effect of timing and duration of grapevine exposure to smoke on the composition and sensory properties of wine. Aust. J. Grape Wine Res. 2009, 15, 228–237. [Google Scholar] [CrossRef]
- Kennison, K.R.; Wilkinson, K.L.; Pollnitz, A.P.; Williams, H.G.; Gibberd, M.R. Effect of smoke application to field-grown Merlot grapevines at key phenological growth stages on wine sensory and chemical properties. Aust. J. Grape Wine Res. 2011, 17, S5–S12. [Google Scholar] [CrossRef]
- Szeto, C.; Ristic, R.; Capone, D.; Puglisi, C.; Pagay, V.; Culbert, J.; Jiang, W.; Herderich, M.; Tuke, J.; Wilkinson, K. Uptake and glycosylation of smoke-derived volatile phenols by Cabernet Sauvignon grapes and their subsequent fate during winemaking. Molecules 2020, 25, 3720. [Google Scholar] [CrossRef] [PubMed]
- Wittkowski, R.; Ruther, J.; Drinda, H.; Rafiei-Taghanaki, F. Formation of smoke flavor compounds by thermal lignin degradation. In Flavor Precursors: Thermal and Enzymatic Conversions; Teranishi, R., Takeoka, G.R., Güntert, M., Eds.; American Chemical Society: Washington, DC, USA, 1992; pp. 232–243. [Google Scholar] [CrossRef]
- Kennison, K.R.; Wilkinson, K.L.; Williams, H.G.; Smith, H.G.; Gibberd, M.R. Smoke-derived taint in wine: Effect of post-harvest smoke exposure of grapes on the chemical composition and sensory characteristics of wine. J. Agric. Food Chem. 2007, 55, 10897–10901. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, Y.; Dungey, K.A.; Baldock, G.A.; Kennison, K.R.; Wilkinson, K.L. Identification of a β-D-glucopyranoside precursor to guaiacol in grape juice following grapevine exposure to smoke. Anal. Chim. Acta 2010, 660, 143–148. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Baldock, G.A.; Pardon, K.H.; Jeffery, D.W.; Herderich, M.J. Investigation into the formation of guaiacol conjugates in berries and leaves of grapevine Vitis vinifera L. cv. Cabernet Sauvignon using stable isotope tracers combined with HPLC-MS and MS/MS analysis. J. Agric. Food Chem. 2010, 58, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Noestheden, M.; Dennis, E.G.; Romero-Montalvo, E.; DiLabio, G.A.; Zandberg, W.F. Detailed characterization of glycosylated sensory-active volatile phenols in smoke-exposed grapes and wine. Food Chem. 2018, 259, 147–156. [Google Scholar] [CrossRef]
- van der Hulst, L.; Munguia, P.; Culbert, J.A.; Ford, C.M.; Burton, R.A.; Wilkinson, K.L. Accumulation of volatile phenol glycoconjugates in grapes following grapevine exposure to smoke and potential mitigation of smoke taint by foliar application of kaolin. Planta 2019, 249, 941–952. [Google Scholar] [CrossRef]
- Caffrey, A.; Lerno, L.; Rumbaugh, A.; Girardello, R.; Zweigenbaum, J.; Oberholster, A.; Ebeler, S.E. Changes in smoke-taint volatile-phenol glycosides in wildfire smoke-exposed Cabernet Sauvignon grapes throughout winemaking. Am. J. Enol. Vitic. 2019, 70, 373–381. [Google Scholar] [CrossRef]
- Härtl, K.; Huang, F.-C.; Giri, A.P.; Franz-Oberdorf, K.; Frotscher, J.; Shao, Y.; Hoffmann, T.; Schwab, W. Glucosylation of smoke-derived volatiles in grapevine (Vitis vinifera) is catalyzed by a promiscuous resveratrol/guaiacol glucosyltransferase. J. Agric. Food Chem. 2017, 65, 5681–5689. [Google Scholar] [CrossRef]
- Mayr, C.M.; Parker, M.; Baldock, G.A.; Black, C.A.; Pardon, K.H.; Williamson, P.O.; Herderich, M.J.; Francis, I.L. Determination of the importance of in-mouth release of volatile phenol glycoconjugates to the flavor of smoke-tainted wines. J. Agric. Food Chem. 2014, 62, 2327–2336. [Google Scholar] [CrossRef]
- Dungey, K.A.; Hayasaka, Y.; Wilkinson, K.L. Quantitative analysis of glycoconjugate precursors of guaiacol in smoke-affected grapes using liquid chromatography–tandem mass spectrometry based stable isotope dilution analysis. Food Chem. 2011, 126, 801–806. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Parker, M.; Baldock, G.A.; Pardon, K.H.; Black, C.A.; Jeffery, D.W.; Herderich, M.J. Assessing the impact of smoke exposure in grapes: Development and validation of an HPLC-MS/MS method for the quantitative analysis of smoke-derived phenolic glycosides in grapes and wine. J. Agric. Food Chem. 2013, 61, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ristic, R.; Pinchbeck, K.A.; Fudge, A.L.; Hayasaka, Y.; Wilkinson, K.L. Effect of leaf removal and grapevine smoke exposure on colour, chemical composition and sensory properties of Chardonnay wines. Aust. J. Grape Wine Res. 2013, 19, 230–237. [Google Scholar] [CrossRef]
- Favell, J.W.; Noestheden, M.; Lyons, S.M.; Zandberg, W.F. Development and evaluation of a vineyard-based strategy to mitigate smoke-taint in wine grapes. J. Agric. Food Chem. 2019, 67, 14137–14142. [Google Scholar] [CrossRef] [PubMed]
- Ristic, R.; Osidacz, P.; Pinchbeck, K.; Hayasaka, Y.; Fudge, A.; Wilkinson, K. The effect of winemaking techniques on the intensity of smoke taint in wine. Aust. J. Grape Wine Res. 2011, 17, S29–S40. [Google Scholar] [CrossRef]
- Fudge, A.L.; Schiettecatte, M.; Ristic, R.; Hayasaka, Y.; Wilkinson, K.L. Amelioration of smoke taint in wine by treatment with commercial fining agents. Aust. J. Grape Wine Res. 2012, 18, 302–307. [Google Scholar] [CrossRef]
- Fudge, A.L.; Ristic, R.; Wollan, D.; Wilkinson, K.L. Amelioration of smoke taint in wine by reverse osmosis and solid phase adsorption. Aust. J. Grape Wine Res. 2011, 17, S41–S48. [Google Scholar] [CrossRef]
- Antolini, A.; Forniti, R.; Modesti, M.; Bellincontro, A.; Catelli, C.; Mencarelli, F. First application of ozone postharvest fumigation to remove smoke taint from grapes. Ozone Sci. Eng. 2021, 43, 254–262. [Google Scholar] [CrossRef]
- Modesti, M.; Szeto, C.; Ristic, R.; Jiang, W.; Culbert, J.; Bindon, K.; Catelli, C.; Mencarelli, F.; Tonutti, P.; Wilkinson, K. Potential mitigation of smoke taint in wines by post-harvest ozone treatment of grapes. Molecules 2021, 26, 1798. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal 2009, 2, ra45. [Google Scholar] [CrossRef] [Green Version]
- Artés-Hernández, F.; Aguayo, E.; Artés, F.; Tomás-Barberán, F. Enriched ozone atmosphere enhances bioactive phenolics in seedless table grapes after prolonged shelf life. J. Sci. Food Agric. 2007, 87, 824–831. [Google Scholar] [CrossRef]
- Carbone, K.; Mencarelli, F. Influence of short-term postharvest ozone treatments in nitrogen or air atmosphere on the metabolic response of white wine grapes. Food Bioprocess Technol. 2015, 8, 1739–1749. [Google Scholar] [CrossRef]
- Karaca, H. The effects of ozone-enriched storage atmosphere on pesticide residues and physicochemical properties of table grapes. Ozone Sci. Eng. 2019, 5, 404–414. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Kaavya, R.; Yasendra, J.; Veenuttranon, K.; Lueprasitsakul, P.; Divya, V.; Kothakota, A.; Ramesh, S.V. Ozone as a novel emerging technology for the dissipation of pesticide residues in foods–a review. Trends Food Sci. Technol. 2020, 97, 38–54. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Margosan, D.M.; Gabler, F.M. Impact of ozonated water on the quality and shelf-life of fresh citrus fruit, stone fruit, and table grapes. Ozone Sci. Eng. 2002, 24, 343–356. [Google Scholar] [CrossRef]
- Botondi, R.; De Sanctis, F.; Moscatelli, N.; Vettraino, A.M.; Catelli, C.; Mencarelli, F. Ozone fumigation for safety and quality of wine grapes in postharvest dehydration. Food Chem. 2015, 188, 641–647. [Google Scholar] [CrossRef]
- Cravero, F.; Englezos, V.; Rantsiou, K.; Torchio, F.; Giacosa, S.; Río Segade, S.; Gerbi, V.; Rolle, L.; Cocolin, L. Ozone treatments of post harvested wine grapes: Impact on fermentative yeasts and wine chemical properties. Food Res. Int. 2016, 87, 134–141. [Google Scholar] [CrossRef]
- Bellincontro, A.; Catelli, C.; Cotarella, R.; Mencarelli, F. Postharvest ozone fumigation of Petit Verdot grapes to prevent the use of sulfites and to increase anthocyanin in wine. Aust. J. Grape Wine Res. 2017, 23, 200–206. [Google Scholar] [CrossRef]
- Río Segade, S.; Vilanova, M.; Giacosa, S.; Perrone, I.; Chitarra, W.; Pollon, M.; Torchio, F.; Boccacci, P.; Gambino, G.; Gerbi, V.; et al. Ozone improves the aromatic fingerprint of white grapes. Sci. Rep. 2017, 7, 16301. [Google Scholar] [CrossRef] [Green Version]
- Modesti, M.; Petriccione, M.; Forniti, R.; Zampella, L.; Mastrobuoi, F.; Scortichini, M.; Mencarelli, F. Methyl jasmonate and ozone affect the antioxidant system and the quality of wine grape during postharvest partial dehydration. Food Res. Int. 2018, 112, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High throughput analysis of red wine and grape phenolics—Adaptation and validation of methyl cellulose precipitable tannin assay and modified Somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef]
- Pollnitz, A.P.; Pardon, K.H.; Sykes, M.; Sefton, M.A. The effects of sample preparation and gas chromatograph injection techniques on the accuracy of measuring guaiacol, 4-methylguaiacol and other volatile oak compounds in oak extracts by stable isotope dilution analyses. J. Agric. Food Chem. 2004, 52, 3244–3252. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Descriptive analysis. In Sensory Evaluation of Food; Springer: New York, NY, USA, 2010; pp. 227–257. [Google Scholar] [CrossRef]
- Ristic, R.; Boss, P.K.; Wilkinson, K.L. Influence of fruit maturity at harvest on the intensity of smoke taint in wine. Molecules 2015, 20, 8913–8927. [Google Scholar] [CrossRef] [Green Version]
- Ristic, R.; van der Hulst, L.; Capone, D.L.; Wilkinson, K.L. Impact of bottle aging on smoke-tainted wines from different grape cultivars. J. Agric. Food Chem. 2017, 65, 4146–4152. [Google Scholar] [CrossRef]
- Wilkinson, K.L.; Ristic, R. Comparing the chemical and sensory consequences of grapevine smoke exposure in grapes and wine from different cultivars and different wine regions in Australia. In Proceedings of the XIIIth International Terroir Congress, Adelaide, Australia, 17–18 November 2020; International Viticulture and Oenology Society: Villenave d’Ornon, France, 2021. [Google Scholar]
- Tiwari, B.K.; O’Donnell, C.P.; Patras, A.; Brunton, N.; Cullen, P.J. Anthocyanins and color degradation in ozonated grape juice. Food Chem. Toxicol. 2009, 47, 2824–2829. [Google Scholar] [CrossRef]

| Control | Control + O3 | Smoke | Smoke + O3 | p | |
|---|---|---|---|---|---|
| guaiacol | tr | 1.0 ± 1.0 c | 30 ± 4.5 a | 23 ± 1.5 b | <0.001 |
| 4-methylguaiacol | nd | nd | 4.3 ± 0.6 a | 3.3 ± 0.6 b | <0.001 |
| o-cresol | nd | nd | 8.3 ± 1.5 | 7.0 ± 0.01 | ns |
| m-cresol | nd | nd | 8.3 ± 1.5 | 7.0 ± 0.01 | ns |
| p-cresol | nd | nd | 4.0 ± 0.01 | 4.0 ± 0.01 | ns |
| syringol | 2.3 ± 0.6 c | 2.0 ± 0.01 c | 6.3 ± 0.6 a | 5.3 ± 0.6 b | <0.001 |
| 4-methylsyringol | nd | nd | nd | nd | – |
| guaiacol glycosides | 9.8 ± 0.9 c | 10.3 ± 1.8 c | 340 ± 22.7 a | 258 ± 15 b | <0.001 |
| 4-methylguaiacol glycosides | 2.9 ± 0.4 c | 2.3 ± 0.4 c | 79 ± 2.4 a | 62 ± 1.2 b | <0.001 |
| phenol glycosides | 3.0 ± 0.2 c | 3.4 ± 0.7 c | 111 ± 2.4 a | 98 ± 2.1 b | <0.001 |
| cresol glycosides | 4.3 ± 0.1 c | 4.3 ± 0.9 c | 117 ± 4.9 a | 100 ± 3.3 b | <0.001 |
| syringol glycosides | 12.2 ± 0.7 c | 13.4 ± 0.6 c | 614 ± 5.1 a | 473 ± 15 b | <0.001 |
| 4-methylsyringol glycosides | tr | tr | 38 ± 1.2 a | 26 ± 0.6 b | <0.001 |
| Control | Control + O3 | Smoke | Smoke + O3 | p | |
|---|---|---|---|---|---|
| pH | 3.65 ± 0.09 | 3.71 ± 0.01 | 3.63 ± 0.04 | 3.62 ± 0.05 | ns |
| TA (g/L) | 7.3 ± 0.2 a | 6.9 ± 0.06 b | 6.9 ± 0.17 b | 6.9 ± 0.16 b | 0.011 |
| alcohol (% abv) | 11.9 ± 0.05 a | 11.3 ± 0.07 b | 10.8 ± 0.05 b | 10.5 ± 0.04 b | <0.001 |
| wine color density (au) | 4.9 ± 0.36 a | 4.2 ± 0.18 b | 4.1 ± 0.09 b | 4.0 ± 0.06 b | 0.002 |
| wine color hue | 0.79 ± 0.01 b | 0.85 ± 0.01 a | 0.87 ± 0.01 a | 0.85 ± 0.01 a | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modesti, M.; Szeto, C.; Ristic, R.; Jiang, W.; Culbert, J.; Catelli, C.; Mencarelli, F.; Tonutti, P.; Wilkinson, K. Amelioration of Smoke Taint in Cabernet Sauvignon Wine via Post-Harvest Ozonation of Grapes. Beverages 2021, 7, 44. https://doi.org/10.3390/beverages7030044
Modesti M, Szeto C, Ristic R, Jiang W, Culbert J, Catelli C, Mencarelli F, Tonutti P, Wilkinson K. Amelioration of Smoke Taint in Cabernet Sauvignon Wine via Post-Harvest Ozonation of Grapes. Beverages. 2021; 7(3):44. https://doi.org/10.3390/beverages7030044
Chicago/Turabian StyleModesti, Margherita, Colleen Szeto, Renata Ristic, WenWen Jiang, Julie Culbert, Cesare Catelli, Fabio Mencarelli, Pietro Tonutti, and Kerry Wilkinson. 2021. "Amelioration of Smoke Taint in Cabernet Sauvignon Wine via Post-Harvest Ozonation of Grapes" Beverages 7, no. 3: 44. https://doi.org/10.3390/beverages7030044
APA StyleModesti, M., Szeto, C., Ristic, R., Jiang, W., Culbert, J., Catelli, C., Mencarelli, F., Tonutti, P., & Wilkinson, K. (2021). Amelioration of Smoke Taint in Cabernet Sauvignon Wine via Post-Harvest Ozonation of Grapes. Beverages, 7(3), 44. https://doi.org/10.3390/beverages7030044