The Impact of Sweetener Type on Physicochemical Properties, Antioxidant Activity and Rheology of Guava Nectar during Storage Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
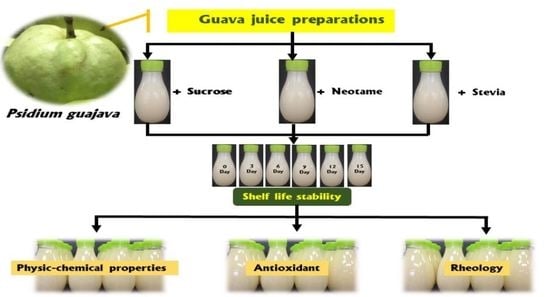
2.2. Preparation Guava Nectar Formulation
2.3. Preparation of Sweetened Guava Nectar
2.4. Analytical Methods for Shelf Life Evaluation
2.5. Physico-Chemical Property
- I is the light density that passes through a sample solution.
- I0 is the light intensity.
2.6. Total Carotenoid Content
2.7. Antioxidant Activity
2.7.1. ABTS•+ Radical Scavenging Activity Assay
- Acontrol is the absorbance of ABTS•+ solution without sample.
- Asample is the absorbance of ABTS•+ mixed with a sample.
2.7.2. DPPH• Radical Scavenging Activity Assay
- Acontrol is the absorbance of DPPH• solution without sample.
- Asample is the absorbance of DPPH• mixed with a sample.
2.8. Flow Behavior and Apparent Viscosity of Guava Juice
2.9. Volatile Compound Gas Chromatography
2.10. Statistical Analysis
3. Results and Discussion
3.1. Cloud Value
3.2. Color Attributes
3.3. pH
3.4. Titratable Acidity (TA)
3.5. Carotenoid Content
3.6. ABTS•+ Radical Scavenging
3.7. DPPH• Radical Scavenging
3.8. Volatile Compound
3.9. Rheology Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bal, L.M.; Ahmad, T.; Senapati, A.K.; Pandit, P.S. Evaluation of quality attributes during storage of guava nectar cv. Lalit from different pulp and TSS ratio. Int. J. Food Sci. 2014, 5, 1–5. [Google Scholar]
- Sethi, V.; Sethi, S. Processing of Fruits and Vegetables for Value Addition; Indus Publishing: Delhi, India, 2006. [Google Scholar]
- Ogden, C.L.; Carroll, M.D.; Flegal, K.M. High body mass index for age among US children and adolescents, 2003–2006. JAMA 2008, 299, 2401–2405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareyt, B.; Talhaoui, F.; Kerckhofs, G.; Brijs, K.; Goesaert, H.; Wevers, M.; Delcour, J.A. The role of sugar and fat in sugar-snap cookies: Structural and textural properties. J. Food Eng. 2009, 90, 400–408. [Google Scholar] [CrossRef]
- Ahmad, U.; Ahmad, R.S.; Imran, A.; Mushtaqq, Z.; Hussian, S.M. Characterization of low calorie ready-to-serve peach beverage using natural sweetener, Stevia (Stevia rebaudiana Bertoni). Prog. Nutr. 2019, 21, 435–444. [Google Scholar]
- Pinheiro, M.V.S.; Oliveira, M.N.; Penna, A.L.B.; Tamime, A.Y. The effect of different sweeteners in low-calorie yogurts e a review. Int. J. Dairy Technol. 2005, 58, 193–199. [Google Scholar] [CrossRef]
- Nabors, L.O. Sweet choices: Sugar replacements for foods and beverages. J. Food Technol. 2002, 56, 28–34. [Google Scholar]
- Dhaka, A.; Sharma, M.; Singh, S.K. Use of additives to reduce browning, microbial load and quality loss of Kinnow Juice under ambient storage. Indian J. Sci. Technol. 2016, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Malik, V.S.; Matthias, B.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic revie. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef]
- Prakash, I.; Corliss, G.; Ponakala, R.; Ishikawa, G. Neotame: The next-generation sweetener. Food Technol. 2002, 56, 36–40. [Google Scholar]
- Dutra, M.B.D.L.; Bolini, H.M.A. Sensory and physicochemical evaluation of acerola nectar sweetened with sucrose and different sweeteners. J. Food Sci. Technol. 2013, 33, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Boileau, A.; Fry, J.C.; Murray, R.A. New calorie-free sugar substitute from the leaf of the stevia plant arrives in the UK. Nutr. Bull. 2012, 37, 47–50. [Google Scholar] [CrossRef]
- Majchrzak, D.; Ipsen, A.; Koenig, J. Sucrose-replacement by rebaudioside a in a model beverage. J. Food Sci. Technol. 2015, 52, 6031–6036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, S.K.; Samsher, R.K. Goyal, Stevia (Stevia rebaudiana) a bio-sweetener: A review. Int. J. Food Sci. 2010, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Oskuyi, A.S.; Amjadi, S. The optimization of prebiotic sucrose-free mango nectar by response surface methodology: The effect of stevia and inulin on physicochemical and rheological properties. Int. J. Food Sci. Technol. 2019, 25, 243–251. [Google Scholar] [CrossRef]
- Cadena, R.S.; Cruz, A.G.; Netto, R.R.; Castro, W.F.; Faria, J.D.A.F.; Bolini, H.M.A. Sensory profile and physicochemical characteristics of mango nectar sweetened with high intensity sweeteners throughout storage time. Food Res. Int. 2013, 54, 1670–1679. [Google Scholar] [CrossRef] [Green Version]
- Miele, N.A.; Cabisidan, E.K.; Blaiotta, G.; Leone, S.; Masi, P.; Di Monaco, R.; Cavella, S. Rheological and sensory performance of a protein-based sweetener (MNEI), sucrose, and aspartame in yogurt. Int. J. Dairy Sci. 2017, 100, 9539–9550. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, S.; Utpal, R.; Runu, C. Artificial sweeteners–a review. J. Food Sci. Technol. 2014, 51, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zhang, X.; Zeng, X. Effect of ultrasound on different quality parameters of apple juice. Ultrason. Sonochem. 2013, 20, 1182–1187. [Google Scholar] [CrossRef]
- Wang, J.; Vanga, S.K.; Raghavan, V. High-intensity ultrasound processing of kiwifruit juice: Effects on the ascorbic acid, total phenolics, flavonoids and antioxidant capacity. LWT 2019, 107, 299–307. [Google Scholar] [CrossRef]
- Kotíková, Z.; Lachman, J.; Hejtmánková, A.; Hejtmánková, K. Determination of antioxidant activity and antioxidant content in tomato varieties and evaluation of mutual interactions between antioxidants. LWT 2011, 44, 1703–1710. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bhat, R.; Goh, K.M. Sonication treatment convalesce the overall quality of hand-pressed strawberry juice. Food Chem. 2017, 215, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.R.; Coutinho, N.M.; Rocha, R.S.; Moraes, J.; Esmerino, E.A.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.; Ranadheera, C.S.; et al. Guava flavored whey-beverage processed by cold plasma: Physical characteristics, thermal behavior and microstructure. Food Res. Int. 2019, 119, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lee, S.C.; Chan, L.Y.; Li, W.M. Risk assessment of exposure to volatile organic compounds in different indoor environments. Environ. Res. 2004, 94, 57–66. [Google Scholar] [CrossRef]
- Pinelo, M.; Zeuner, B.; Meyer, A.S. Juice clarification by protease and pectinase treatments indicates new roles of pectin and protein in cherry juice turbidity. Food Bioprod. Process. 2010, 88, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Ertan, K.; Türkyılmaz, M.; Özkan, M. Effects of natural copigment sources in combination with sweeteners on the stability of anthocyanins in sour cherry nectars. Food Chem. 2019, 294, 423–432. [Google Scholar] [CrossRef]
- Ma, Y.H.; Ma, F.W.; Wang, Y.H.; Zhang, J.K. The responses of the enzymes related with ascorbate–glutathione cycle during drought stress in apple leaves. Acta Physiol. Plant. 2011, 33, 173–180. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Han, Z.; Sun, D.W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Influence of pulsed electric field processing on the quality of fruit juice beverages sweetened with Stevia rebaudiana. Food Bioprod. Process. 2017, 101, 214–222. [Google Scholar] [CrossRef]
- Jabeen, F.; Wahab, S.; Hashmi, M.S.; Mehmood, Z.; Riaz, A.; Ayub, M.; Muneeb, M. Liquid stevia extract as a substitute of sucrose in the preparation of guava drink. Fresenius Environ. Bull. 2019, 28, 233–243. [Google Scholar]
- Satyavathi, K.; Raju, P.B.; Bupesh, K.V.; Kiran, T.N.R. Neotame: High intensity low caloric sweetener. Asian J. Chem. 2010, 22, 5792–5796. [Google Scholar]
- Phillips, K.M.; Council-Troche, M.R.; McGinty, C.; Rasor, A.S.; Tarrago-Trani, M.T. Stability of vitamin C in fruit and vegetable homogenates stored at different temperatures. J. Food Compos. Anal. 2016, 45, 147–162. [Google Scholar] [CrossRef]
- Sethi, V.; Anand, J.C.; Saxena, S.K. Kinnow orange in juice and beverage making. Indian J. Hortic. 1980, 25, 13. [Google Scholar]
- Pareek, S.; Paliwal, R.; Mukherjee, S. Effect of juice extraction methods, potassium meta-bi-sulphite concentration and storage temperature on the extent of degradation and reactivity of chemical constituents in mandarin (Citrus reticulata Blanco) juice. J. Food Agric. Environ. 2015, 13, 39–44. [Google Scholar]
- Galani Yamdeu, J.H.; Gupta, P.H.; Patel, N.J.; Shah, A.K.; Talati, J.G. Effect of storage temperature on carbohydrate metabolism and development of cold-induced sweetening in indian potato (Solanum tuberosum L.) Varieties. J. Food Biochem. 2016, 40, 71–83. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Zura-Bravo, L.; Ah-Hen, K. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef]
- Ordóñez-Santos, L.E.; Vázquez-Riascos, A. Effect of processing and storage time on the vitamin C and lycopene contents of nectar of pink guava (Psidium guajava L.). Arch. Latinoam. Nutr. 2010, 60, 280. [Google Scholar]
- Yen, G.C.; Lin, H.T.; Yang, P. Volatile constituents of guava fruits (Psidium guajava L.) and canned puree. J. Food Sci. 1992, 57, 679–681. [Google Scholar] [CrossRef]
- Ryo, S.; Barringer, S.A. Effect of storage, oxygen and concentration on the levels of key volatiles in processed orange juice. J. Food Process. Preserv. 2015, 39, 495–507. [Google Scholar] [CrossRef]
- Bodini, R.B.; Montini, E.M.; de Carvalho, C.C.; de Moraes, L.A.; Meirelles, A.J.D.A.; de Oliveira, A.L. Sensory and composition analyses of the aqueous phases from the concentration of guava (Psidium guava L.) and Mango (Mangifera indica L.) Juices and the process-induced losses of vitamin C. Open Food Sci. J. 2019, 28, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Helmig, D.; Ortega, J.; Duhl, T.; Tanner, D.; Guenther, A.; Harley, P.; Wiedinmyer, C.; Milford, J.; Sakulyanontvittaya, T. Sesquiterpene emissions from pine trees—Identifications, emission rates and flux estimates for the contiguous United States. Environ. Sci. Technol. 2007, 41, 1545–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, W.K.; Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Zhao, K.; Zhang, Z.; Liu, F.; Hu, H.; Pan, S. Changes on the rheological properties of pectin-enriched mango nectar by high intensity ultrasound. LWT 2016, 91, 414–422. [Google Scholar] [CrossRef]
- Al-Dabbas, M.M.; Al-Qudsi, J.M. Effect of partial replacement of sucrose with the artificial sweetener sucralose on the physico-chemical, sensory, microbial characteristics, and final cost saving of orange nectar. Int. Food Res. J. 2012, 19, 1–6. [Google Scholar]
- Rad, A.H.; Delshadian, Z.; Arefhosseini, S.R.; Alipour, B.; Jafarabadi, M.A. Effect of inulin and stevia on some physical properties of chocolate milk. Health Promot. Perspect. 2012, 2, 42–47. [Google Scholar]



| Storage Day | Guava Nectar with Sucrose | Sweetened Guava Nectar | |||
|---|---|---|---|---|---|
| 0.01% Neotame | 0.01% Stevia | 0.05% Neotame | 0.05% Stevia | ||
| 0 | 30.42 ± 0.25 C,a | 31.01 ± 0.55 B,a | 30.93 ± 1.35 C,a | 32.49 ± 0.21 A,a | 30.94 ± 0.57 C,a |
| 3 | 26.43 ± 0.4 A,b | 20.46 ± 0.6 B,b | 19.53 ± 0.64 C,b | 20.48 ± 1.30 B,b | 19.67 ± 0.52 C,b |
| 6 | 25.29 ± 0.08 A,c | 19.80 ± 0.19 C,c | 17.88 ± 0.88 D,c | 20.35 ± 012 B,b | 18.46 ± 0.15 C,c |
| 9 | 24.13 ± 0.04 A,d | 19.10 ± 0.06 B,d | 18.38 ± 0.02 C,d | 18.73 ± 0.55 C,c | 17.57 ± 0.41 D,d |
| 12 | 22.09 ± 0.42 A,e | 18.74 ± 0.30 B,e | 17.90 ± 0.06 C,e | 17.29 ± 0.06 D,d | 17.18 ± 0.14 D,e |
| 15 | 13.84 ± 0.04 D,f | 18.10 ± 0.08 A,f | 17.36 ± 0.12 C,e | 17.51 ± 0.11 B,e | 17.71 ± 0.30 B,d |
| Storage Day | Guava Nectar with Sucrose | Sweetened Guava Nectar | |||
|---|---|---|---|---|---|
| 0.01% Neotame | 0.01% Stevia | 0.05% Neotame | 0.05% Stevia | ||
| 0 | Pulp separated from the juice | Pulp uniformly distributed | Pulp does not precipitate | Pulp uniformly distributed | Pulp does not precipitate |
| 3 | Pulp separated from the juice and settled at the bottom of the | Pulp did not precipitate | Pulp does not precipitate | Pulp does not precipitate | Pulp does not precipitate |
| 6 | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle | Pulp does not precipitate | Pulp does not precipitate |
| 9 | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle |
| 12 | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle |
| 15 | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle | Pulp separated from the juice and settled at the bottom of the bottle |
| Storage Day | Guava Nectar with Sucrose | Sweetened Guava Nectar | |||
|---|---|---|---|---|---|
| 0.01% Neotame | 0.01% Stevia | 0.05% Neotame | 0.05% Stevia | ||
| ΔE (0 day) | 0 ± 0 A,f | 0 ± 0 A,f | 0 ± 0 A,e | 0 ± 0 A,e | 0 ± 0 A,f |
| ΔE (3 days) | 6.72 ± 0.03 A,e | 6.08 ± 0.15 B,e | 5.18 ± 0.07 D,d | 6.22 ± 0.04 A,d | 5.81 ± 0.10 C,e |
| ΔE 6 days) | 7.11 ± 0.15 A,d | 6.22 ± 0.05 B,d | 5.20 ± 0.01 D,d | 6.24 ± 0.06 B,d | 5.92 ± 0.07 C,d |
| ΔE (9 days) | 8.09 ± 0.08 A,c | 6.41 ± 0.09 B,c | 5.48 ± 0.03 D,c | 6.41 ± 0.02 B,c | 6.22 ± 0.05 C,c |
| ΔE (12 days) | 8.55 ± 0.04 A,b | 6.68 ± 0.07 B,b | 5.71 ± 0.04 D,b | 6.68 ± 0.03 B,b | 6.35 ± 0.05 C,b |
| ΔE (15 days) | 9.03 ± 0.05 A,a | 6.88 ± 0.07 B,a | 6.08 ± 0.08 D,a | 6.82 ± 0.02 B,a | 6.51 ± 0.02 C,a |
| Storage Day | Guava Nectar with Sucrose | Sweetened Guava Nectar | |||
|---|---|---|---|---|---|
| 0.01% Neotame | 0.01% Stevia | 0.05% Neotame | 0.05% Stevia | ||
| 0 | 3.51 ± 0.01 A,d | 3.52 ± 0.02 A,d | 3.18 ± 0.09 B,e | 3.51 ± 0.02 A,e | 3.56 ± 0.02 A,b |
| 3 | 3.55 ± 0.01 A,c | 3.52 ± 0.02 B,d | 3.53 ± 0.02 B,d | 3.52 ± 0.02 B,d | 3.54 ± 0.01 A,d |
| 6 | 3.60 ± 0.02 A,b | 3.55 ± 0.01 B,c | 3.56 ± 0.02 B,c | 3.50 ± 0.11 C,c | 3.55 ± 0.01 B,c |
| 9 | 3.61 ± 0.01 A,b | 3.55 ± 0.04 B,c | 3.57 ± 0.02 B,b | 3.56 ± 0.01 B,b | 3.53 ± 0.03 C,d |
| 12 | 3.66 ± 0.01 A,a | 3.56 ± 0.01 B,b | 3.57 ± 0.02 B,b | 3.57 ± 0.01 B,a | 3.41 ± 0.25 C,e |
| 15 | 3.67 ± 0.02 A,a | 3.57 ± 0.02 C,a | 3.58 ± 0.03 B,a | 3.57 ± 0.01 C,a | 3.57 ± 0.01 C,a |
| Storage Day | Guava Nectar with Sucrose | Sweetened Guava Nectar | |||
|---|---|---|---|---|---|
| 0.01% Neotame | 0.01% Stevia | 0.05% Neotame | 0.05% Stevia | ||
| 0 | 0.59 ± 0.01 C,a | 0.60 ± 0.01 B,a | 0.61 ± 0.01 A,a | 0.59 ± 0.01 C,a | 0.61 ± 0.01 A,a |
| 3 | 0.59 ± 0 A,a | 0.59 ± 0.01 B,b | 0.56 ± 0.01 D,b | 0.58 ± 0.02 C,b | 0.59 ± 0.01 B,b |
| 6 | 0.57 ± 0.01 A,b | 0.57 ± 0.02 A,c | 0.55 ± 0.01 C,c | 0.55 ± 0.01 C,c | 0.56 ± 0.01 B,c |
| 9 | 0.55 ± 0.01 B,c | 0.56 ± 0.01 A,d | 0.55 ± 0.01 B,c | 0.56 ± 0.01 A,d | 0.56 ± 0.01 A,c |
| 12 | 0.55 ± 0.01 B,c | 0.56 ± 0.01 A,d | 0.53 ± 0.01 D,d | 0.53 ± 0.01 D,e | 0.54 ± 0.01 C,d |
| 15 | 0.52 ± 0.01 D,d | 0.55 ± 0.01 A,e | 0.53 ± 0.01 C,d | 0.53 ± 0.01 C,e | 0.54 ± 0.01 B,d |
| Volatile Compound | % Area | |||||
|---|---|---|---|---|---|---|
| Guava Nectar with Sucrose | Guava Nectar with Neotame | Guava Nectar with Stevia | ||||
| 0.01% | 0.05% | 0.01% | 0.05% | |||
| 0 Day | 15 Days | 0 Day | 15 Days | 0 Day | 15 Days | |
| 1. β-Caryophyllene | 26.71 | 10.15 | 26.71 | 13.55 | 26.71 | 12.95 |
| 2. α-Caryophyllene | 2.84 | 1.58 | 2.84 | 1.92 | 2.84 | 1.95 |
| 3. Bisabolene | 2.89 | - | 2.89 | - | 2.89 | - |
| 4. Aromadendrene | - | - | - | 2.98 | - | 3.87 |
| 5. α- Humulene | 2.84 | 1.52 | 2.84 | 1.93 | 2.84 | 1.54 |
| 6. Nerolidol | 0.44 | - | 0.44 | 0.39 | 0.44 | 0.33 |
| 7. β-ocimene | 0.07 | ND | 0.07 | ND | 0.07 | ND |
| 8. Acetic acid | 1.37 | 0.02 | 1.05 | 0.03 | 1.05 | 0.02 |
| 9. octanoic acid | 1.00 | ND | 1.00 | ND | 1.00 | ND |
| 10. ethyl acetate | 0.12 | ND | 0.12 | ND | 0.12 | ND |
| 11. 2-methyl-1-propanol | 0.53 | ND | 0.53 | 0.02 | 0.53 | ND |
| 12. 2-pentanone | 0.35 | 0.02 | 0.35 | 0.01 | 0.35 | ND |
| 13. ethyl propanoate | 0.22 | ND | 0.22 | ND | 0.22 | ND |
| 14. n-propyl acetate | 0.13 | ND | 0.13 | ND | 0.13 | ND |
| 15. ethyl butyrate | 0.11 | ND | 0.11 | ND | 0.11 | ND |
| 16. methyl butyrate | 0.20 | 0.02 | 0.20 | ND | 0.20 | 0.01 |
| 17. pentanol | 0.18 | ND | 0.18 | ND | 0.18 | 0.01 |
| 18. 1-penten-3-ol | 0.09 | ND | 0.09 | ND | 0.09 | ND |
| 19. ethyl-2-butenoate | 0.18 | ND | 0.18 | ND | 0.18 | ND |
| 20. 2-hexenal | 0.26 | 0.01 | 0.26 | 0.02 | 0.26 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peasura, N.; Sinchaipanit, P. The Impact of Sweetener Type on Physicochemical Properties, Antioxidant Activity and Rheology of Guava Nectar during Storage Time. Beverages 2022, 8, 24. https://doi.org/10.3390/beverages8020024
Peasura N, Sinchaipanit P. The Impact of Sweetener Type on Physicochemical Properties, Antioxidant Activity and Rheology of Guava Nectar during Storage Time. Beverages. 2022; 8(2):24. https://doi.org/10.3390/beverages8020024
Chicago/Turabian StylePeasura, Napassorn, and Pornrat Sinchaipanit. 2022. "The Impact of Sweetener Type on Physicochemical Properties, Antioxidant Activity and Rheology of Guava Nectar during Storage Time" Beverages 8, no. 2: 24. https://doi.org/10.3390/beverages8020024
APA StylePeasura, N., & Sinchaipanit, P. (2022). The Impact of Sweetener Type on Physicochemical Properties, Antioxidant Activity and Rheology of Guava Nectar during Storage Time. Beverages, 8(2), 24. https://doi.org/10.3390/beverages8020024