Combined Pulsed Electric Field with Antimicrobial Caps for Extending Shelf Life of Orange Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Juice Samples
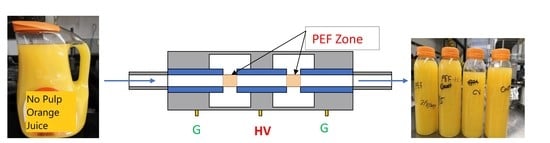
2.2. Treatments of Pulsed Electric Field
2.3. Antimicrobial Caps
2.4. Microbiological Analysis
2.5. Determinations of pH, Titratable Acidity, and Total Soluble Solids
2.6. Determination of Color
2.7. Determination of Ascorbic Acid (Vitamin C) Content
2.8. Determination of Total Phenolics Content
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Treatments on Microbial Reductions
3.2. Effect of Treatments on Quality Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurtler, J.B.; Rivera, R.B.; Zhang, H.Q.; Geveke, D.J. Selection of surrogate bacteria in place of E. coli O157:H7 and Salmonella Typhimurium for pulsed electric field treatment of orange juice. Int. J. Food Microbiol. 2010, 139, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Cheng, Z.; Mittal, G.S. Inactivation of spoilage microorganisms in apple cider using a continuous flow pulsed electric field system. LWT Food Sci. Technol. 2006, 39, 351–357. [Google Scholar] [CrossRef]
- Mendes-Oliveira, G.; Jin, Z.T.; Campanella, O.H. Modeling the inactivation of Escherichia coli O157:H7 and Salmonella Typhimurium in juices by pulsed electric fields: The role of the energy density. J. Food Eng. 2020, 282, 110001. [Google Scholar] [CrossRef]
- Mosqueda-Melgar, J.; Raybaudi-Massilia, R.M.; Martín-Belloso, O. Microbiological shelf life and sensory evaluation of fruit juices treated by high-intensity pulsed electric fields and antimicrobials. Food Bioprod. Process. 2012, 90, 205–214. [Google Scholar] [CrossRef]
- Mosqueda-Melgar, J.; Raybaudi-Massilia, R.M.; Martín-Belloso, O. Influence of treatment time and pulse frequency on Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes populations inoculated in melon and watermelon juices treated by pulsed electric fields. Int. J. Food Microbiol. 2007, 117, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Wang, L.-H.; Zeng, X.-A.; Wen, Q.-H.; Brennan, C.S.; Tang, Z.-S.; Wang, M.-S. Effect of ethanol adaption on the inactivation of Acetobacter sp. by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2019, 52, 25–33. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Shen, X.; Zhang, S.; Chen, X. Lethal and sublethal injury and kinetics of Escherichia coli, Listeria monocytogenes, and Staphylococcus aureus in milk by pulsed electric fields. Food Control 2013, 32, 6–12. [Google Scholar] [CrossRef]
- Caminiti, I.M.; Noci, F.; Muñoz, A.; Whyte, P.; Morgan, D.J.; Cronin, D.A.; Lyng, J.G. Impact of selected combinations of non-thermal processing technologies on the quality of an apple and cranberry juice blend. Food Chem. 2011, 124, 1387–1392. [Google Scholar] [CrossRef]
- Elez-Martínez, P.; Martín-Belloso, O. Effects of high intensity pulsed electric fields processing conditions on Vitamin C and antioxidant capacity of orange juice and gazpacho, a cold vegetable soup. Food Chem. 2007, 102, 201–209. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Lebovka, N.; Foucart, F.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field treatment of citrus fruits: Improvement of juice and polyphenols extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.; Geveke, D.J.; Fan, X.; Sites, J.E.; Wang, L. Evaluation of microbial stability, bioactive compounds, physicochemical properties, and consumer acceptance of pomegranate juice processed in a commercial scale pulsed electric field system. Food Bioprocess Technol. 2014, 7, 2112–2120. [Google Scholar] [CrossRef]
- Jin, T.Z. Pulsed electric fields for pasteurization: Defining processing conditions. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer International: Cham, Switzerland, 2017; pp. 2271–2295. [Google Scholar] [CrossRef]
- Jin, T.; Guo, M.; Zhang, H.Q. Upscaling from benchtop processing to industrial scale production: More factors to be considered for pulsed electric field food processing. J. Food Eng. 2015, 146, 72–80. [Google Scholar] [CrossRef]
- Jin, T.Z.; Guo, M.; Yang, R. Combination of pulsed electric field processing and antimicrobial bottle for extending microbiological shelf-life of pomegranate juice. Innov. Food Sci. Emerg. Technol. 2014, 26, 153–158. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, H.; Boyd, G. Incorporation of preservatives in polylactic acid films for inactivating Escherichia coli O157:H7 and extending microbiological shelf-life of strawberry puree. J. Food Prot. 2010, 73, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Chi-Zhang, Y.; Yam, K.L.; Chikindas, M.L. Effective control of Listeria monocytogenes by combination of nisin formulated and slowly released into a broth system. Int. J. Food Microbiol. 2004, 90, 15–22. [Google Scholar] [CrossRef]
- Jin, T.; Liu, L.S.; Zhang, H.; Hicks, K. Antimicrobial activity of nisin incorporated in pectin and polylactic acid composite films against List. Monocytogenes. Int. J. Food Sci. Technol. 2009, 44, 322–329. [Google Scholar] [CrossRef]
- Salmaso, S.; Elvassore, N.; Bertucco, A.; Lante, A.; Caliceti, P. Nisin-loaded poly-L-lactide nano-particles produced by CO2 anti-solvent precipitation for sustained antimicrobial activity. Int. J. Pharm. 2004, 287, 163–173. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, H.; Hermawan, N.; Dantzer, B. Effects of pH and temperature on inactivation of Salmonella DT104 in liquid whole egg by pulsed electric fields. Int. J. Food Sci. Technol. 2009, 44, 367–372. [Google Scholar] [CrossRef]
- Jin, T. Inactivation of Listeria monocytogenes in skim milk and liquid egg white by antimicrobial bottle coating with polylactic acid and nisin. J. Food Sci. 2010, 75, M83–M88. [Google Scholar] [CrossRef]
- Jin, T.; Gurtler, J.B. Inactivation of Salmonella in liquid egg albumen by antimicrobial bottle coatings infused with allyl isothiocyanate, nisin and zinc oxide nanoparticles. J. Appl. Microbiol. 2011, 110, 704–712. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Martínez-López, A.; Rodrigo, D. Cinnamon antimicrobial effect against Salmonella typhimurium cells treated by pulsed electric fields (PEF) in pasteurized skim milk beverage. Food Res. Int. 2012, 48, 777–783. [Google Scholar] [CrossRef]
- McNamee, C.; Noci, F.; Cronin, D.A.; Lyng, J.G.; Morgan, D.J.; Scannell, A.G.M. PEF based hurdle strategy to control Pichia fermentans, Listeria innocua and Escherichia coli K12 in orange juice. Int. J. Food Microbiol. 2010, 138, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ouazzou, A.; Cherrat, L.; Espina, L.; Lorán, S.; Rota, C.; Pagán, R. The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation. Innov. Food Sci. Emerg. Technol. 2011, 12, 320–329. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Espina, L.; García-Gonzalo, D.; Pagán, R. Synergistic combination of physical treatments and carvacrol for Escherichia coli O157:H7 inactivation in apple, mango, orange, and tomato juices. Food Control 2013, 32, 159–167. [Google Scholar] [CrossRef]
- Jin, T.Z.; Aboelhaggag, R.M.; Guo, M. Apple juice preservation using combined nonthermal processing and antimicrobial packaging. J. Food Prot. 2021, 84, 1528–1538. [Google Scholar] [CrossRef]
- Bhat, R.; Kamaruddin, N.S.B.C.; Liong, M.-T.; Karim, A. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason. Sonochem. 2011, 18, 1295–1300. [Google Scholar] [CrossRef]
- Ranganna, S. Proximate constituents. In Handbook of Analysis and Quality Control for Fruit and Vegetable Products, 2nd ed.; Ranganna, S., Ed.; Tata McGraw-Hill: New Delhi, India, 2001; pp. 12–17. [Google Scholar]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Sackey, A.S.; Wu, M.; Xiao, L. Effect of pulsed light treatment on the phytochemical, volatile, and sensorial attributes of lactic-acid-fermented mulberry juice. Int. J. Food Prop. 2018, 21, 213–228. [Google Scholar] [CrossRef]
- Aneja, K.R.; Dhiman, R.; Aggarwal, N.K.; Kumar, V.; Kaur, M. Microbes associated with freshly prepared juices of citrus and carrots. Int. J. Food Sci. 2014, 2014, 408085. [Google Scholar] [CrossRef] [Green Version]
- Schottroff, F.; Krottenthaler, A.; Jaeger, H. Stress induction and response, inactivation, and recovery of vegetative microorganisms by pulsed electric fields. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer Publisher: Berlin, Germany, 2017. [Google Scholar]
- García, D.; Hassani, M.; Mañas, P.; Condón, S.; Pagán, R. Inactivation of Escherichia coli O157:H7 during the storage under refrigeration of apple juice treated by pulsed electric fields. J. Food Saf. 2005, 25, 30−42. [Google Scholar] [CrossRef]
- Somolinos, M.; Garcia, D.; Condón, S.; Mañas, P.; Pagán, R. Relationship between sublethal injury and inactivation of yeast cells by the combination of sorbic acid and pulsed electric fields. Appl. Environ. Microbiol. 2007, 73, 3814−3821. [Google Scholar] [CrossRef] [Green Version]
- Somolinos, M.; Garcia, D.; Mañas, P.; Condón, S.; Pagán, R. Effect of environmental factors and cell physiological state on Pulsed Electric Fields resistance and repair capacity of various strains of Escherichia Coli. Int. J. Food Microbiol. 2008, 124, 260−267. [Google Scholar] [CrossRef] [PubMed]
- Mafeti, N.A.; Ramos-Villaroel, A.Y.; Nicolau, A.I.; Martín-Belloso, O.; Soliva-Fortuny, R. Influence of process parameters on the pulsed light inactivation of Penicillium expansum in apple juice. Food Control 2014, 41, 27–31. [Google Scholar] [CrossRef]
- Noci, F.; Riener, J.; Walkling-Ribeiro, M.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. Ultraviolet irradiation and pulsed electric fields (PEF) in a hurdle strategy for the preservation of fresh apple juice. J. Food Eng. 2008, 85, 141–146. [Google Scholar] [CrossRef]
- Palgan, I.; Caminiti, I.M.; Muñoz, A.; Noci, F.; Whyte, P.; Morgan, D.J.; Cronin, D.A.; Lyng, J.G. Effectiveness of High Intensity Light Pulses (HILP) treatments for the Control of Escherichia coli and Listeria innocua in apple juice, orange juice and milk. Food Microbiol. 2011, 28, 14–20. [Google Scholar] [CrossRef]
- Preetha, P.; Varadharaju, N.; Kennedy, Z.J.; Malathi, D.; Shridar, B. Evaluation of non-thermal process for decontamination of orange juice using a pulsed light system. Int. J. Food Ferment. Technol. 2015, 5, 121–128. [Google Scholar] [CrossRef]
- Pala, Ç.U.; Toklucu, A.K. Effect of UV-C light on anthocyanin content and other quality parameters of pomegranate juice. J. Food Compos. Anal. 2011, 24, 790–795. [Google Scholar] [CrossRef]
- Azhuvalappil, Z.; Fan, X.; Geveke, D.; Zhang, Q.H. Thermal and nonthermal processing of apple cider: Storage quality under equivalent process conditions. J. Food Qual. 2010, 33, 612–631. [Google Scholar] [CrossRef]
- Tran, M.T.T.; Farid, M. Ultraviolet treatment of orange juice. Innov. Food Sci. Emerg. Technol. 2004, 5, 495–502. [Google Scholar] [CrossRef]
- Schoenbach, K.H.; Joshi, R.P.; Stark, R.H. Bacterial decontamination of liquids with pulsed electric fields. IEEE Trans. Dieletrics Electr. Insul. 2000, 7, 637–645. [Google Scholar] [CrossRef]
- Rems, L.; Miklavčič, D. Tutorial: Electroporation of cells in complex materials and tissue. J. Appl. Phys. 2016, 119, 201101. [Google Scholar] [CrossRef]
- Zimmermann, U. Electric breakdown, electropermeabilization and electrofusion. Rev. Physiol. Biochem. Pharmacol. 1986, 105, 175–256. [Google Scholar]
- Kranjc, M.; Miklavčič, D. Electric Field Distribution and Electroporation Threshold. In Handbook of Electroporation; Damijan Miklavcic, D., Ed.; Springer Publisher: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Food and Drug Administration (FDA). Guidance for Industry: Juice Haccp Hazards and Controls Guidance, 1st ed.; Final Guidance. 2004. Available online: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/Juice/ucm072557.htm (accessed on 14 October 2022).



| Storage (Weeks) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| Control | 3.91 ± 0.01 a | 3.93 ± 0.01 a | 3.80 ± 0.02 a | 3.88 ± 0.01 a | 3.91 ± 0.01 a | 3.76 ± 0.01 a | |
| pH | PEF | 3.90 ± 0.01 a | 3.89 ± 0.01 b | 3.79 ± 0.02 a | 3.88 ± 0.01 a | 3.90 ± 0.01 a | 3.76 ± 0.01 a |
| AP | 3.91 ± 0.01 a | 3.87 ± 0.01 b | 3.78 ± 0.02 a | 3.88 ± 0.01 a | 3.90 ± 0.01 a | 3.76 ± 0.01 a | |
| PEF+AP | 3.90 ± 0.01 a | 3.88 ± 0.01 b | 3.78 ± 0.02 a | 3.88 ± 0.01 a | 3.90 ± 0.01 a | 3.76 ± 0.01 a | |
| Control | 3.15 ± 0.05 a | 3.14 ± 0.03 b | 3.49 ± 0.02 a | 3.53 ± 0.03 a | 3.51 ± 0.02 a | 3.76 ± 0.02 a | |
| Acidity | PEF | 3.18 ± 0.06 a | 3.23 ± 0.03 a | 3.48 ± 0.04 a | 3.50 ± 0.02 a | 3.53 ± 0.03 a | 3.68 ± 0.02 b |
| (mg/mL) | AP | 3.15 ± 0.05 a | 3.21 ± 0.03 a | 3.51 ± 0.02 a | 3.51 ± 0.04 a | 3.51 ± 0.01 a | 3.71 ± 0.02 b |
| PEF+AP | 3.18 ± 0.06 a | 3.21 ± 0.02 a | 3.49 ± 0.02 a | 3.55 ± 0.02 a | 3.50 ± 0.01 a | 3.69 ± 0.02 b | |
| Control | 11.23 ± 0.06 a | 11.03 ± 0.06 a | 10.93 ± 0.12 a | 11.17 ± 0.06 a | 11.00 ± 0.01 a | 11.03 ± 0.06 a | |
| TSS | PEF | 11.10 ± 0.11 a | 10.90 ± 0.10 a | 11.07 ± 0.12 a | 11.27 ± 0.12 a | 10.97 ± 0.06 a | 10.90 ± 0.01 a |
| AP | 11.23 ± 0.06 a | 11.10 ± 0.10 a | 11.17 ± 0.06 a | 11.33 ± 0.12 a | 11.10 ± 0.10 a | 11.00 ± 0.01 a | |
| PEF+AP | 11.10 ± 0.10 a | 11.13 ± 0.12 a | 11.13 ± 0.12 a | 11.30 ± 0.02 a | 11.10 ± 0.01 a | 11.17 ± 0.07 a | |
| Control | 31.17 ± 0.31 a | 29.87 ± 0.55 a | 28.53 ± 0.67 a | 29.13 ± 0.45 a | 28.80 ± 0.26 a | 28.47 ± 0.21 a | |
| Color L* value | PEF | 30.20 ± 0.56 a | 29.80 ± 0.53 a | 29.97 ± 0.93 a | 29.13 ± 0.15 a | 28.57 ± 0.31 a | 28.10 ± 0.10 a |
| AP | 31.17 ± 0.32 a | 28.37 ± 0.15 a | 27.97 ± 0.23 a | 28.37 ± 0.61 a | 28.57 ± 0.38 a | 28.53 ± 0.23 a | |
| PEF+AP | 30.20 ± 0.45 a | 30.27 ± 0.25 a | 29.47 ± 1.21 a | 28.43 ± 0.49 a | 29.07 ± 0.15 a | 27.97 ± 0.15 a | |
| Control | 14.43 ± 0.51 a | 14.60 ± 1.01 a | 14.30 ± 0.36 a | 15.67 ± 0.85 a | 14.97 ± 0.31 a | 16.40 ± 0.60 a | |
| Color a* value | PEF | 15.97 ± 0.41 a | 16.33 ± 0.42 a | 13.73 ± 1.10 a | 15.23 ± 1.33 a | 14.17 ± 0.90 a | 15.30 ± 0.46 b |
| AP | 14.43 ± 0.55 a | 15.17 ± 0.25 a | 13.77 ± 1.84 a | 13.80 ± 0.26 b | 13.97 ± 0.50 a | 15.13 ± 0.49 b | |
| PEF+AP | 15.97 ± 0.46 a | 14.93 ± 0.25 a | 14.73 ± 0.55 a | 13.93 ± 0.12 b | 14.60 ± 0.36 a | 15.60 ± 0.87 b | |
| Control | 4.67 ± 0.35 a | 4.27 ± 0.21 a | 4.33 ± 0.31 b | 3.73 ± 0.06 a | 4.53 ± 0.35 a | 3.73 ± 0.12 a | |
| Color b* value | PEF | 3.97 ± 0.25 a | 3.53 ± 0.31 b | 4.57 ± 1.04 b | 3.83 ± 0.70 a | 4.17 ± 0.35 a | 3.97 ± 0.15 a |
| AP | 4.67 ± 0.24 a | 3.47 ± 0.15 b | 4.23 ± 1.05 b | 3.83 ± 0.35 a | 4.37 ± 0.38 a | 4.03 ± 0.61 a | |
| PEF+AP | 3.97 ± 0.33 a | 3.93 ± 0.25 a | 5.10 ± 0.30 a | 4.20 ± 0.56 a | 4.57 ± 0.21 a | 4.00 ± 0.20 a | |
| Total phenol(µg/100µL) | Control | 75.47 ± 3.21 a | 65.59 ± 2.88 a | 77.21 ± 3.32 b | 74.97 ± 1.55 a | 66.32 ± 3.08 b | 66.15 ± 1.65 a |
| PEF | 73.55 ± 3.25 a | 65.71 ± 2.97 a | 76.53 ± 3.44 b | 73.55 ± 2.12 a | 67.03 ± 2.79 b | 67.74 ± 2.33 a | |
| AP | 75.47 ± 3.33 a | 65.32 ± 1.99 a | 78.01 ± 1.09 b | 75.11 ± 2.33 a | 68.89 ± 2.55 b | 68.53 ± 1.12 a | |
| PEF+AP | 73.55 ± 3.09 a | 66.30 ± 2.03 a | 88.28 ± 1.77 a | 75.12 ± 2.55 a | 72.34 ± 2.12 a | 69.24 ± 1.34 a | |
| Control | 34.44 ± 1.12 a | 26.83 ± 1.23 a | 16.47 ± 1.77 b | 12.58 ± 1.22 b | 5.91 ± 0.77 b | 0.83 ± 0.05 b | |
| Vitamin C | PEF | 33.98 ± 1.09 a | 26.86 ± 0.98 a | 22.58 ± 1.45 a | 16.86 ± 2.09 a | 16.86 ± 1.13 a | 5.55 ± 0.30 a |
| (mg/100 mL) | AP | 34.44 ± 1.11 a | 27.30 ± 1.21 a | 15.83 ± 0.79 b | 16.66 ± 1.39 a | 4.25 ± 0.59 b | 0.92 ± 0.12 b |
| PEF+AP | 33.98 ± 1.03 a | 27.11 ± 1.07 a | 22.41 ± 1.10 a | 8.52 ± 1.32 c | 2.97 ± 0.32 c | 1.02 ± 0.16 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, T.Z.; Aboelhaggag, R.M. Combined Pulsed Electric Field with Antimicrobial Caps for Extending Shelf Life of Orange Juice. Beverages 2022, 8, 72. https://doi.org/10.3390/beverages8040072
Jin TZ, Aboelhaggag RM. Combined Pulsed Electric Field with Antimicrobial Caps for Extending Shelf Life of Orange Juice. Beverages. 2022; 8(4):72. https://doi.org/10.3390/beverages8040072
Chicago/Turabian StyleJin, Tony Z., and Ramadan M. Aboelhaggag. 2022. "Combined Pulsed Electric Field with Antimicrobial Caps for Extending Shelf Life of Orange Juice" Beverages 8, no. 4: 72. https://doi.org/10.3390/beverages8040072
APA StyleJin, T. Z., & Aboelhaggag, R. M. (2022). Combined Pulsed Electric Field with Antimicrobial Caps for Extending Shelf Life of Orange Juice. Beverages, 8(4), 72. https://doi.org/10.3390/beverages8040072