Abstract
This work presents the study of the effectiveness of different inert gases applied during racking to prevent oxygen uptake by wine. Inert gases were used for the purging of empty tanks and hoses before the start of each racking, as well as for blanketing in the full racked tank. After analyzing these operations with the different inert gases, the required volumes of each gas were optimized. The CO2:Ar (20:80) mixture proved to be the most effective for the complete purging of the empty tank, while CO2 was the most cost-effective gas. Purging the empty tank with 25% vessel volume gas was sufficient to achieve useful inerting with all the gases studied, as well as to maintain low levels of dissolved oxygen (DO) in the wine filling the tank. Applying 0.5 of vessel volume of Ar, CO2:Ar (20:80), and CO2 gases during blanketing allowed the headspace oxygen (HSO) of the racked tank to be protected throughout. During the racking of a white wine in a commercial winery, Ar showed the highest efficiency, compared to N2, for both the inerting of empty hoses and destination tank and for maintaining low levels of DO and HSO in the tank.
Keywords:
inert gases; racking; purging; blanketing; flushing; dissolved oxygen; wine; nitrogen; carbon dioxide; argon 1. Introduction
The presence of atmospheric oxygen generates oxidation phenomena in wine that affect their physicochemical and sensory evolution. During the winemaking process, there are multiple stages where the concentration of dissolved oxygen is increased, intentionally or unintentionally, through the treatments or equipment used, from the crushing of the grapes to the bottling or packaging of the final wine [1,2,3,4], and the contribution of oxygen is unavoidable. The oxygen uptake in the winemaking stages (racking, fining, pumping, filtration, low temperature treatments, and bottling) is generally considered to be negative for the sensory characteristics of white and rosé wines [1,5,6,7]. Wine racking is a critical point of oxygen uptake, as the large surface area of the wine exposed during this operation and the inability to maintain an effective inert gas blanket over it result in the exchange of large amounts of gases [1,5,6,7]. All this causes more oxygen to be dissolved in the wine, which can gain between 2.5 and 5 mg/L of dissolved oxygen, depending on the technique and the technology used [8,9]. The speed of moving the wine during racking, the type of pump, the material of the hoses, and the type of fittings and gaskets used are critical factors to be controlled in order to minimize the transfer of oxygen to the wine [10]. During transfer, there are three main areas for consideration in preventing wine oxidation: the tank being emptied, the tank being filled, and the hoses, lines, and pumps [11]. This uptake of oxygen affects the protection of the wine, due to the consumption of the sulfur dioxide present in it. It is known that 1.7 moles of SO2 react with one mole of oxygen [12], so that, in this operation, the wine would lose between 10 and 20 mg/L of free SO2. In recent years, the demand for softer, fruitier, and more aromatic wines has forced the industry to make great efforts to produce wines of the highest quality. To this end, inert gases, such as nitrogen (N2), carbon dioxide (CO2), or a mixture of both in different proportions, are essential in modern winemaking technology. N2 is the most commonly used gas, but its disadvantage is that it has a similar density to air (air: 1.20 kg/m3; N2: 1.17 kg/m3 at 20 °C and 1 bar), so it mixes with air, depleting its oxygen, rather than displacing it, making it less effective [13]. CO2 is denser (1.84 kg/m3 at 20 °C and 1 bar) and displaces air. However, when high purity CO2 is used, its solubility in wine is a drawback, so it is mainly mixed with N2 (CO2:N2 (20:80) is 1.30 kg/m3 at 20 °C and 1 bar) [13]. Other gases, such as argon (Ar) (1.66 kg/m3 at 20 °C and 1 bar) mixed with CO2, have also been recommended for their good results in inerting processes because they are denser (CO2:Ar (20:80) is 1.70 kg/m3 at 20 °C and 1 bar) and heavier than air and can displace the O2 more effectively [10,13,14]. However, CO2 is very soluble in wines (107 L/hL at 20 °C) [10], so it is not highly recommended for inerting the various vessels containing still wine in a winery. Ar is barely soluble in wine (4 L/hL at 20 °C) and has a limited practical use in the winemaking sector, due to its high price [10], but as it is more efficient than other gases, less volume is required to achieve the inerting of tanks and hoses, so it could be more economical. On the other hand, N2 is the most frequently used inert gas and is easily obtained from air via adsorption by pressure change [15]. The purpose of the application of these gases is the preservation of wine from oxidation, since the exhaustive control of the oxygen that can dissolve allows the wine to remain as unaltered as possible for long periods of time, avoiding the occurrence of chemical and enzymatic oxidations, which can lead to wine browning [16], certain aromatic modifications that generate unpleasant aromas, or the loss of fruity aromas in wines [17].
Very few studies report on the control of oxygen uptake into the wine during racking or transfer of wine between tanks, and operations always present in the winemaking process. The wine in the starting tank, which is being emptied, is in contact with the air that occupies the headspace between the surface of the wine and the top of the tank. A common practice, in order to inert this zone by blanketing, is to introduce an undetermined amount of a common inert gas, such as N2 or CO2, until the gas coming out of the tank extinguishes a flame, for example, with a lighter or match. The minimum oxygen concentration (MOC) is defined as the limiting concentration of oxygen below which combustion is not possible, which for N2-air/propane-wood is 11.5–17% and for CO2-air/propane-wood is 14.5–17%, respectively [18]. That inevitably creates a false sense of protection from O2. Another common option is to burn a “sulfur straw-pill” to inertize that headspace or simply do nothing. A practice that is quite common in other sectors, but hardly used in oenology, is the inerting of the destination tank to which a wine is to be racked. This inerting process consists of removing the air in the tank with inert gases to prevent the wine that enters the tank from coming into contact with the air [13,19,20,21].
Therefore, there is a need within the winemaking sector to know, from a scientific–technical point of view, which are the critical points of oxygen uptake during racking when no inert gas is applied. Once these points have been identified in terms of the amount of oxygen that the wine receives during racking, it is necessary and of significant importance for the sector to establish a protocol on how, when, and in what quantities the inert gases most highly recommended for the winemaking sector should be added during racking. The ultimate goal is to minimize the amount of oxygen that is added to the wine in this process, in order to be able to apply it in commercial wineries and, thus, avoid undesired and unknown oxygen uptakes in the wine, which can lead to an overdose of sulfur dioxide.
Therefore, the aim of this work was to study the uptake of oxygen during the racking of a model wine without applying any inert gas and to compare it with what happens when different inert gases are applied, both in the inerting of the destination tank (purging or flushing) and during the protection by gaseous blanket (blanketing) of the wine in the racking tank. Although there is abundant information for the petrochemical industry, there is very little bibliography on the optimization of the volume of gas to be applied in these inerting operations with oenological criteria. Finally, a full-scale inerting study was carried out in a commercial winery during the racking of a white wine: the objective was to evaluate the effectiveness of the use of different gases to minimize the uptake of oxygen in this process.
2. Materials and Methods
2.1. Wine
2.1.1. Model Wine
A model wine consisting of a 12.5% v/v hydroalcoholic solution (food-grade alcohol) with characteristics similar to those of wine was used (pH = 3.5). It does not consume oxygen and was used in order to quantify oxygen incorporation. The model wine was degassed before each racking through two membrane contactors, i.e., Liqui-Cel® 4 × 13 extra-flow modules (3M, Maplewood, Ramsey, MN, USA), which were used in series mode. The Liqui-Cel® modules were operated in the so-called transverse-flow for the liquid, meaning that the model wine flowed on the shell side of the membrane module. A low pressure of Pa = 60 mbar (vacuum) was maintained inside the hollow-fiber membranes (tube or lumen side of the module), and nitrogen was supplied as a stripping gas, working together in combo mode at a flow rate of 10 L/min until the oxygen content was <1 hPa. The model wine was then treated in a liquid disinfection system with a high-intensity germicidal ultraviolet radiation system.
2.1.2. Commercial Wine
Tests in a real situation were carried out at the Bodega Emina sited in Rueda appellation of origin (Medina del Campo, Spain) during the production of white wine elaborated with Verdejo grapes.
2.2. Inert Gases
The inert gases used were those usually indicated for cellar use: N2, CO2, Ar, and the mixtures CO2:N2 (20:80) and CO2:Ar (20:80), whose properties have been described in previous works [10] and were provided by Carburos Metálicos (Air Products Group, Barcelona, Spain). The gases were supplied in 50L High Pressure Standard Cylinders and a high flow pressure reducer (flow rate 0–15 m3/h referred to N2 and an inlet pressure of 50 bar) mod. RBDI-XL 200 0-2 bar supplied by Carburos Metálicos (Air Products Group, Barcelona, Spain) was used. These gases were dosed with a rotameter calibrated for air (flow rate 0–10 m3/h), together with an absolute pressure manometer mod. CPG1500 WIKA (Barcelona, Spain) that also measures temperature, which allowed calculation of the volumetric flow rate qair (m3/h). Using the specific gas constant R (J/kg⋅K) for each gas, the correction factor Cf = (Rgas/Rair) 0.5 was determined, and the equivalent flow qe = Cf. qair measured for each gas at all times was calculated (with Cf for N2 = 1.01683; CO2 = 0.81127; Ar = 0.85152; CO2:N2 = 0.97918; and CO2:Ar = 0.84362).
2.3. Racking
The studies of optimization and effect of the application of inert gases during the racking of the model wine were carried out in the experimental winery of the University of Valladolid (Palencia Campus) using two 1800 L capacity tanks. Two DN32 butyl rubber hoses were used for the connections, one 7 m long and the other 3 m long, and the connections were all DIN 11851 with Viton seals. Butyl rubber was used for the hoses and Viton for the seals, due to the fact that these were the materials that gave the best results in a previous work published by our group [10]. A flexible impeller pump was used (Volumex 30/40, BCM Enología s.r.l., Padova, Italy) with a flow rate of 6000 L/h. For the studies carried out at the commercial winery, tanks of 25,000 L, butyl DN50 hoses, and an impeller pump at a nominal flow rate of 18,750 L/h were used.
2.4. Dissolved Oxygen Measurement Equipment
For the oxygen measurements carried out both in the commercial winery and at the winery in the University10-m-long Oxygen Dipping Probes DP-PSt6, accurate to ±1 ppb or ±3% of the respective concentration, were used connected to two OXY-4 trace measuring devices (PreSens GmbH, Regensburg, Germany). All equipment was periodically calibrated at two points, according to the manufacturers’ instructions, using a GM-3 gas mixer (SensorSense, Nijmegen, The Netherlands).
2.5. Purging of Tanks and Hoses
One of the methods of inerting is purging or flushing, which consists of eliminating, by displacement, air or any unwanted gas from a given system by introducing an inert gas into the system [13,19,20,21,22]. Inerting can be total or partial, the latter being the one in which the oxygen concentration is reduced to the level considered acceptable for each application. In oenology, this is when the oxygen concentration in the air is less than 0.5% [13,19,20,21,22,23]. As mentioned in the literature, there are several different purging methods, such as displacement, dilution, pressure swing, and vacuum purging. In the winery, the first two are the most common: displacement purging requires a low flow rate to avoid turbulences with velocities lower than 10 m/s and is recommended for tall and narrow vessels, with a high height/diameter (H/D) ratio. The use of an inert gas with a density higher than that of the gas to be displaced, in our case air is, thus, ideal. When the vessel to be inerted has a low H/D ratio, it is more advisable to use dilution purging, which consists of injecting an inert gas to reduce the oxygen concentration, and more vessel volume changes are needed. The number of vessel changes i can be determined according to Equation (1), and the volume of inert gas required VN can be determined according to Equation (2) [22]:
where Ca is the initial concentration, Ce is the final concentration
and VB is the vessel volume. For the case of N2 in a dilution purging i varies between 3.2 and 4.6 [22].
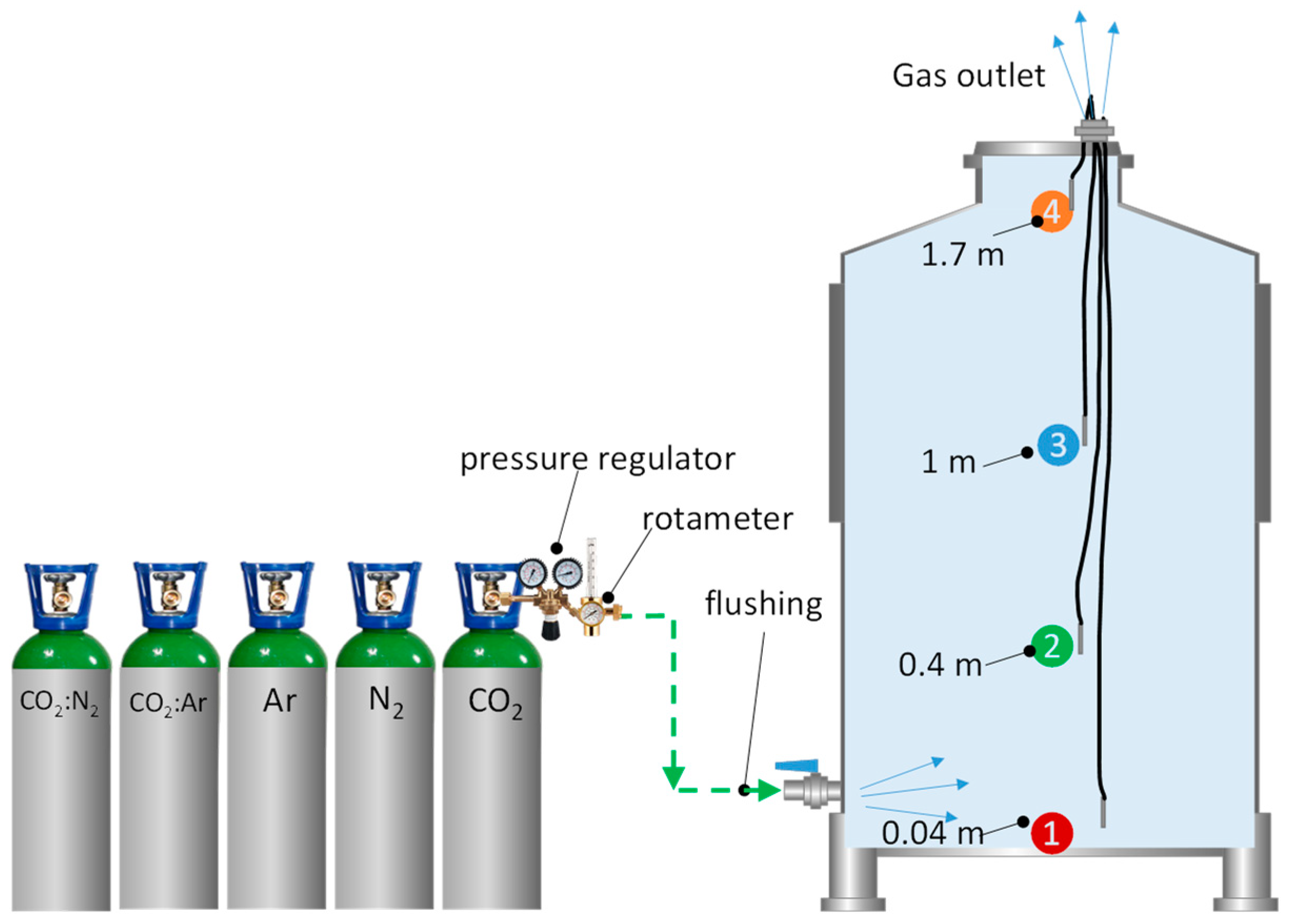
In this work, 4 probes were placed to monitor the inertization of the tank (Figure 1). Inertization by purging of the entire volume of the destination tank with the different gases was monitored with the probe placed at the top of the tank at 1.7 m (Figure 1, number 4). Inerting of the tank was optimized by forming layers of different thickness with the different inert gases studied and monitored with the probes placed inside the tank, at 0.04 m, 0.4 m, and 1 m in height from the bottom, and introduced through a connector in the upper lid (Figure 1). Each gas was injected through a valve at the bottom of the tank, since the most efficient way to gas a tank is through the bottom valve [19] via a connector that allows for monitoring.
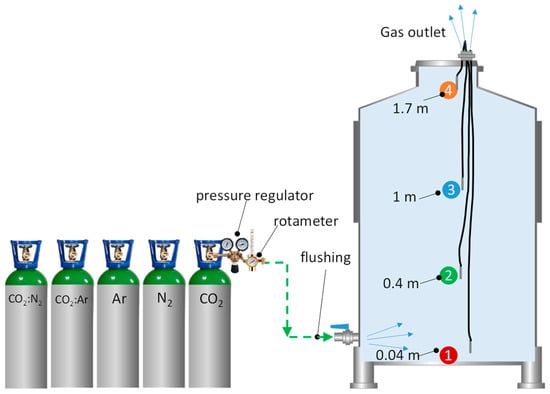
Figure 1.
Schematic diagram of the monitoring system for total and sequential inerting in an empty tank to compare the different inert gases (Purging), as well as the different probes placed at different heights.
Next, the inert gas bottle to be used was opened, and the pressure was adjusted with a pressure gauge to 1013 mba (standard atmospheric pressure) by regulating the desired gas flow rate. Purging was terminated when the different probes placed at different heights that measured the partial pressure of oxygen (pO2) reached a value of pO2 < 1 hPa (Figure 1). The probes were then carefully removed, and the connector through which the probes were inserted was closed to prevent the entry of oxygen.
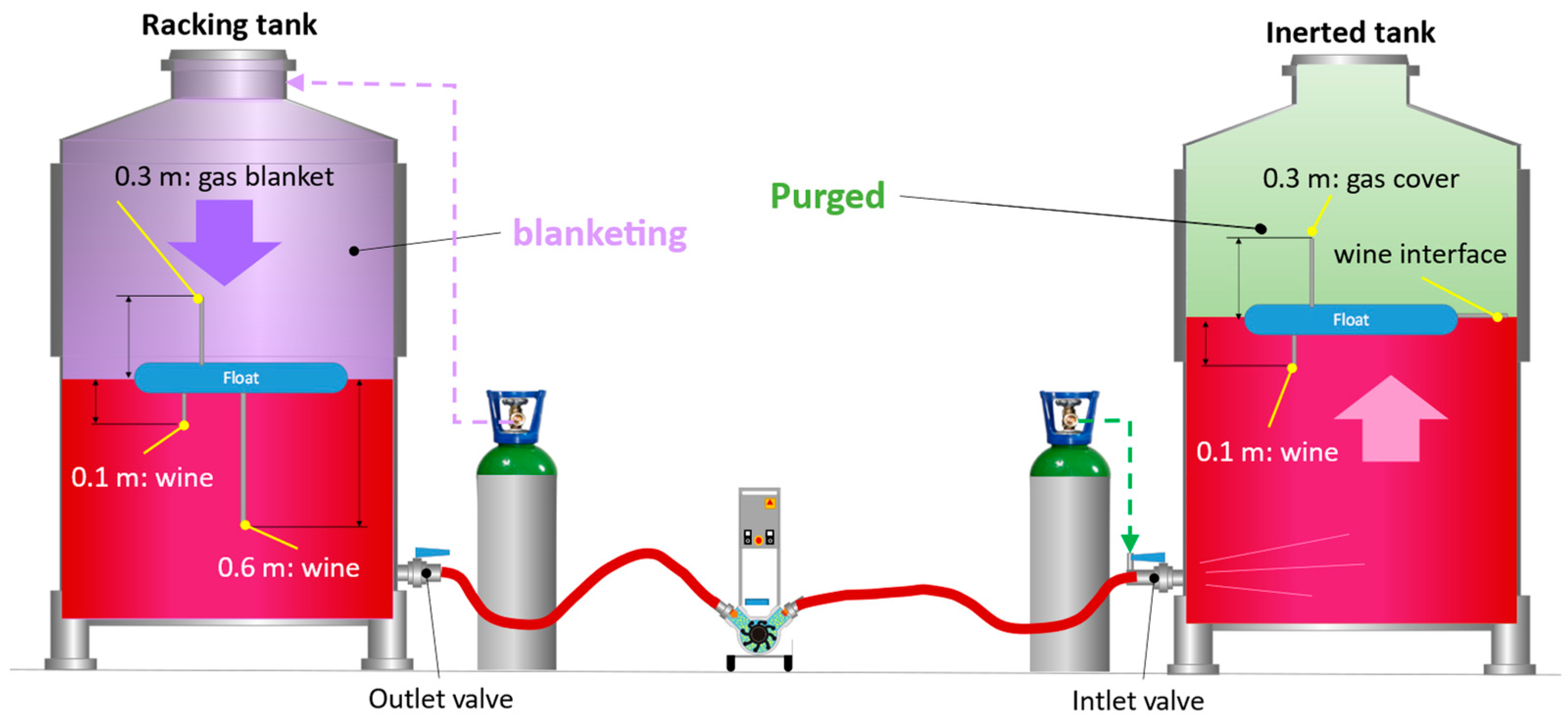
Once the oxygen had been evacuated from the interior of the destination tank, and before starting the wine racking, the hoses were also inerted until pO2 < 1 hPa values were reached. For the inertization of the hoses, the protocol established in the previously published work [10] was followed and monitored with the probes located in the outlet and inlet valves, as shown in Figure 2. Once inertization of the hoses was completed and the whole unit was free of oxygen, racking was started with the usual precaution of opening an outlet at the top for the air/gas displaced by the wine when filling the tank. The position of the probes in the racking and target tanks are attached to the respective floats (Figure 2). The floats move as the racking tank empties or the empty one fills, but the position measurement of the probes does not change: 0.1 m below the surface of the wine, 0.6 m below the surface of the wine, and 0.3 m above the surface of the wine.
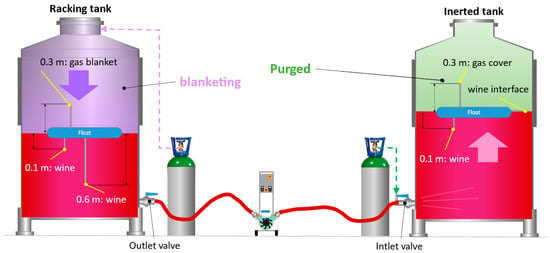
Figure 2.
Arrangement of the application of gases for purging, blanketing, and oxygen monitoring during wine racking. Detail of the placement of oxygen measurement probes fixed on the float.
According to the literature consulted, a perfect inerting of the empty destination tank is one in which the replacement of air by a purge gas is carried out completely by displacement, and only a volume of the tank to be inerted is required [19]. However, in the winemaking industry, when this type of inerting is carried out by diluting purging with N2, between 2 and 5 of vessel volume changes are usually applied [21]. In order to optimize the inerting process of the large tanks of most wineries (usually with an H/D > 1), in addition to the inerting of the entire tank volume, smaller volumes were tested. The strategy tested was to perform a displacement purging by forming a blanket of inert gas (mostly denser than air) at the bottom of the destination tank at a low flow rate, which, when filled with wine at the bottom, allowed the blanket of inert gas to accompany the wine until the tank was completely filled. For this purpose, in addition to the study of inerting with the total volume of the tank, we studied the formation of a blanket of inert gas (pO2 < 1 hPa) of only 0.04 m (3% of the tank volume), also with a blanket of 0.4 m (25% of the tank volume), and finally with a blanket of 1 m (60% of the tank volume).
2.6. Blanketing: Inert Gas Cover of the Headspace in the Racking Tank
Blanketing consists of creating a blanket of inert gas to cover the wine in the racking tank, a layer that will accompany the wine during the emptying of the tank to protect it from the oxygen in the air that enters through the upper opening of the tank. In the petrochemical industry, a blanketing process with N2 must be carried out with a gas volume several times the volume of liquid that is removed from the tank, since it is still a dilution purging process. In the case of the winemaking industry, the aim is not to avoid explosions, and it seems logical to think that it will be sufficient to introduce a volume of gas into the tank equal to the volume of liquid to be extracted, thus avoiding the entry of air and the possibility of the oxygen it contains being incorporated into the wine. In this work, the volumes of each inert gas injected for blanketing during the emptying of the raking tank were 1, 0.5, and 0.25 of the vessel volume. The objective was to find the lower limit of the number of vessel volumes of gas that would allow an accompaniment of the wine protecting it from oxygen from the air entering the tank during emptying. This seemed feasible, since several of the gases studied are denser than air, and presumably, an inert blanket could form on top of the wine during tank emptying. To inject each gas, the tank lids were adapted with quick connectors, allowing the inert gas to be introduced easily, while keeping the tank lid closed, thus preventing the entry of air. At the beginning of the operation, the tank was completely filled with wine, and there was a small ullage between the surface of the wine and the top of the tank. During tests, it was ensured that this small gas space was practically free of O2 (pO2 < 10 hPa) by flushing with the gas to be tested prior to racking. For this purpose, the gas cylinder first had to be opened, and the tank ullage was flushed with the tested inert gas. Then racking was started by activating the pump and the volume of gas corresponding to the volume of wine being racked into the destination tank was injected. Once the volume of gas to be tested was injected, the gas inlet was closed, and air was allowed to enter until the end of racking in the cases of 0.5 and 0.25 of vessel volume of inert gas.
3. Results and Discussion
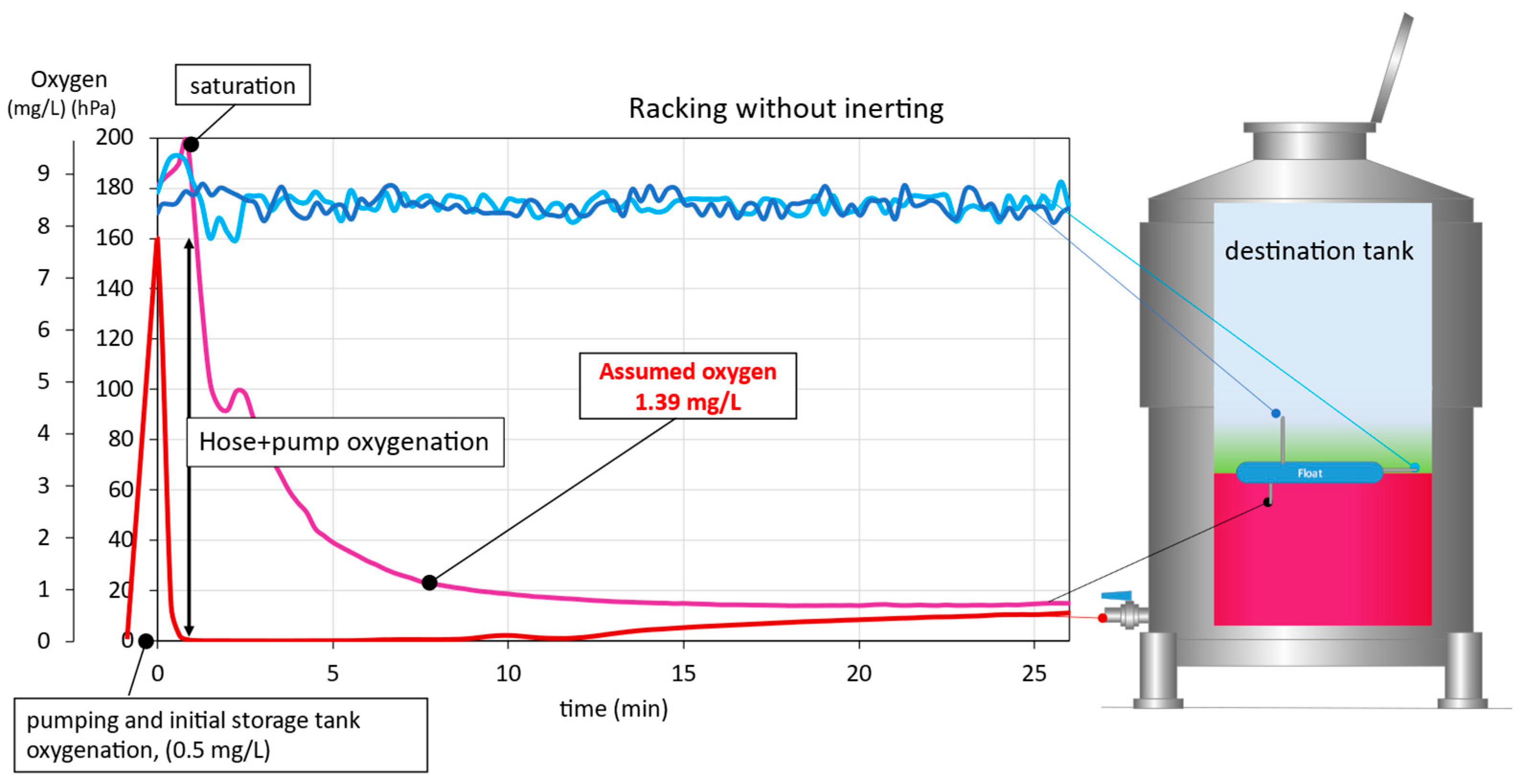
As can be seen in Figure 3, during racking without the use of inert gas, the destination tank was filled with air. The model wine arrived at the inlet valve of the destination tank virtually free of DO, but upon entering the destination tank during the first 2 min of racking the wine reached the DO saturation level (O2 > 200 hPa; roughly > 9 mg/L since O2 has different solubilities in water, juice, and wine, with solubility varying with temperature, pressure, alcoholic strength, sugars, polysaccharides, and phenols) [24]. This increase was most likely due to the violent entry of the wine into the destination tank, as droplets formed on the tank walls and a large surface area of liquid was in contact with the gas inside the tank (air, in this case), increasing the DO content in the wine. As the destination tank was filled, the DO concentration in the model wine decreased. Taking into account the DO incorporated during that time and the volume of wine affected, the mean equivalent oxygenation received by the wine was estimated at 1.39 mg/L. In this case, this oxygen incorporation would affect the protection of a real wine, since the wine would lose 5.6 mg/L of SO2, due to the consumption of the sulfur dioxide present [12]. A continuous incorporation of DO could be observed as the wine was racked, since after 12 min, the wine reaching the inlet valve of the destination tank showed certain levels of DO that could only have been acquired in the racking tank (Figure 3). This could have been due to the incorporation of O2 from the air, while the racking tank was being emptied in the absence of blanketing.
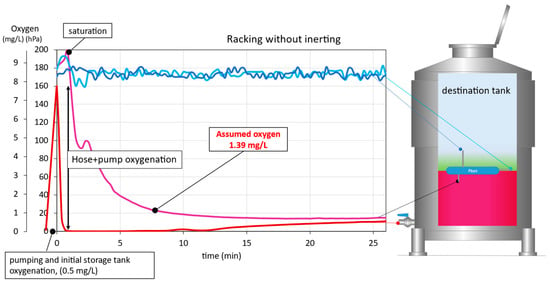
Figure 3.
Wine DO evolution in the destination tank during racking without inertization.
Based on this first reference racking, different tests were proposed to inertize both the destination tank and the headspace of the racking tank, which increased as it was emptied. Oxygen content monitoring was carried out as shown in Figure 2.
3.1. Determination of the Number of Vessel Volumes of Inert Gas for Purging
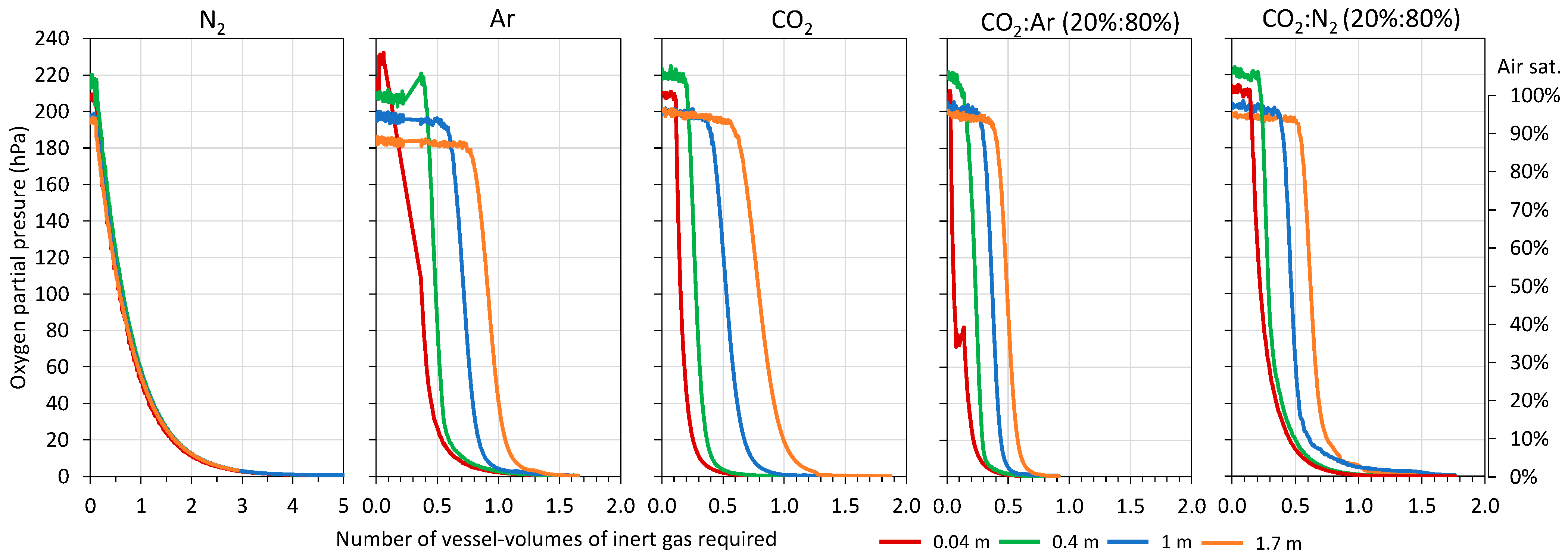
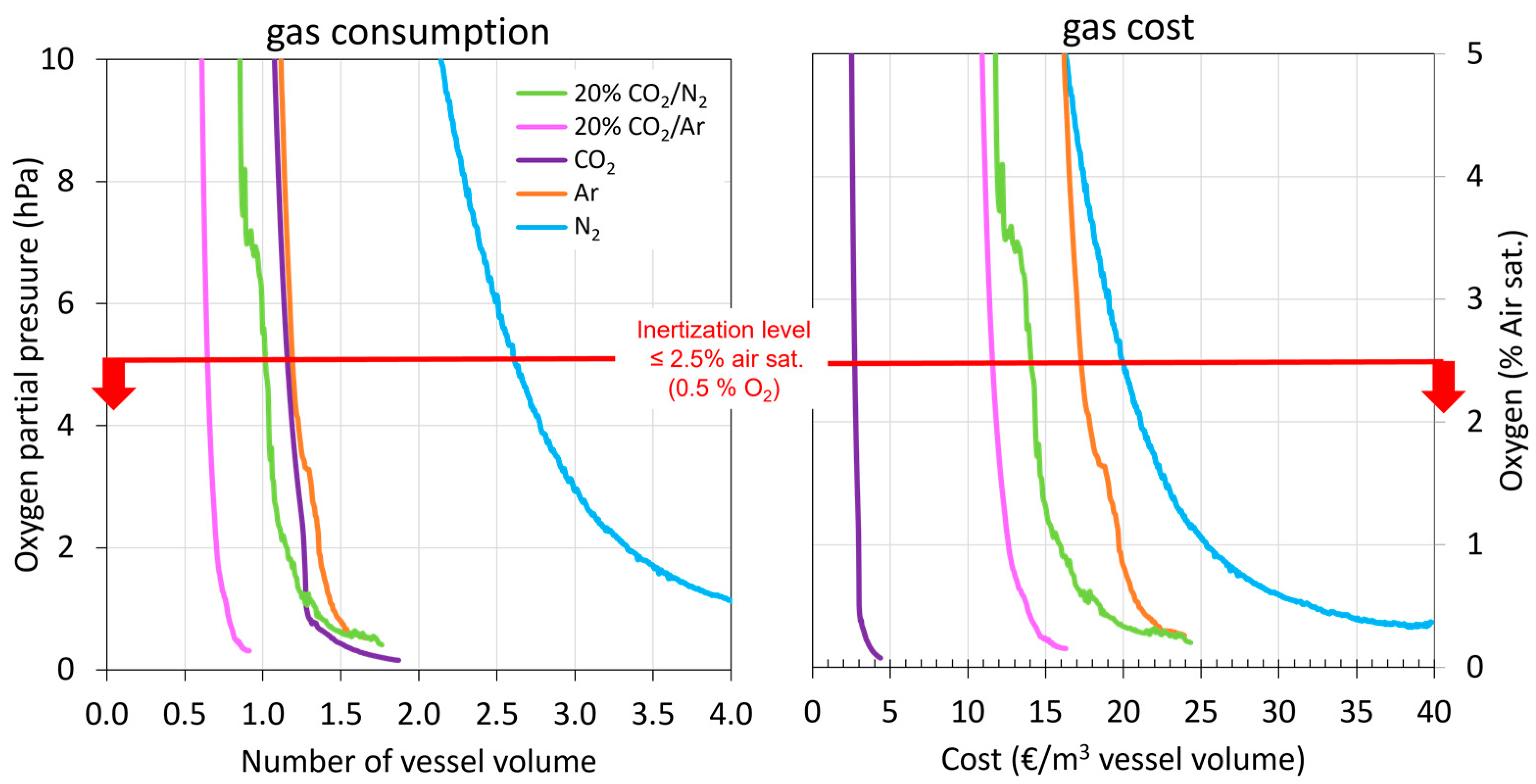
Initially, the necessary volume of each gas was determined to achieve complete inerting of the destination tank (1800 L) to be filled with model wine (Figure 4). The literature recommends O2 values in wine tanks lower than 2.5 % air sat. (0.5% O2 = 5 hPa) to be considered inertized, and for this, between 3 and 7 vessel volumes of N2 as inert gas need to be incorporated [20,21,22]. First, the volume of each gas needed to achieve the recommended inertization throughout the destination tank (blanket of inert gas corresponding to 1.7 m height at the top of the tank) that was studied, and Figure 4 shows the evolution of the O2 content when the different gases were injected to achieve values of 5 < hPa, equivalent to an O2 content of less than 0.5%. In addition, Figure 4 shows how the inert gas progressively formed a blanket from the bottom of the tank that increased in height with the volume injected. It is possible to see the volume of each gas studied that was necessary in order to form a protective blanket of inert gas with thicknesses of 0.04 m (corresponding to 3% of the tank volume), 0.4 m (corresponding to 25% of the tank volume), and 1 m (corresponding to 60% of the tank volume) and that allowed O2 values lower than 0.5% to be maintained. The results obtained showed that it is possible to make blankets of different thicknesses and that they are maintained in the lower part of the tank. Thus, the volume of gas needed to inertize increased as the gas blanket to be formed increased (0.04 m, 0.4 m, 1 m, and 1.7 m). For the complete inerting of the destination tank (1.7 m), the lowest volume of inert gas used was with the CO2:Ar (20:80) mixture with 0.7 vessel volume. Next, and in increasing order, the CO2:N2 mixture (20:80) with 1 vessel volume, CO2, Ar with 1.25 vessel volume, and finally, N2 with 2.7 vessel volume were in agreement with the values offered by the literature and showed that this gas is more suitable for diluting purging than for displacement purging. Therefore, the lowest inert gas consumption was obtained using the CO2:Ar mixture, clearly the most effective. These results were to be expected, since CO2 and Ar are the denser and heavier gases and, therefore, may be able to perform displacement purging faster [25,26]. In contrast, N2 tends to dissolve with O2 and fails to displace it [10,13].
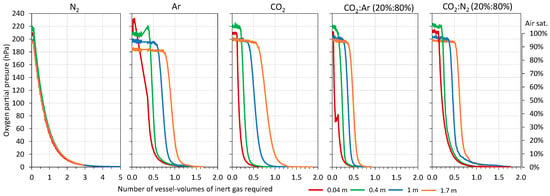
Figure 4.
Monitoring of oxygen content during purging of the empty destination tank with different gases.
Apart from the volume of inert gas used, it is important to take into account the economic cost of applying these gases for the wineries in the oenological sector. Therefore, in terms of economic cost (€/m3 of tank capacity), CO2 inerting was clearly the best, while Ar and N2 inerting were the most expensive to completely inert the tank (Figure 5).
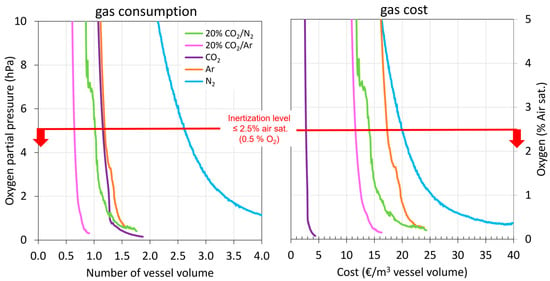
Figure 5.
Number of vessel volumes of inert gas required to achieve total inerting and their economic cost.
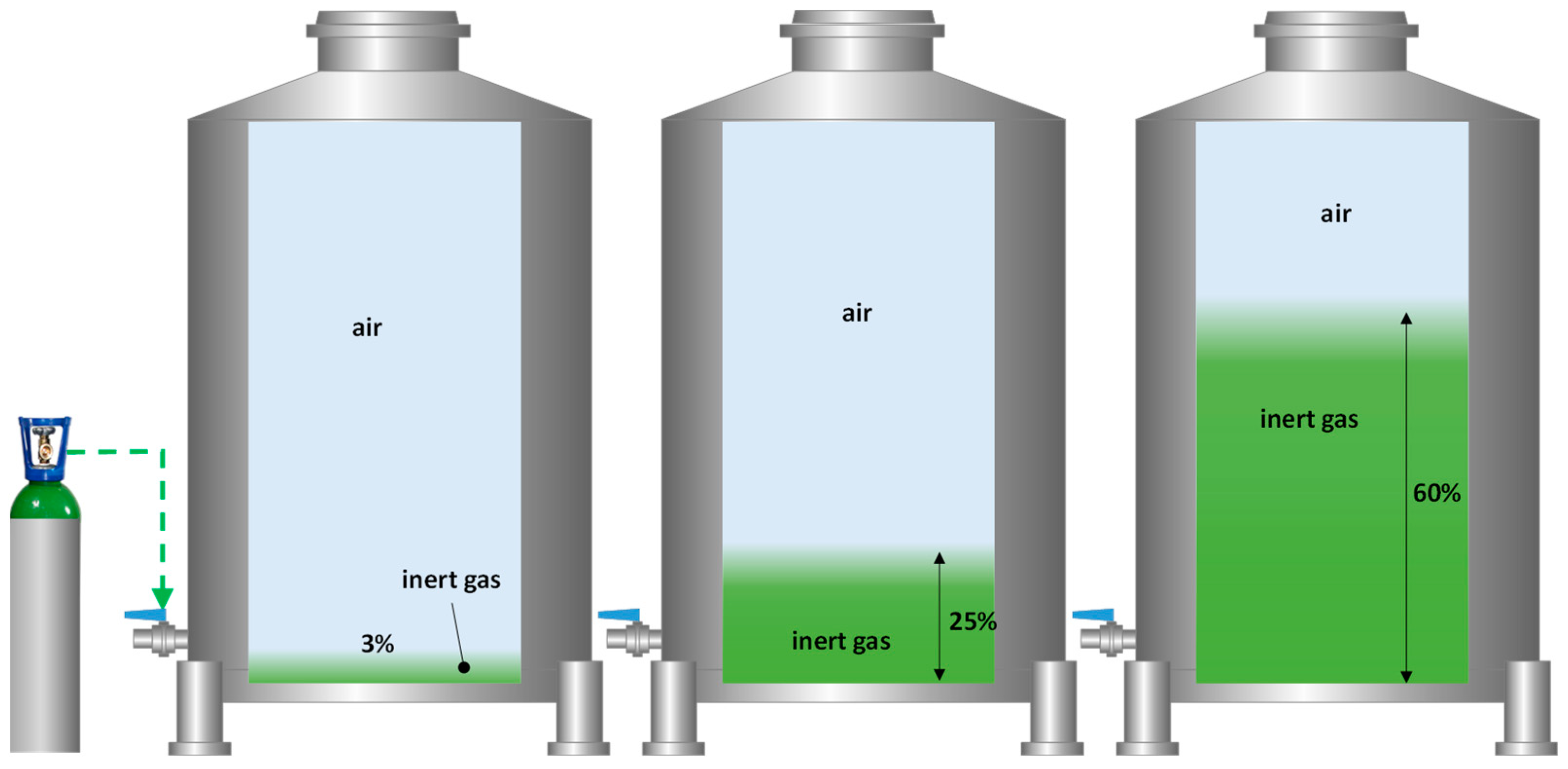
Once the inert gas cost for a complete inertization of a tank with the different gases studied was evaluated (Figure 5), it was proposed to optimize this inertization process by using smaller volumes of each gas. The results of total inertization showed a clear stratification of inert gas blankets of a thickness proportional to the volume of gas injected and which accumulated in the lower part of the tank. The next step was to perform a blanketing with different intensities (thickness of the blanket) and with different inert gases of the wine racked in the destination tank. This optimization seems interesting because of the time involved and the amount of gas required for the complete inerting of a tank [20]. For this reason, the effectiveness of each inert gas was tested by applying the necessary volume to take advantage of its higher specific density to form a stable inert gas blanket of different thicknesses (0.04 m, 0.4 m, and 1 m) on top of the wine (Figure 6).
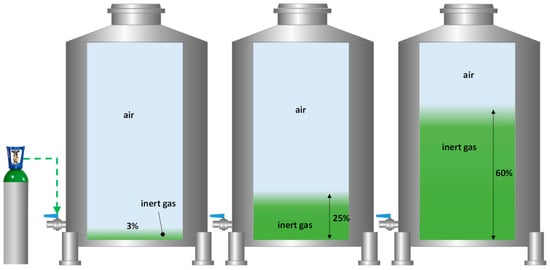
Figure 6.
Diagram of the purging process with different inert gases and different thicknesses of the applied gas cover. A total of 3% corresponds to a thickness of 0.04 m of each gas; 25% to a thickness of 0.4 m; and 60% to 1 m.
3.2. Optimization of Tank Purging by Blanketing the Racked Wine
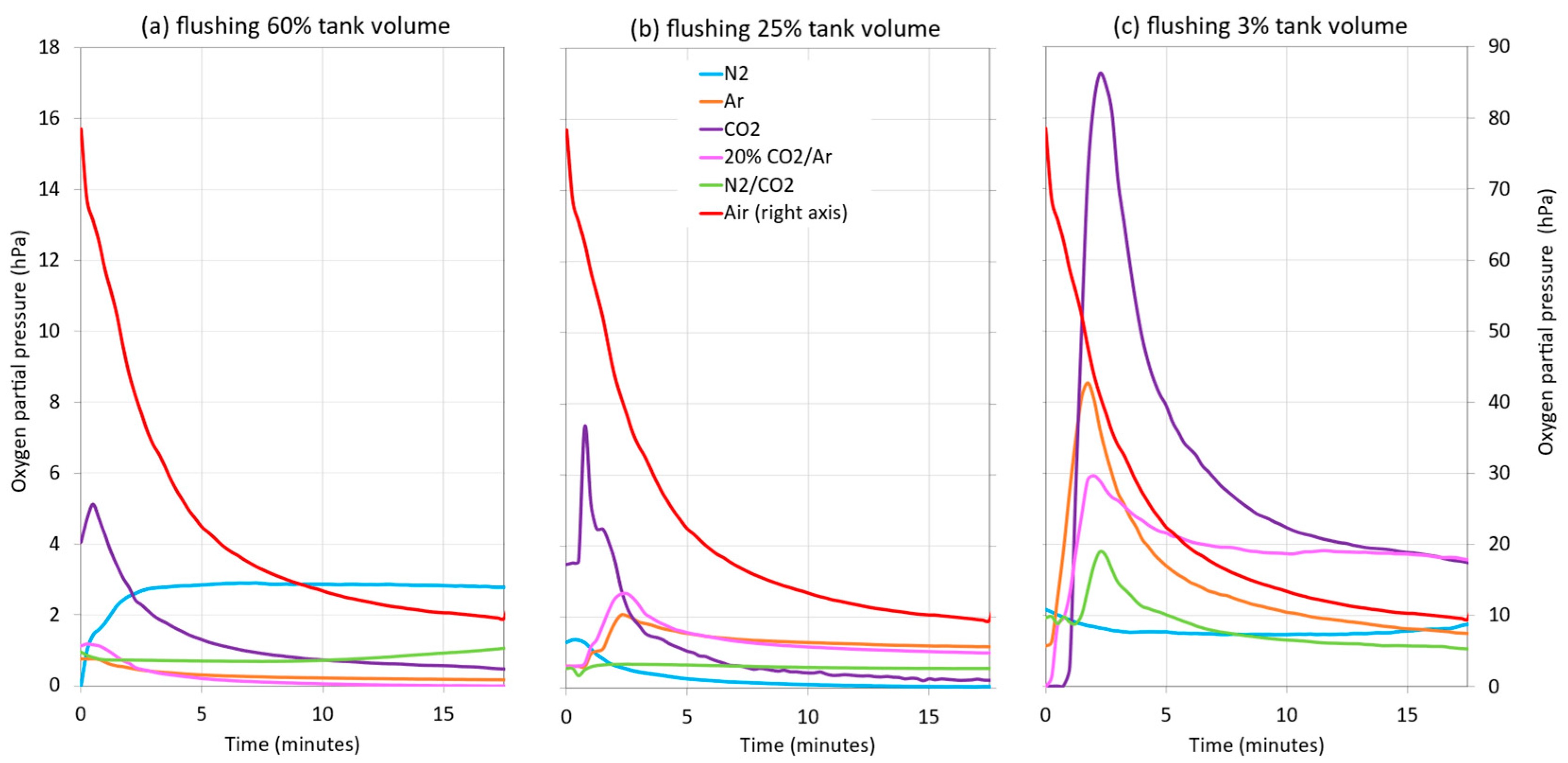
Figure 7 shows the evolution of the DO of the wine when racked into a tank with different purging intensities by forming different blanket thicknesses. When the tank was not inerted, the O2 uptake into the wine (submersible probe located 0.1 m inside the wine) was quite high during the first minutes of racking (between 78.5 and 27.3 hPa, right axis, Figure 7), mainly due to the formation of turbulences when entering the tank. These cause the incorporation of O2 on the way from the racking tank, thus increasing its content in the wine. As the racking progressed, the DO values decreased, due to the homogenization of the DO as the volume of wine in the tank increased, until racking was completed at 9.5 hPa. When the different inert gases were applied for purging, it was seen that, in general, as the thickness of the blanket of each gas decreased, so did the level of protection because an increase in the DO content of the wine was observed, except in the case of the use of N2 at 1m. It was generally observed that, during the first few minutes of racking, CO2 was the gas that provided the least protection in the three blanket thicknesses used (1, 0.4, and 0.04 m) against the incorporation of DO into the wine. On the other hand, when the wine was racked after inerting with N2 and with CO2:N2 (20:80), the values of O2 transfer to the wine were lower. Observing the racking section where the DO measurement begins to be more stable (from the middle of racking to the end), the level of protection provided by the 1 and 0.4 m thick blankets were seen to be very similar. Therefore, it could be said that a thickness of 0.4 m (25% of the tank volume) would be sufficient to maintain low DO contents in the wine. On the other hand, the level of protection exerted by the 0.04 m thick blanket was much lower, since the DO values increased in all the cases studied.
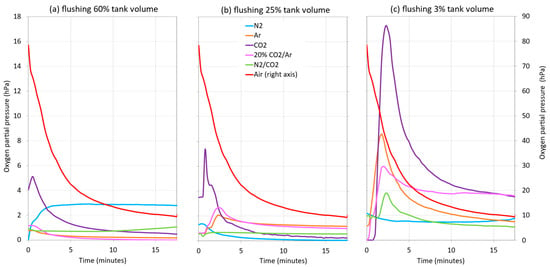
Figure 7.
Influence of purging with different gases and reduction of gas blanket thickness on wine DO when filling the target tank during a wine racking with 60% tank volume, i = 0.6 (a); with 25% tank volume; i = 0.25 (b); and with 3% tank volume, i = 0.03 (c).
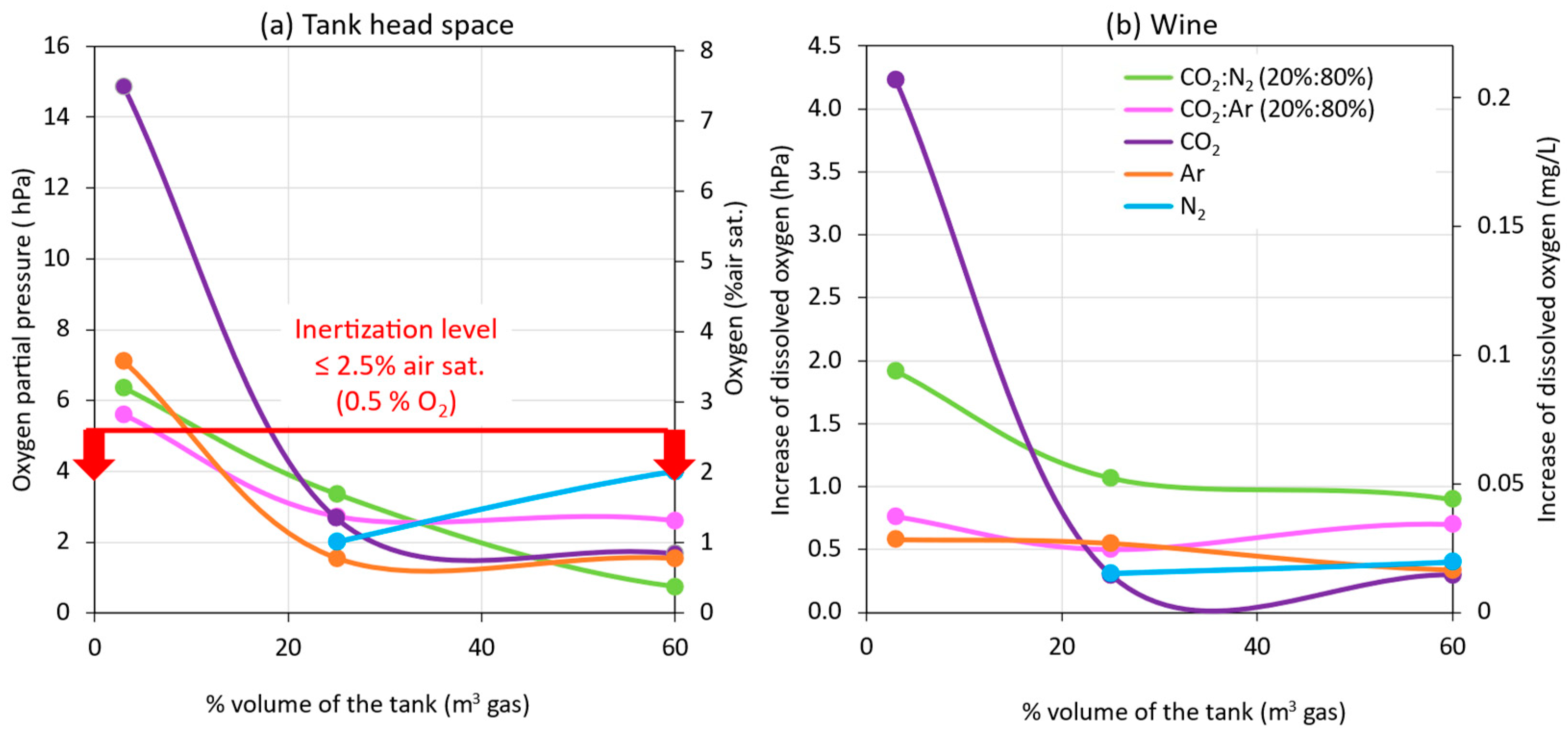
Figure 8 presents the effect of the thickness of the inert gas blankets applied in the empty destination tank during a model wine racking. Figure 8a shows the O2 values recorded by the probe placed in the inert gas blanket formed 0.3 m above the wine surface, and Figure 8b presents the difference between the values read at the inlet valve of the empty tank and the probe that was immersed in the wine at 0.1 m (under surface; Figure 2). In general, it was observed that, in all rackings (including the one without inerting) at the inlet valve to the empty destination tank, the DO values were very low, since the wine arrived from the racking tank with low DO levels. On the other hand, when the wine began to enter the destination tank, the submersible probe recording DO at 0.1 m into the wine showed significant differences, depending on whether the tank was inerted or not. Therefore, the entry of the wine into the empty destination tank seems to be a critical point of O2 transfer to the wine that will depend on whether or not some type of inert gas (purging) is used. In addition, differences were seen depending on the gas used, as well as the thickness of the blanket generated, with each gas performing the inerting of the empty destination tank.
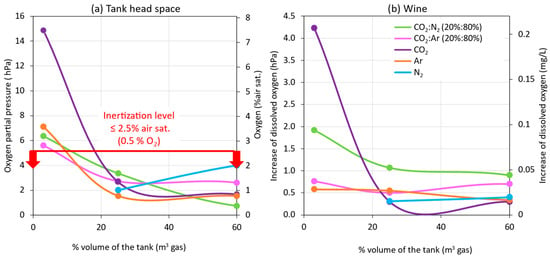
Figure 8.
Comparison of the efficiency of inerting (purging or flushing) the destination tank with different gases and intensities during a racking, 0.3 m over wine surface (a), and 0.1 m under racked wine surface (b).
As can be seen in Figure 8, the formation of a gas blanket of 0.04 m (3% volume of the tank) with all the gases was not sufficient for maintaining the gas blanket inert (0.5% O2 or pO2 ≤ 5 hPa) with a thickness of 0.3 m above the surface of the wine (Figure 2). However, when the thickness of the inert gas blanket was 0.4 m with the injection of 0.25 vessel volume in the empty tank, the gas blanket was kept inerted 0.3 m above the wine surface for all gases, and the DO increase of the racked wine was practically less than 1 hPa. It was observed that, when 0.6 of vessel volume (60% or 1 m thick) was injected, a similar inertization was achieved, both in the blanket above the wine and in the DO incorporated in the racked wine. This avoided injecting the volume of gas necessary for the complete inerting of the tank shown in Figure 5. Ar was the gas that allowed the 0.4 m gas blanket to be maintained with lower O2 values, probably because it displaced more O2, due to its higher density. However, as previously explained, from an economic point of view and due to its cost-benefit ratio or effectiveness in displacing O2, CO2 is the most recommended inert gas for purging. However, the high solubility of CO2 in wine must be taken into account [13], so the application of this gas will depend on the type of wine to be preserved from oxidation.
3.3. Efficiency of Blanketing in the Racked Tank with Different Gases and Different Volumes of Added Gas
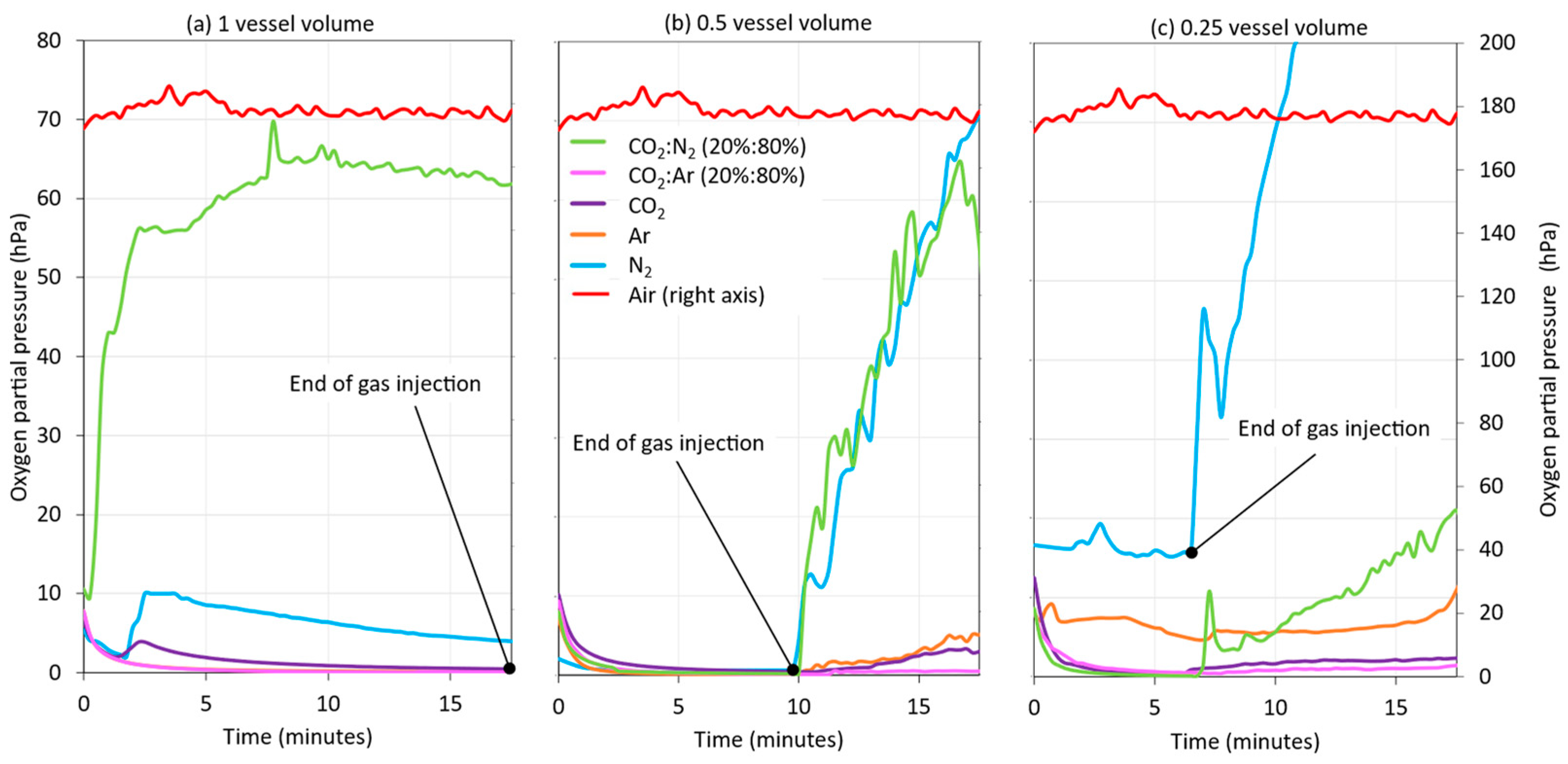
As with purging, the practice of incorporating an inert gas blanket (blanketing) into the starting tank while emptying it during wine racking can contribute significantly to preventing the incorporation of oxygen into the wine and, thus, protect it. In this operation, the intensity of blanketing was also optimized by injecting different vessel volumes of gas during racking. For this purpose, instead of injecting the same volume of gas as of racked wine during the entire racking process, the injection times were shortened, in order to achieve a half- and quarter-thickness blanketing or vessel volume. As shown in Figure 9, the greater the volume of gas incorporated into the racking tank while emptying, the greater the level of HSO protection generated between the wine and the top of the starting tank, with the exception of CO2:N2 (20:80). In general, it was found that, when inert gas was no longer injected in the 0.5 and 0.25 vessel volumes, the level of HSO protection decreased, and this protective effect was much lower in the tests performed with N2 and CO2:N2 (20:80). Therefore, based on these results, it can be stated that, by applying 0.5 of vessel volume of Ar, CO2, and CO2:Ar (20:80) inert gases, the HSO of the starting tank can be protected during the entire wine racking process.
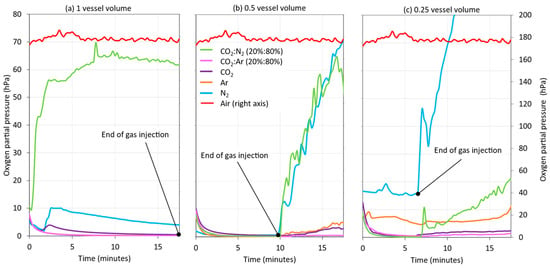
Figure 9.
Monitoring of the oxygen content in the HSO of the racked tank by inerting the wine with different gases and different volumes of each gas added: one vessel volume of inert gas (a); 0.5 vessel volume of inert gas (b); and 0.25 vessel volume of inert gas (c).
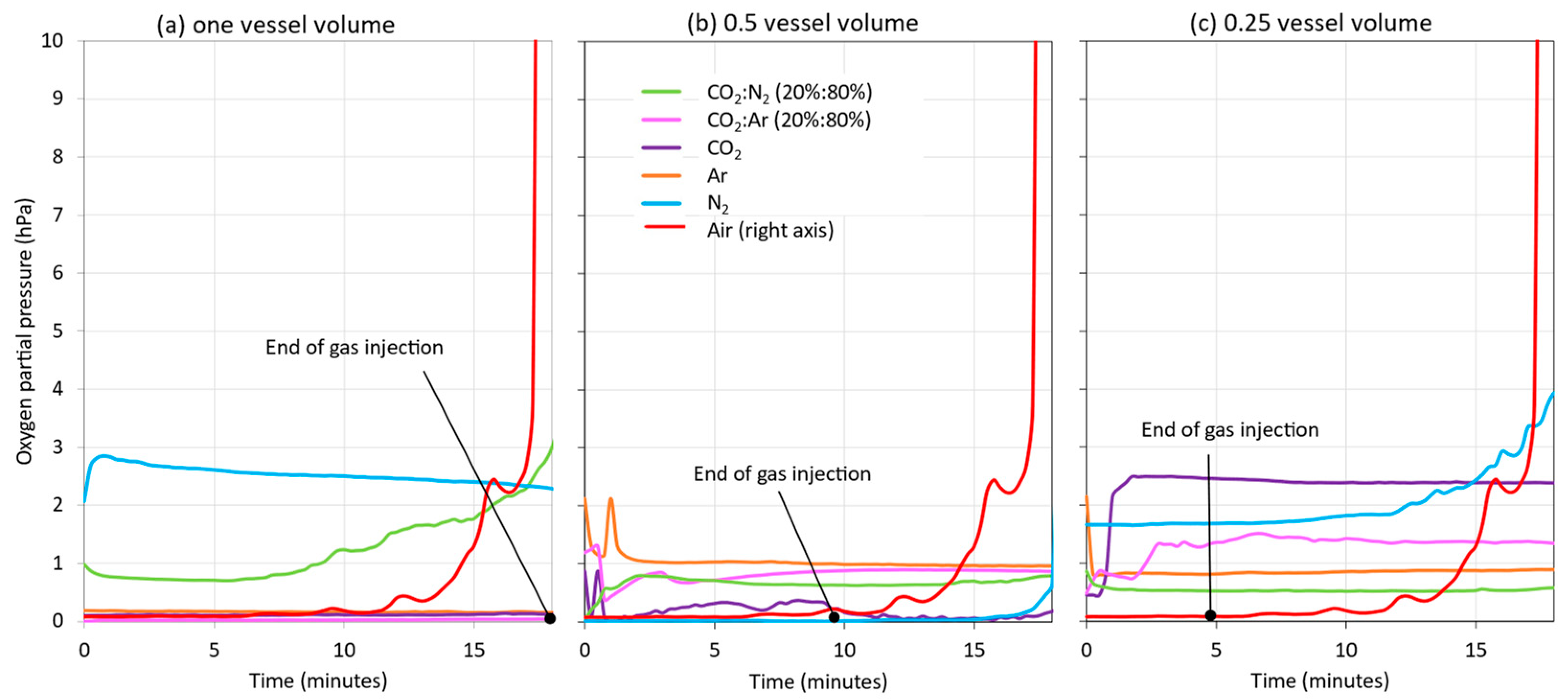
In addition to the HSO values during blanketing, the DO present in the wine at the starting tank outlet was monitored. Measurement at the outlet valve made it possible to measure the amount of oxygen incorporated into the wine when no blanketing was applied and when different vessel volumes of each of the gases studied were applied during wine racking (Figure 10). In general, few differences in DO were seen when different volumes of the gases studied were applied. In the case of racking without blanketing (red line), it was clearly seen that, when the tank was half full, the effect of the incorporation of the air entering the tank while it was being emptied became noticeable. When inert gas was injected for the same duration as the racking (Figure 10a), all the gases injected reduced the incorporation of oxygen into the wine, except N2 and the CO2:N2 mixture, which performed worse than the other gases. The presence of N2, a gas not recommended for blanketing [16], was probably responsible for the poorer results. Figure 10b shows the evolution of the DO of the wine at the racked tank outlet valve when half of the vessel volume was injected and then air was allowed to enter. In this case, the behavior in the evolution of the DO level of the wine being racked remained relatively constant, evidencing that there was hardly any incorporation from the headspace, and the same occurred with the injection of ¼ vessel volume of inert gas (Figure 10c). In general, N2 and its mixture with CO2 showed a more uneven behavior. In all the studies carried out, the model wine remained at the starting tank outlet valve with very low DO values (between 0 and 3 hPa). Therefore, the application of the different gases in different volumes did not seem to have had much effect on the DO of the wine during its transfer from the racking tank.
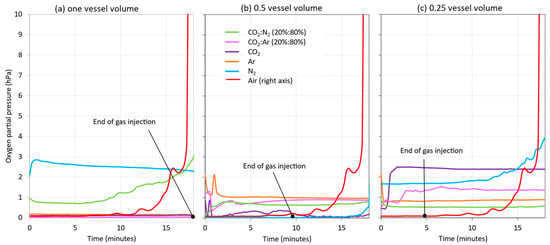
Figure 10.
Influence of blanketing with different inert gases and different number of vessel volumes of gas on the DO of the wine monitored at the outlet valve of the source tank during the racking of a model wine: 1 vessel volume (a); 0.5 vessel volume (b); and 0.25 vessel volume (c).
3.4. Monitoring of Oxygen Content during an Inerted Racking of White Wine in a Commercial Winery
The study was carried out in 25,000 L stainless steel tanks. The oxygen content of white wine in a commercial winery was monitored during three rackings, from a full tank to an empty tank. Butyl DN50 hoses and an impeller pump at flow rates between 15,000 L/h and 18,000 L/h were used. The purging of the hoses and the inerting of the destination tank were monitored under three different situations: a wine racking without purging the destination tank, a second inerting the destination tank with i = 0.6 vessel volume of N2, and a third purging the destination tank with i = 0.6 vessel volume of Ar (recommended volume, based on the results obtained in the optimization of the purging for racking a model wine).
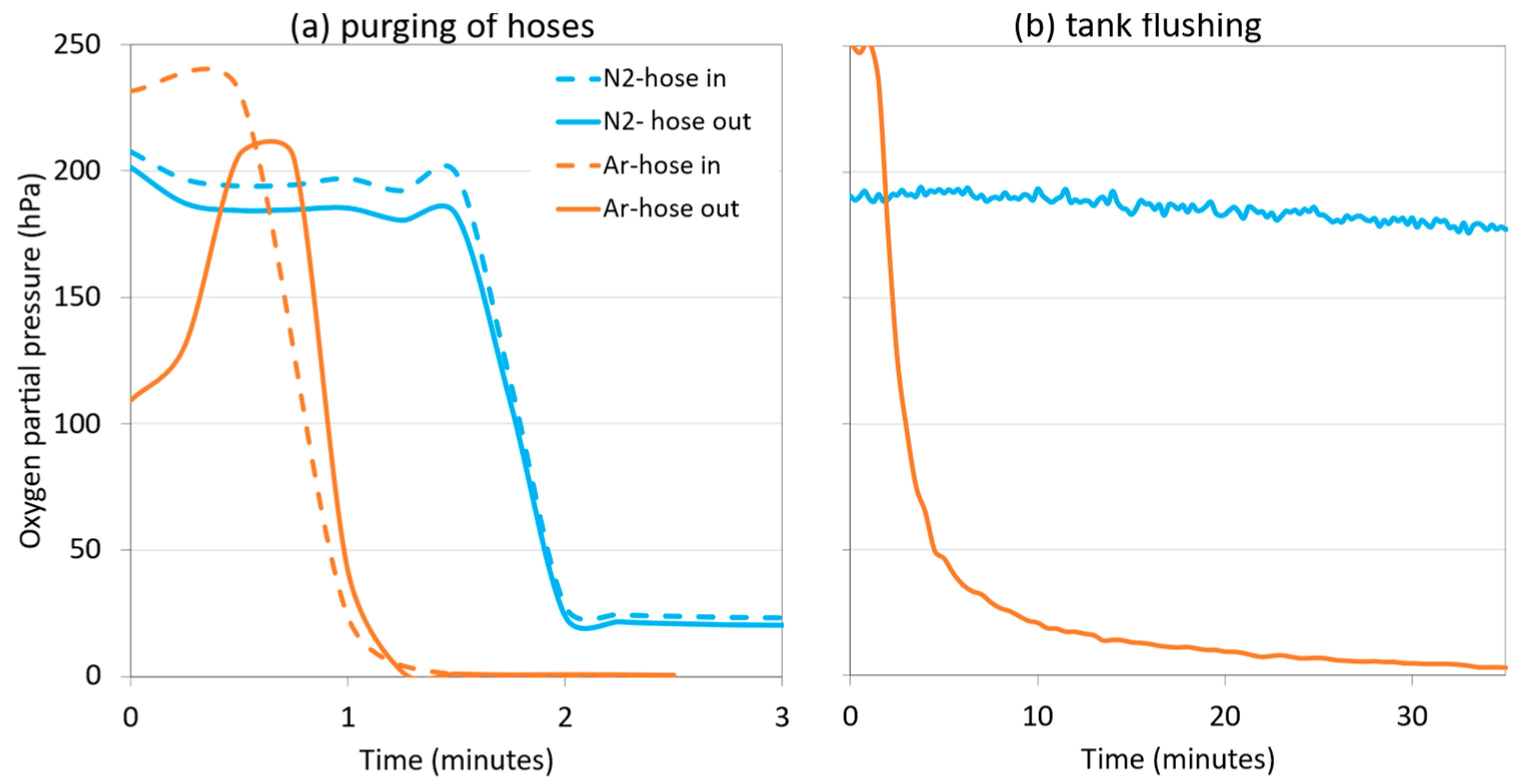
Figure 11a shows the evolution of the oxygen content as the hoses were inerted with both inert gases for the time required until the oxygen measurement remained stable. Figure 11b shows the oxygen level in the HSO of the empty tank by introducing i = 0.6 vessel volume (15 m3) of N2 or Ar. When inerting the hoses, N2 was almost as effective as Ar. This was due to a very long and narrow section with a very high H/D ratio, which allowed the air to be displaced out of the hoses by a plug of inert gas, instead of being diluted [22]. In contrast, Ar was clearly more effective than N2 in the inerting of the destination tank, due to the fact that the H/D ratio was lower, and the N2 gas acted by dilution, while the Ar acted as blanket flushing. As explained in previous sections, since Ar is a denser and heavier gas, it can displace oxygen more effectively in the destination tank, since it does not mix, while N2 acts by dissolution, mixing with the air and decreasing the oxygen concentration.
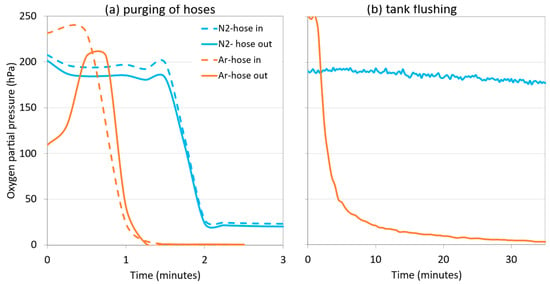
Figure 11.
Comparison of oxygen content during purging of hoses (DN50 and L = 20 m) (a) and of an empty 25,000 L tank with N2 and Ar in a commercial white wine cellar (b).
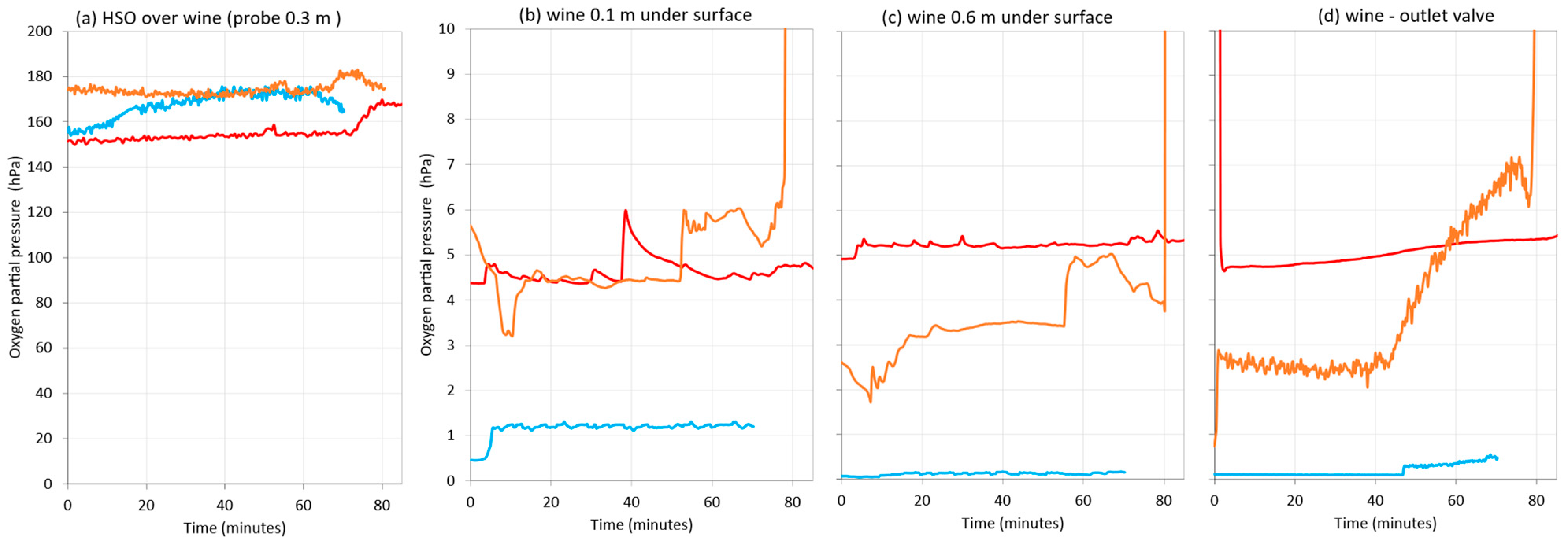
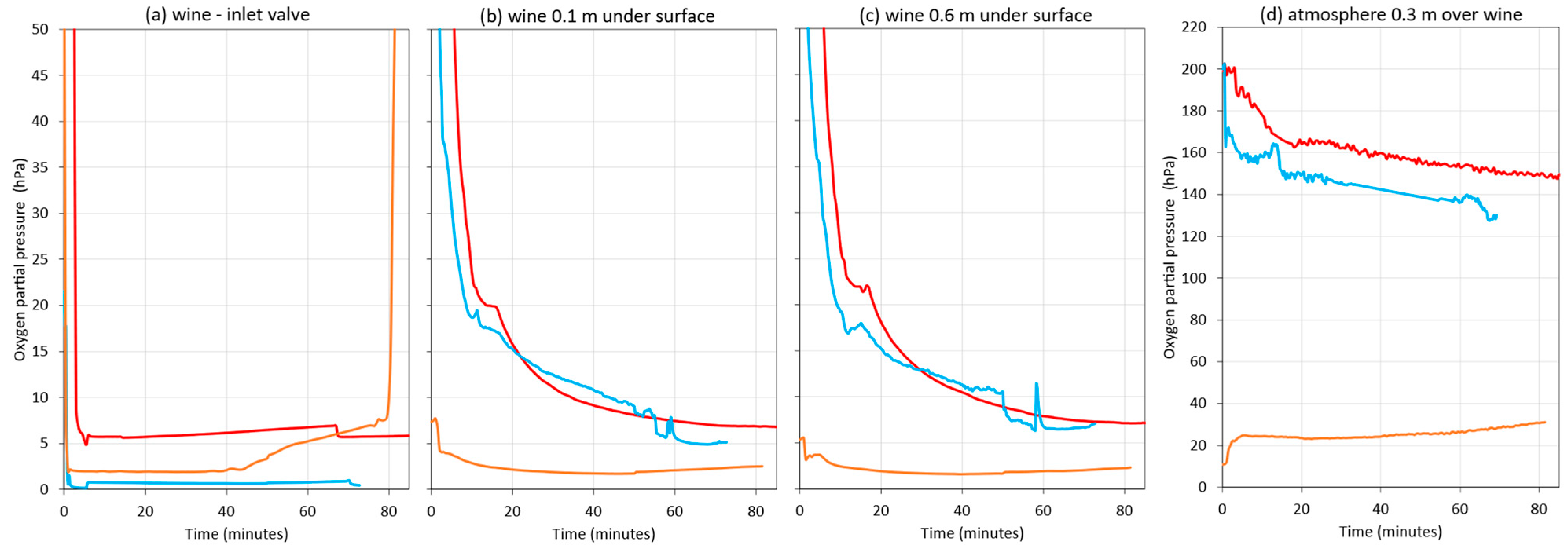
Figure 12 shows the oxygen content in the starting tank, where no gas was applied, while the wine was moving to the destination tank during the three rackings. At this time, it was observed that the probe in the headspace above the wine was measuring air (Figure 12a)—the one submerged 0.1 m in wine varied between 1 hPa and 5 hPa (Figure 12b), and the one submerged 0.6 m indicated that the wine had between 0.5 and 5 hPa (Figure 12c). While the wine was flowing out to the destination tank, the DO level varied between 0.05 hPa and 5 hPa (Figure 12d). These differences may be due to the fact that the wines from the different rackings were not exactly the same.
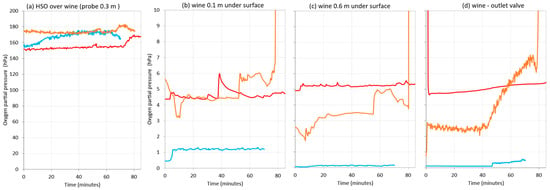
Figure 12.
Comparison of oxygen content in the racked tank during wine racking in a commercial winery (Bodega Emina, Medina del Campo, Spain). Without inerting (red), using nitrogen as inerting gas (blue) or argon (orange). Measures made in the atmosphere at 0.3 m above the surface of the wine (a); 0.1 m depth inside the wine (b); 0.6 m depth inside the wine (c) and wine at the outlet valve (d).
Figure 13 presents the oxygen content of the wine in the destination tank, showing the oxygen content in the three different situations. When the wine racking was started, the oxygen content hardly changed during the hose run and up to its arrival at the inlet valve of the destination tank, since the wine arrived with oxygen levels between 0.05 hPa and 5 hPa (Figure 13a). It should be noted that, when the destination tank was purged with Ar, at around minute 45, an increase in DO was observed at the outlet valve of the racked tank (Figure 12d), which was reflected in the inlet valve of the destination tank (Figure 13a). This increase could be due to the formation of turbulence caused by the suction exerted by the pump. This was also observed when purging with N2 or when not inerting, but it was much lower. When the wine entered the destination tank without inerting or purging with a 0.5 vessel volume of N2, during the first 12 min, the probe immersed 0.1 m into the racked wine showed DO values from saturation to approximately 40 hPa. Subsequently, the DO decreased to end the racking, with DO values of 10 hPa when the tank was not purged and 16 hPa if it was purged with N2. However, in the purged tank with Ar, DO values were much lower from the beginning of the racking, being 8 to 4 hPa during the first 5 min and then about 2 hPa until the end of racking.
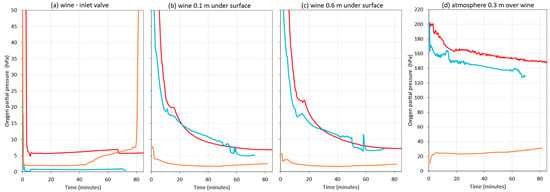
Figure 13.
Oxygen content in the 25,000 L destination tank during a white wine racking in a commercial winery (Bodega Emina, Medina del Campo, Spain). Without inerting (red), using nitrogen as inerting gas (blue) or argon (orange). Measures made in wine in the inlet valve (a); 0.1m depth inside the wine (b); 0.6m depth inside the wine (c) and in the atmosphere at 0.3m above the surface of the wine (d).
When the O2 uptake during the filling of the empty destination tank without inerting was determined, the contact of the wine with the air in the tank resulted in the uptake of 1.37 mg/L during this racking. In the case of N2 inerting of the tank before the start of racking with i = 0.6 vessel volume, the N2 worked as a diluting purging and the DO uptake was still 0.78 mg/L. However, when flushing with Ar was performed, as this inert gas worked as a blanket flushing with the formation of layers, the DO uptake dropped drastically to 0.09 mg/L. Therefore, it can be stated that purging with Ar allowed the racked wine to be stored in the target tank with significantly lower dissolved oxygen values than in the case of purging with N2 or not being inerted (Figure 12b,c). As for the protection exerted on the HSO with the two inert gases used, Ar was clearly more effective than N2 (Figure 13d), maintaining values around 25 hPa throughout racking, while in the case of N2, the values were similar to the case without inerting, i.e., it hardly exerted any protection on the wine, maintaining an inert blanket on the HSO.
4. Conclusions
Inerting an empty tank with different gases was effective in displacing O2 from the tank, with the CO2:Ar (20:80) mixture clearly being the most effective and requiring less gas volume to displace O2. The opposite result was found with N2 requiring much more volume to dilute O2, instead of displacing it like the other gases tested. On the other hand, from an economic viewpoint, the most recommendable gas was CO2.
The level of protection of the racked wine and the headspace in the empty destination tank differed depending on the gas used and the thickness (% of the vessel volume) of the blanket formed with each gas. Based on the results obtained, purging with 25% of the vessel volume of each inert gas is recommended to blanket the racked wine, in order to obtain good cost-benefit results during wine racking.
In the blanketing, adding 0.5 vessel volume of Ar or CO2 and the mixture of both were sufficient to keep the headspace of the racking tank inert. Applying different volumes of gas had little effect on the DO of the wine at the tank outlet.
The study of a white wine racking in a commercial winery demonstrated the greater efficacy of Ar versus N2 in the purging of the destination tank. For the inerting of the hoses, the differences between both gases were minor. In addition, Ar was able to maintain the wine at lower DO levels, as well as to provide a higher level of HSO protection in the destination tank during the racking process.
The results obtained in this work have made it possible to optimize the inerting of tanks with different gases, thus minimizing the volume necessary to avoid the uptake of oxygen during racking.
Author Contributions
Conceptualization, R.d.B.-G., I.N. and M.d.A.-S.; methodology, R.d.B.-G., I.N. and M.d.A.-S.; validation, R.d.B.-G., I.N. and M.d.A.-S.; formal analysis, R.d.B.-G.; investigation, R.d.B.-G. and I.N.; resources, I.N. and M.d.A.-S.; data curation, R.d.B.-G.; writing—original draft preparation, R.d.B.-G. and I.N.; writing—review and editing, R.d.B.-G., I.N. and M.d.A.-S.; visualization, R.d.B.-G., I.N. and M.d.A.-S.; supervision, I.N. and M.d.A.-S.; project administration, I.N. and M.d.A.-S.; funding acquisition, I.N. and M.d.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministerio de Ciencia e Innovación (Ministry of Science and Innovation) (RTC2019-007319-2 project) and Conserjería de Agricultura de la Junta de Castilla y León (Convenio de colaboración entre el Instituto Tecnológico Agrario de Castilla y León (ITACyL), la Universidad de Valladolid (UVa), y la Fundación Parque Científico Universidad de Valladolid (PCUVa) para la realización de actividades de investigación, fomento de la innovación y transferencia de conocimiento sobre productos alimentarios, y optimización de procesos productivos en sectores estratégicos de Castilla y León: el sector vitivinícola).
Data Availability Statement
The data are available upon request to the authors.
Acknowledgments
The authors thank Carburos Metálicos (Air Products) for the provision of the inert gases, Bodega Emina (Medina del Campo, Spain) for collaboration, P. Franch for analysis assistance, and Ann Holliday for revising the English.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Calderón, J.F.; del Alamo-Sanza, M.; Nevares, I.; Laurie, F. The influence of selected winemaking equipment and operations on the concentration of dissolved oxygen in wines. Cienc. Investig. Agrar. 2014, 41, 273–280. [Google Scholar] [CrossRef]
- Catarino, A.; Alves, S.; Mira, H. Influence of technological operations in the dissolved oxygen content of wines. J. Chem. Chem. Eng. 2014, 8, 390–394. [Google Scholar] [CrossRef]
- Masella, P.; Angeloni, A.; Guerrini, L.; Spadi, A.; Maioli, F.; Calamai, L.; Parenti, A. A deeper understanding of the qualitative consequences of food pumping: A case study of wine. Food Bioprod. Process. 2022, 131, 13–21. [Google Scholar] [CrossRef]
- Day, M.P.; Schmidt, S.A.; Smith, P.A.; Wilkes, E.N. Use and impact of oxygen during winemaking. Aust. J. Grape Wine Re. 2015, 21, 693–704. [Google Scholar] [CrossRef]
- Moutounet, M.; Mazauric, J.P. Lóxygène dissous dans les vins. Rev. Fr. Oenol. 2001, 186, 12–15. [Google Scholar]
- Castellari, M.; Simonato, B.; Tornielli, G.B.; Spinelli, P.; Ferrarini, R. Effects of different enological treatments on dissolved oxygen in wines. Ital. J. Food Sci. 2004, 16, 387–396. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Duboudieu, D. The Chemistry of Wine Stabilization and Treatments, Volume 1. In Handbook of Enology, 3rd ed.; Chimie du vin stabilisation et Traitments; John Wiley & Sons: Chichester, UK, 2021; p. 519. ISBN 9781119587668-1. [Google Scholar]
- Vivas, N.; Glories, Y. Les phenomenes d’Oxydroreduction lies a l’elevage en barrique des vins rouges: Aspects technologiques. Rev. Fr. Oenol. 1993, 142, 33–38. [Google Scholar]
- Available online: https://www.awri.com.au/industry_support/winemaking_resources/storage-and-packaging/pre-packaging-preparation/gas-adjustment/ (accessed on 12 November 2022).
- Nevares, I.; Fernández-Díaz, A.; del Alamo-Sanza, M. Characterization and control of hidden micro-oxygenation in the winery: Wine racking. Foods 2021, 10, 386–410. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.L. Wine transfer using inert gas for prevention of oxidation. Aust. Grapegrow. Winemak. 1985, 256, 110–111. [Google Scholar]
- Goode, J. Oxygen & Wine. Research Gets Specific about Oxygen, Closures, Bottling and Aging. Wines & Vines, August 2009. pp. 26–32. Available online: https://winesvinesanalytics.com/sections/printout_article.cfm?content=66003&article=feature (accessed on 14 December 2022).
- Girardon, P. Gases in Enology. In Gases in Agro-Food Processes, 1st ed.; Cachon, R., Girardon, P., Voilley, A., Eds.; Academic Press: London, UK, 2019; pp. 433–449. ISBN 9780128124659. [Google Scholar] [CrossRef]
- Westrick, M. Managing oxygen in white wine production. Pract. Winery Vineyard 1996, 49–52. [Google Scholar]
- Báleš, V.; Furman, D.; Timár, P.; Ševčík, M. Oxygen Removal from the White Wine in Winery. Acta Chim. Pharm. Indica 2017, 7, 107. [Google Scholar]
- Lopes, P.; Silva, M.A.; Pons, A.; Tominaga, T.; Lavigne, V.; Saucier, C.; Darriet, P.; Teissedre, P.-L.; Dubourdieu, D. Impact of oxygen dissolved at bottling and transmitted through closures on the composition and sensory properties of a sauvignon blanc wine during bottle storage. J. Agric. Food Chem. 2009, 57, 10261–10270. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, C.; Du Toit, W.J. Sauvignon blanc wine: Contribution of ageing and oxygen on aromatic and non-aromatic compounds and sensory composition: A review. S. Afr. J. Enol. Vitic. 2015, 36, 347–365. [Google Scholar] [CrossRef]
- Green, D.W.; Robert, H.P. Perry’s Chemical Engineers’ Handbook, McGraw-Hill Professional, 8th ed.; McGraw-Hill: New York, NY, USA, 2007; Chapter 23; ISBN 978-0-07-142294-9. [Google Scholar]
- American Gas Association. Purging Principles and Practices, 3rd ed.; American Gas Association: Washington, DC, USA, 2001; Available online: https://law.resource.org/pub/us/cfr/ibr/001/aga.purging.2001.pdf (accessed on 10 November 2022).
- Dharmadhikari, M. Use of Inert Gases. Midwest Grape and Wine Industry Institute. Available online: https://www.extension.iastate.edu/wine/use-inert-gases (accessed on 18 December 2022).
- Moroney, M. Use of Inert Gases. Available online: https://www.extension.iastate.edu/wine/publications/use-of-inert-gases-2 (accessed on 18 December 2022).
- Reinhardt, H.J.; Himmen, H.R.; Kaltenegger, J. Inerting in the Chemical Industry, Ed. Linde Gases Division. Available online: https://www.lindegas.com/en/processes/inerting_purging_and_blanketing/index.html# (accessed on 14 December 2022).
- Lewis, D. Blanketing in storage tanks. Aust. Grapegrow. Winemak. 1990, 4, 100–101. [Google Scholar]
- del Alamo-Sanza, M.; Pando, V.; Nevares, I. Investigation and correction of the interference of ethanol, sugar and phenols on dissolved oxygen measurement in wine. Anal. Chim. Acta 2014, 809, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Zoecklein, B. Gases: Carbon Dioxide, Argon, and Nitrogen. (n.d.). Department of Food Science & Technology, Virginia Tech. Available online: https://www.apps.fst.vt.edu/extension/enology/downloads/wm_issues/Winery%20Gases/Winery%20Gases1.pdf (accessed on 14 January 2023).
- Stamp, C. Indispensable Inert Gas. Wines & Vines. 2009. Available online: https://winesvinesanalytics.com/sections/printout_article.cfm?article=feature&content=66992 (accessed on 14 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).