Isotonic Drinks Based on Organic Grape Juice and Naturally Flavored with Herb and Spice Extracts
Abstract
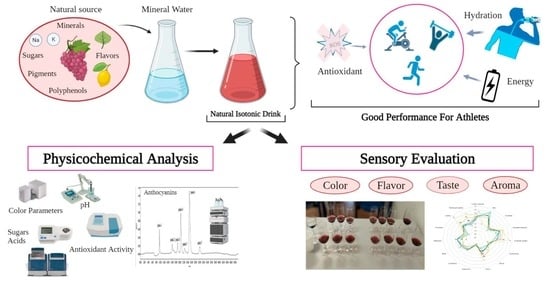
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Isotonic Drink Design
2.3. Color Parameters and Total Phenolic Index
2.4. pH Measurement
2.5. Nutritional Composition
2.6. Total Soluble Solids
2.7. Determination of Anthocyanins
2.8. Determination of Antioxidant Capacity
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results
3.1. Nutritional Composition
3.2. Physicochemical Characteristics
3.3. Anthocyanin Content
3.4. Antioxidant Activity
3.5. Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geraldini, S.; de Freitas Cruz, I.; Romero, A.; Fonseca, F.L.A.; de Campos, M.P. Isotonic sports drink promotes rehydration and decreases proteinuria following karate training. J. Bras. Nefrol. 2017, 39, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Bovi, G.G.; Petrus, R.R.; Pinho, S.C. Feasibility of incorporating buriti (Mauritia flexuosa L.) oil nanoemulsions in isotonic sports drink. Intrn. J. Food Sci. Technol. 2017, 52, 2201–2209. [Google Scholar] [CrossRef]
- Świtalski, M.; Rybowska, A. Product innovation in isotonic drinks-expectations of tri-city university students. Sci. J. Gdynia Maritime Univrst. 2021, 118, 62–74. [Google Scholar] [CrossRef]
- Ferreira, L.R.; Fontes, E.A.F.; Marinho, L.M.G.; de Barros, F.A.R.; Stringheta, P.C.; Ramos, A.M. Elaboration, characterization and color stability of an isotonic beverage based on whey permeate with carotenoid powder from pequi. Res. Soc. Dev. 2021, 10, e41810817233. [Google Scholar] [CrossRef]
- Pivnenko, T.N.; Esipenko, R.V.; Kovalev, A.N. Functional Isotonic Drinks Based on the Tissue Fluid of Rhopilema Jellyfish. Procd. Unvers Appl. chem. Biotechnol. 2018, 8, 141–149. [Google Scholar] [CrossRef]
- Bonetti, D.; Hopkins, W.; Jeukendrup, A. Effects of Hypotonic and Isotonic Sports Drinks on Endurance Performance and Physiology. Sport sci. 2010, 14, 63–70. [Google Scholar]
- D’Angelo, S. Polyphenols and athletic performance: A review on human data. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Tenango, M.P., Eds.; IntechOpen: London, UK, 2019; pp. 1–24. ISBN 978-1-78984-033-9. [Google Scholar] [CrossRef] [Green Version]
- Cerezal Mezquita, P.; Espinosa Álvarez, C.; Palma Ramírez, J.; Bugueño Muñoz, W.; Salinas Fuentes, F.; Ruiz-Domínguez, M.D.C. Isotonic Beverage Pigmented with Water-Dispersible Emulsion from Astaxanthin Oleo-resin. Molecules 2020, 25, 841. [Google Scholar] [CrossRef] [Green Version]
- Gironés-Vilaplana, A.; Villaño, D.; Moreno, D.A.; García-Viguera, C. New isotonic drinks with antioxidant and biological capacities from berries (maqui, açaí and blackthorn) and lemon juice. Int. J. Food Sci. Nutr. 2013, 64, 897–906. [Google Scholar] [CrossRef]
- Porfírio, M.C.P.; Gonçalves, M.S.; Borges, M.V.; Leite, C.X.D.S.; Santos, M.R.C.; da Silva, A.G.; Fontan, G.C.R.; Leão, D.J.; de Jesus, R.M.; Gualberto, S.A.; et al. Development of isotonic beverage with functional attributes based on extract of Myrciaria jabuticaba (Vell) berg. Food Sci. Technol. 2020, 40, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.D.S.; Da Conceição Prudêncio Dutra, M.; Toaldo, I.M.; Corrêa, L.C.; Pereira, G.E.; De Oliveira, D.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic compounds, organic acids and antioxidant activity of grape juices produced in industrial scale by different processes of maceration. Food Chem. 2015, 188, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Cosme, F.; Pinto, T.; Vilela, A. Phenolic Compounds and Antioxidant Activity in Grape Juices: A Chemical and Sensory View. Beverages 2018, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.D.S.; Silani, I.D.S.V.; Toaldo, I.M.; Corrêa, L.C.; Biasoto, A.C.T.; Pereira, G.E.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic compounds, organic acids and antioxidant activity of grape juices produced from new Brazilian varieties planted in the Northeast Region of Brazil. Food Chem. 2014, 161, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toscano, L.T.; Silva, A.S.; Toscano, L.T.; Tavares, R.L.; Biasoto, A.C.T.; de Camargo, A.C.; da Silva, C.S.O.; Gonçalves, M.d.C.R.; Shahidi, F. Phenolics from purple grape juice increase serum antioxidant status and im-prove lipid profile and blood pressure in healthy adults under intense physical training. J. Funct. Foods 2017, 33, 419–424. [Google Scholar] [CrossRef]
- Rodrigues, A.D.; Scheffel, T.B.; Scola, G.; dos Santos, M.T.; Fank, B.; de Freitas, S.C.V.; Dani, C.; Vanderlinde, R.; Henriques, J.A.P.; Coitinho, A.S.; et al. Neuroprotective and anticonvulsant effects of organic and conventional purple grape juices on seizures in Wistar rats induced by pentylenetetrazole. Neurochem. Int. 2012, 60, 799–805. [Google Scholar] [CrossRef] [Green Version]
- da Conceição Prudêncio Dutra, M.; Viana, A.C.; Pereira, G.E.; de Cássia Mirella Resende Nassur, R.; dos Santos Lima, M. Whole, concentrated and reconstituted grape juice: Impact of processes on phenolic composition, “foxy” aromas, organic acids, sugars and antioxidant capacity. Food Chem. 2021, 343, 128399. [Google Scholar] [CrossRef]
- Granato, D.; de Magalhães Carrapeiro, M.; Fogliano, V.; van Ruth, S.M. Effects of geographical origin, varietal and farming system on the chemical composition and functional properties of purple grape juices: A review. Trends Food Sci. Technol. 2016, 52, 31–48. [Google Scholar] [CrossRef]
- Skąpska, S.; Marszałek, K.; Woźniak, Ł.; Szczepańska, J.; Danielczuk, J.; Zawada, K. The development and consumer acceptance of functional fruit-herbal beverages. Foods 2020, 9, 1819. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A.; Cosme, F. Chemical and sensory characteristics of fruit juice and fruit fermented beverages and their consumer acceptance. Beverages 2022, 8, 33. [Google Scholar] [CrossRef]
- Bendaali, Y.; Vaquero, C.; González, C.; Morata, A. Elaboration of an organic beverage based on grape juice with positive nutritional properties. Food Sci. Nutr. 2022, 10, 1768–1779. [Google Scholar] [CrossRef]
- Sowbhagya, H.B.; Chitra, V.N. Enzyme-assisted extraction of flavorings and colorants from plant materials. Food Sci. Nutr. 2010, 50, 146–161. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A. Healthy drinks with lovely colors: Phenolic compounds as constituents of functional beverages. Beverages 2021, 7, 12. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Nedamani, A.R. Stability Enhancement of Natural Food Colorants-A Review. J. Res. Innov. Food Sci. Technol. 2021, 10, 369–388. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; Julian, D. Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem. 2016, 212, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Escott, C.; Loira, I.; López, C.; Palomero, F.; González, C. Emerging non-thermal technologies for the extraction of grape anthocyanins. Antioxidants 2021, 10, 1863. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Morata, A.; López, C.; Tesfaye, W.; González, C.; Escott, C. Anthocyanins as Natural Pigments. In Value—Added Ingredients and Enrichments of Beverages; Academic Press: Cambridge, MA, USA, 2019; Volume 14, pp. 383–428. [Google Scholar] [CrossRef]
- Tan, C.; Dadmohammadi, Y.; Lee, M.C.; Abbaspourrad, A. Combination of copigmentation and encapsulation strategies for the synergistic stabilization of anthocyanins. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3164–3191. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Youssef, A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef] [Green Version]
- Burin, V.M.; Falcão, L.D.; Gonzaga, L.V.; Fett, R.; Rosier, J.P.; Bordignon-Luiz, M.T. Colour, phenolic content and antioxidant activity of grape juice. Food Sci. Technol. 2010, 30, 1027–1032. [Google Scholar] [CrossRef] [Green Version]
- Milella, R.A.; Basile, T.; Alba, V.; Gasparro, M.; Giannandrea, M.A.; Debiase, G.; Genghi, R.; Antonacci, D. Optimized ultrasonic-assisted extraction of phenolic antioxidants from grape (Vitis vinifera L.) skin using response surface methodology. J. Food Sci. Technol. 2019, 56, 4417–4428. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Escott, C.; Vaquero, C.; del Fresno, J.M.; Bañuelos, M.A.; Loira, I.; Han, S.Y.; Suárez-Lepe, J.A. Pulsed light effect in red grape quality and fermentation. Food Biopr. Technol. 2017, 10, 1540–1547. [Google Scholar] [CrossRef]
- Leśniewicz, A.; Grzesiak, M.; Żyrnicki, W.; Borkowska-Burnecka, J. Mineral Composition and Nutritive Value of Isotonic and Energy Drinks. Biol. Trace Elem. Res. 2016, 170, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Gironés-Vilaplana, A.; Mena, P.; Moreno, D.A.; García-Viguera, C. Evaluation of sensorial, phytochemical and biological properties of new isotonic beverages enriched with lemon and berries during shelf life. J. Sci. Food. Agric. 2014, 94, 1090–1100. [Google Scholar] [CrossRef]
- Estupiñan, D.C.; Schwartz, S.J.; Garzón, G.A. Antioxidant Activity, Total Phenolics Content, Anthocyanin, and Color Stability of Isotonic Model Beverages Colored with Andes Berry (Rubus glaucus Benth) Anthocyanin Powder. J. Food Sci. 2011, 76, S26–S34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbuMoh’d, M.F. Influence of an Isotonic Sports Drink during Exercise and Recovery on Subsequent Endurance Capacity and Aldosterone Response in the Heat in Well-Trained Endurance Athletes. Sport Mont 2020, 18, 25–31. [Google Scholar] [CrossRef]
- Demirhan, B.; Cengiz, A.; Gunay, M.; Türkmen, M.; Geri, S. The effect of drinking water and isotonic sports drinks in elite wrestlers. Anthropologist 2015, 21, 213–218. [Google Scholar] [CrossRef]
- Del Coso, J.; Estevez, E.; Baquero, R.A.; Mora-Rodriguez, R. Anaerobic performance when rehydrating with water or commercially available sports drinks during prolonged exercise in the heat. App. Physiol. Nutr. Metab. 2008, 33, 290–298. [Google Scholar] [CrossRef]
- Singh, A.; Chaudhary, S.; Sandhu, J.S. Efficacy of Pre-Exercise Carbohydrate Drink (Gatorade) on the Recovery Heart Rate, Blood Lactate and Glucose Levels in Short Term Intensive Exercise. Serbian J. Sport Sci. 2011, 5, 29–34. Available online: https://search.ebscohost.com/login.aspx?direct=true&db=s3h&AN=66385377&lang=es&site=ehost-live&scope=site (accessed on 5 June 2023).
- Galvão, L.M.V.; Sousa, M.d.M.; Nascimento, A.M.d.C.B.; de Souza, B.V.C.; Nunes, L.C.C. Evaluation of shelf life of isotonic beverage enriched with cajuína. Food Sci. Technol. 2020, 42, 1–6. [Google Scholar] [CrossRef]
- Chen, J.; Du, J.; Li, M.; Li, C. Degradation kinetics and pathways of red raspberry anthocyanins in model and juice systems and their correlation with color and antioxidant changes during storage. LWT-Food Sci. Technol. 2020, 128, 109448. [Google Scholar] [CrossRef]
- Pinto, E.P.; Perin, E.C.; Schott, I.B.; Düsman, E.; da Silva Rodrigues, R.; Lucchetta, L.; Manfroi, V.; Rombaldi, C.V. Phenolic compounds are dependent on cultivation conditions in face of UV-C radiation in ‘Concord’ grape juices (Vitis labrusca). Lwt 2022, 154, 112681. [Google Scholar] [CrossRef]
- González-Molina, E.; Gironés-Vilaplana, A.; Mena, P.; Moreno, D.A.; García-Viguera, C. New Beverages of Lemon Juice with Elderberry and Grape Concentrates as a Source of Bioactive Compounds. J. Food Sci. 2012, 77, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Bingöl, A.; Türkyılmaz, M.; Özkan, M. Increase in thermal stability of strawberry anthocyanins with amino acid copigmentation. Food Chem. 2022, 384, 132518. [Google Scholar] [CrossRef] [PubMed]
- Gironés-vilaplana, A.; Mena, P.; García-viguera, C.; Moreno, D.A. A novel beverage rich in antioxidant phenolics: Maqui berry (Aristotelia chilensis) and lemon juice. LWT-Food Sci. Techol. 2012, 47, 279–286. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Huertas, J.P.; Moreno, D.A.; Periago, P.M.; García-Viguera, C. Quality and microbial safety evaluation of new isotonic beverages upon thermal treatments. Food Chem. 2016, 194, 455–462. [Google Scholar] [CrossRef]
- Bendokas, V.; Stanys, V.; Mažeikienė, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the field to the antioxidants in the body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef]
- Vidana Gamage, G.C.; Lim, Y.Y.; Choo, W.S. Sources and relative stabilities of acylated and nonacylated anthocyanins in beverage systems. J. Food Sc. Technol. 2021, 59, 831–845. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; He, K.; Huang, C.; Tang, S. Consumption of sugar-sweetened beverages and fruit juice and human cancer: A systematic review and dose-response meta-analysis of observational studies. J. Cancer 2021, 12, 3077–3088. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Zhou, H.; Muriel Mundo, J.L.; McClements, D.J. Protection of anthocyanin-rich extract from pH-induced color changes using water-in-oil-in-water emulsions. J. Food Eng. 2019, 254, 1–9. [Google Scholar] [CrossRef]
- Bendaali, Y.; Vaquero, C.; González, C.; Morata, A. Contribution of Grape Juice to Develop New Isotonic Drinks with Antioxidant Capacity and Interesting Sensory Properties. Front. Nutr. 2022, 9, 1–9. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Bezerra, F.F.; de Araujo Eleutherio, E.C.; Bouskela, E.; Koury, J. Organic grape juice intake improves functional capillary density and postocclusive reactive hyperemia in triathletes. Clinics 2011, 66, 1537–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalla Corte, C.L.; de Carvalho, N.R.; Amaral, G.P.; Puntel, G.O.; Silva, L.F.A.; Retamoso, L.T.; Royes, L.F.F.; Bresciani, G.B.; da Cruz, I.B.M.; Rocha, J.B.T.; et al. Antioxidant effect of organic purple grape juice on exhaustive exercise. Appl. Physiol. Nutr. Metab. 2013, 38, 558–565. [Google Scholar] [CrossRef]
- Goulart, M.J.V.C.; Pisamiglio, D.S.; Möller, G.B.; Dani, C.; Alves, F.D.; Bock, P.M.; Schneider, C.D. Effects of grape juice consumption on muscle fatigue and oxidative stress in judo athletes: A randomized clinical trial. An. Acad. Bras. Cienc. 2020, 92, e20191551. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.C.; Dorneles, G.P.; Blembeel, A.S.; Marinho, J.P.; Proença, I.C.T.; da Cunha Goulart, M.J.V.; Moller, G.B.; Marques, E.P.; Pochmann, D.; Salvador, M.; et al. Effects of grape juice consumption on oxidative stress and inflammation in male volleyball players: A randomized, double-blind, placebo-controlled clinical trial. Complement. Ther. Med. 2020, 54, 102570. [Google Scholar] [CrossRef] [PubMed]
- Toscano, L.T.; Tavares, R.L.; Toscano, L.T.; da Silva, C.S.O.; de Almeida, A.E.M.; Biasoto, A.C.T.; Gonçalves, M.d.C.R.; Silva, A.S. Potential ergogenic activity of grape juice in runners. Appl. Physiol. Nutr. Metab. 2015, 40, 899–906. [Google Scholar] [CrossRef] [Green Version]
- de Lima Tavares Toscano, L.; Silva, A.S.; de França, A.C.L.; de Sousa, B.R.V.; de Almeida Filho, E.J.B.; da Silveira Costa, M.; Marques, A.T.B.; da Silva, D.F.; de Farias Sena, K.; Cerqueira, G.S.; et al. A single dose of purple grape juice improves physical performance and antioxidant activity in runners: A randomized, crossover, double-blind, placebo study. Eur. J. Nutr. 2020, 59, 2997–3007. [Google Scholar] [CrossRef]
- Neto, M.M.; da Silva, T.F.; de Lima, F.F.; Siqueira, T.M.Q.; Toscano, L.T.; de Moura, S.K.M.S.F.; Silva, A.S. Whole Red Grape Juice Reduces Blood Pressure at Rest and Increases Post-exercise Hypotension. J. Am. Coll. Nutr. 2017, 36, 533–540. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- O’Connor, E.; Mündel, T.; Barnes, M.J. Nutritional Compounds to Improve Post-Exercise Recovery. Nutrients 2022, 14, 5069. [Google Scholar] [CrossRef]
- Martins, Z.E.; Machado, J.C., Jr.; Cunha, S.C.; Barata, A.M.; Ferreira, I.M. A chemometric approach to compare Portuguese native hops with worldwide commercial varieties. J. Chemometrics 2020, 34, e3285. [Google Scholar] [CrossRef]
- Rodrigues Arruda, T.; Fontes Pinheiro, P.; Silva, P.I.; Campos Bernardes, P. A new perspective of a well-recognized raw material: Phenolic content, antioxidant and antimicrobial activities and α-and β-acids profile of Brazilian hop (Humulus lupulus L.) extracts. LWT 2021, 141, 110905. [Google Scholar] [CrossRef]
- Baji, M.; Jal, H.; Travan, A. Chitosan-based films with incorporated supercritical CO2 hop extract: Structural, physicochemical, and antibacterial properties. J. Carbohydr. Polym. 2019, 219, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Swarnalakshmi, C.S.; Manisha, C.P.; Harini, B.; Akshara, J.; Joshika, G.; Keerthana, R. Optimization and standardization of lemon grass incorporated into pseudostem and mint extracts based isotonic drink. Int. J. Adv. Res. Ideas Innov. Technol. 2019, 5, 1089–1093. Available online: https://www.academia.edu/download/59980481/V5I3-162220190711-94310-12ubpoy.pdf (accessed on 5 June 2023).
- Baruah, S.; Bordoloi, A.K.; Gogoi, R.C.; Gogoi, M.K.; Hazarika, M. An integrated approach to the extraction of natural tea color, flavor and evaluation of antioxidant properties of tea. Two Bud 2012, 59, 126–129. [Google Scholar]



| Formulation | Group | Composition |
|---|---|---|
| BAN BBN | A B | Water + Grape juice + Lemon juice (Control) |
| BAHTC1 BBHTC1 | A B | Water + Grape juice + Lemon juice + Hops + Tea + Concentration 1 of salt |
| BAHTC2 BBHTC2 | A B | Water + Grape juice + Lemon juice + Hops + Tea + Concentration 2 of salt |
| BAHTC3 BBHTC3 | A B | Water + Grape juice + Lemon juice + Hops + Tea + Concentration 3 of salt |
| BAHMC1 BBHMC1 | A B | Water + Grape juice + Lemon juice + Hops + Mint + Concentration 1 of salt |
| BAHMC2 BAHMC2 | A B | Water + Grape juice + Lemon juice + Hops + Mint + Concentration 2 of salt |
| BAHMC3 BAHMC3 | A B | Water + Grape juice + Lemon juice + Hops + Mint + Concentration 3 of salt |
| Sugar (g/L) | Total Soluble Solids (°Brix) | Total Acid (g/L) | Malic Acid (g/L) | Alpha Amino Acids (mg/L) | Ammonia (mg/L) | |
|---|---|---|---|---|---|---|
| BAN | 73.90 ± 1.39 a | 4.23 ± 0.06 a | 2.24 ± 0.12 c | 1.43 ± 0.06 ab | 17.57 ± 2.00 b | 4.73 ± 0.93 a |
| BAHTC1 | 78.30 ± 0.17 d | 4.57 ± 0.06 abc | 2.30 ± 0.07 c | 1.50 ± 0.10 bc | 14.50 ± 0.69 a | 4.63 ± 0.32 a |
| BAHTC2 | 78.20 ± 0.17 cd | 4.67 ± 0.31 bc | 2.19 ± 0.15 bc | 1.50 ± 0.10 bc | 17.17 ± 2.69 b | 5.23 ± 0.78 a |
| BAHTC3 | 77.90 ± 0.17 cd | 4.47 ± 0.35 abc | 2.15 ± 0.20 bc | 1.53 ± 0.12 bc | 17.23 ± 1.68 b | 4.67 ± 0.29 a |
| BAHMC1 | 78.43 ± 0.06 d | 4.70 ± 0.10 bc | 2.39 ± 0.08 c | 1.50 ± 0.17 bc | 18.53 ± 0.49 b | 6.83 ± 0.47 b |
| BAHMC2 | 72.73 ± 0.23 a | 4.53 ± 0.32 abc | 2.24 ± 0.06 c | 1.50 ± 0.10 bc | 18.50 ± 0.53 b | 6.53 ± 0.55 b |
| BAHMC3 | 74.27 ± 0.42 a | 4.67 ± 0.29 bc | 2.21 ± 0.11 bc | 1.40 ± 0.10 ab | 17.00 ± 1.92 b | 7.90 ± 0.17 c |
| BBN | 77.60 ± 0.95 bcd | 4.47 ± 0.06 abc | 1.90 ± 0.18 a | 1.50 ± 0.10 bc | 31.97 ± 2.54 d | 20.67 ± 0.29 d |
| BBHTC1 | 76.47 ± 0.40 bc | 4.47 ± 0.21 abc | 1.98 ± 0.07 ab | 1.63 ± 0.12 c | 17.50 ± 0.00 b | 20.57 ± 0.40 d |
| BBHTC2 | 73.67 ± 1.23 a | 4.33 ± 0.32 ab | 1.76 ± 0.19 a | 1.37 ± 0.15 ab | 18.97 ± 0.64 b | 20.47 ± 0.65 d |
| BBHTC3 | 76.10 ± 0.17 b | 4.47 ± 0.35 abc | 1.90 ± 0.20 a | 1.50 ± 0.12 bc | 22.13 ± 1.68 c | 20.33 ± 0.29 d |
| BBHMC1 | 76.10 ± 2.00 b | 4.33 ± 0.12 ab | 1.82 ± 0.19 a | 1.43 ± 0.15 ab | 22.60 ± 1.39 c | 20.87 ± 0.47 d |
| BBHMC2 | 74.30 ± 1.73 a | 4.20 ± 0.20 a | 1.80 ± 0.21 a | 1.40 ± 0.00 ab | 22.23 ± 0.58 c | 20.83 ± 0.58 d |
| BBHMC3 | 73.43 ± 1.07 a | 4.83 ± 0.29 c | 1.75 ± 0.02 a | 1.30 ± 0.10 a | 18.23 ± 0.29 b | 20.10 ± 0.36 d |
| SAMPLES | RC | CI | T | pH | TPI | |
|---|---|---|---|---|---|---|
| BAN | Beginning End | 0.24 ± 0.01 b 0.43 ± 0.01 g | 0.46 ± 0.01 b 1.04 ± 0.01 g | 0.70 ± 0.01 bc 0.88 ± 0.01 ef | 3.30 ± 0.01 c 3.29 ± 0.01 c | 2.47 ± 0.01 ab 2.50 ± 0.03 bcde |
| BAHTC1 | Beginning End | 0.25 ± 0.01 bc 0.26 ± 0.01 a | 0.49 ± 0.01 def 0.56 ± 0.03 a | 0.71 ± 0.02 bcd 0.81 ± 0.02 ab | 3.29 ± 0.01 c 3.28 ± 0.01 b | 2.55 ± 0.01 cd 2.45 ± 0.03 abc |
| BAHTC2 | Beginning End | 0.24 ± 0.01 b 0.29 ± 0.02 ab | 0.47 ± 0.01 cde 0.67 ± 0.06 ab | 0.72 ± 0.02 d 0.89 ± 0.05 ef | 3.27 ± 0.01 b 3.27 ± 0.01 a | 2.55 ± 0.07 cd 2.51 ± 0.02 cde |
| BAHTC3 | Beginning End | 0.25 ± 0.01 b 0.30 ± 0.01 bc | 0.46 ± 0.02 cd 0.68 ± 0.03 b | 0.69 ± 0.01 b 0.82 ± 0.01 bc | 3.27 ± 0.01 b 3.28 ± 0.01 b | 2.54 ± 0.04 bcd 2.47 ± 0.05 abcd |
| BAHMC1 | Beginning End | 0.26 ± 0.01 cd 0.33 ± 0.02 bcd | 0.49 ± 0.01 ef 0.74 ± 0.07 bcd | 0.69 ± 0.02 b 0.83 ± 0.02 bcd | 3.25 ± 0.01 a 3.26 ± 0.01 a | 2.63 ± 0.04 e 2.47 ± 0.04 abcd |
| BAHMC2 | Beginning End | 0.28 ± 0.01 e 0.34 ± 0.03 cde | 0.52 ± 0.01 g 0.73 ± 0.06 bc | 0.67 ± 0.01 a 0.77 ± 0.02 a | 3.25 ± 0.01 a 3.26 ± 0.01 a | 2.51 ± 0.07 cd 2.44 ± 0.04 a |
| BAHMC3 | Beginning End | 0.27 ± 0.01 de 0.36 ± 0.02 de | 0.51 ± 0.02 fg 0.83 ± 0.06 cde | 0.69 ± 0.02 b 0.81 ± 0.02 b | 3.24 ± 0.01 a 3.26 ± 0.01 a | 2.57 ± 0.08 de 2.48 ± 0.02 abcd |
| BBN | Beginning End | 0.23 ± 0.01 b 0.30 ± 0.03 abc | 0.44 ± 0.02 a 0.67 ± 0.07 ab | 0.72 ± 0.01 cd 0.86 ± 0.01 def | 3.47 ± 0.01 g 3.49 ± 0.01 f | 2.47 ± 0.01 ab 2.45 ± 0.04 ab |
| BBHTC1 | Beginning End | 0.24 ± 0.01 b 0.37 ± 0.03 ef | 0.47 ± 0.01 cd 0.88 ± 0.09 ef | 0.71 ± 0.01 bcd 0.86 ± 0.02 def | 3.45 ± 0.01 ef 3.45 ± 0.01 d | 2.54 ± 0.04 bcd 2.46 ± 0.02 abc |
| BBHTC2 | Beginning End | 0.24 ± 0.01 b 0.43 ± 0.04 g | 0.46 ± 0.01 b 1.06 ± 0.13 g | 0.70 ± 0.01 bcd 0.87 ± 0.01 ef | 3.45 ± 0.01 f 3.47 ± 0.01 e | 2.44 ± 0.03 a 2.48 ± 0.01 abcd |
| BBHTC3 | Beginning End | 0.24 ± 0.01 b 0.36 ± 0.03 de | 0.46 ± 0.02 ab 0.86 ± 0.09 ef | 0.70 ± 0.02 bc 0.90 ± 0.02 f | 3.47 ± 0.01 g 3.50 ± 0.01 f | 2.49 ± 0.01 abc 2.54 ± 0.05 e |
| BBHMC1 | Beginning End | 0.25 ± 0.01 b 0.36 ± 0.02 de | 0.47 ± 0.01 cd 0.85 ± 0.04 def | 0.70 ± 0.01 bc 0.89 ± 0.01 ef | 3.45 ± 0.01 f 3.50 ± 0.01 f | 2.54 ± 0.03 bcd 2.48 ± 0.04 abcde |
| BBHMC2 | Beginning End | 0.27 ± 0.02 de 0.41 ± 0.01 g | 0.51 ± 0.03 fg 0.97 ± 0.02 fg | 0.66 ± 0.02 a 0.86 ± 0.02 cde | 3.43 ± 0.01 d 3.47 ± 0.01 e | 2.51 ± 0.03 abcd 2.53 ± 0.04 de |
| BBHMC3 | Beginning End | 0.27 ± 0.01 de 0.41 ± 0.04 fg | 0.51 ± 0.01 fg 0.96 ± 0.12 fg | 0.66 ± 0.01 a 0.86 ± 0.03 cde | 3.44 ± 0.01 de 3.49 ± 0.01 f | 2.45 ± 0.01 a 2.47 ± 0.03 abcd |
| BAN | BAHTC1 | BAHTC2 | BAHTC3 | BAHMC1 | BAHMC2 | BAHMC3 | BBN | BBHTC1 | BBHTC2 | BBHTC3 | BBHMC1 | BBHMC2 | BBHMC3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Color I | 3.3 a | 3.5 ab | 3.5 ab | 3.6 ab | 3.6 ab | 3.9 b | 3.6 ab | 3.5 ab | 3.6 ab | 3.8 ab | 3.3 a | 3.8 ab | 3.8 ab | 3.8 ab |
| Tonality | 2.4 a | 2.8 a | 2.8 a | 2.8 a | 2.3 a | 2.4 a | 2.5 a | 2.4 a | 2.1 a | 2.3 a | 2.0 a | 1.8 a | 2.0 a | 1.8 a |
| Turbidity | 1.4 a | 1.6 a | 1.5 a | 1.6 a | 1.4 a | 1.3 a | 1.6 a | 2.0 a | 1.6 a | 1.5 a | 1.4 a | 1.5 a | 1.5 a | 1.4 a |
| Aromatic I | 3.1 abc | 3.5 c | 3.0 abc | 3.1 abc | 3.1 abc | 3.3 abc | 3.4 abc | 2.6 a | 3.0 abc | 2.8 ab | 3.0 abc | 3.5 c | 3.0 abc | 3.4 bc |
| Aromatic Q | 2.6 ab | 3.1 abc | 3.0 abc | 3.3 abc | 3.1 abc | 3.3 abc | 3.4 abc | 2.6 ab | 3.1 abc | 2.5 a | 3.0 abc | 3.8 c | 3.6 bc | 3.4 abc |
| Herbaceous | 1.3 a | 2.0 abc | 1.9 abc | 1.8 abc | 1.6 ab | 1.6 ab | 1.6 ab | 1.8 abc | 2.4 bc | 2.0 abc | 2.5 c | 2.4 bc | 2.5 c | 2.1 bc |
| Floral | 1.8 a | 2.3 a | 2.1 a | 2.4 a | 2.6 a | 2.3 a | 2.6 a | 2.1 a | 2.3 a | 2.1 a | 2.3 a | 2.8 a | 2.9 a | 2.8 a |
| Fruity | 2.3 a | 2.1 a | 2.0 a | 2.1 a | 2.4 a | 2.4 a | 2.5 a | 1.8 a | 2.0 a | 2.1 a | 2.1 a | 1.9 a | 2.0 a | 2.1 a |
| Reduction | 1.0 a | 1.0 a | 1.0 a | 1.0 a | 1.1 a | 1.1 a | 1.0 a | 1.0 a | 1.0 a | 1.1 a | 1.0 a | 1.0 a | 1.0 a | 1.0 a |
| Oxidation | 1.3 ab | 1.5 b | 1.5 b | 1.5 b | 1.3 ab | 1.4 ab | 1.3 ab | 1.0 a | 1.1 ab | 1.1 ab | 1.1 ab | 1.1 ab | 1.1 ab | 1.1 ab |
| Body | 2.1 a | 2.8 a | 2.8 a | 2.5 a | 2.6 a | 2.8 a | 2.8 a | 2.3 a | 2.6 a | 2.5 a | 2.8 a | 3.0 a | 2.4 a | 2.8 a |
| Bitterness | 1.4 a | 1.4 a | 1.4 a | 1.6 a | 1.8 a | 1.5 a | 1.5 a | 1.4 a | 1.8 a | 1.8 a | 1.8 a | 1.8 a | 1.9 a | 1.6 a |
| Sweetness | 2.5 a | 2.1 a | 2.0 a | 2.3 a | 2.1 a | 2.3 a | 2.3 a | 2.3 a | 2.1 a | 2.4 a | 2.3 a | 2.3 a | 2.1 a | 2.0 a |
| Salinity | 1.0 a | 2.0 b | 2.9 bc | 2.3 bc | 2.5 bc | 2.8 bc | 2.5 bc | 1.0 a | 2.6 bc | 2.5 bc | 2.4 bc | 2.4 bc | 3.0 c | 2.6 bc |
| Acidity | 2.5 a | 2.3 a | 2.4 a | 2.4 a | 2.5 a | 2.5 a | 2.8 a | 2.5 a | 2.5 a | 2.8 a | 2.6 a | 2.5 a | 2.4 a | 2.6 a |
| Global P | 2.9 a | 3.6 bc | 3.3 abc | 3.5 abc | 3.0 ab | 3.5 abc | 3.8 c | 2.9 a | 2.9 a | 3.0 ab | 3.4 abc | 3.9 c | 3.3 abc | 3.9 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendaali, Y.; Vaquero, C.; Escott, C.; González, C.; Morata, A. Isotonic Drinks Based on Organic Grape Juice and Naturally Flavored with Herb and Spice Extracts. Beverages 2023, 9, 49. https://doi.org/10.3390/beverages9020049
Bendaali Y, Vaquero C, Escott C, González C, Morata A. Isotonic Drinks Based on Organic Grape Juice and Naturally Flavored with Herb and Spice Extracts. Beverages. 2023; 9(2):49. https://doi.org/10.3390/beverages9020049
Chicago/Turabian StyleBendaali, Yasmina, Cristian Vaquero, Carlos Escott, Carmen González, and Antonio Morata. 2023. "Isotonic Drinks Based on Organic Grape Juice and Naturally Flavored with Herb and Spice Extracts" Beverages 9, no. 2: 49. https://doi.org/10.3390/beverages9020049
APA StyleBendaali, Y., Vaquero, C., Escott, C., González, C., & Morata, A. (2023). Isotonic Drinks Based on Organic Grape Juice and Naturally Flavored with Herb and Spice Extracts. Beverages, 9(2), 49. https://doi.org/10.3390/beverages9020049