Polyphenols Profile and Antioxidant Activity Characterization of Commercial North Italian Ciders in Relation to Their Geographical Area of Production and Cidermaking Procedures
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Total Polyphenolic Contents by the Folin-Ciocalteau Reagent
2.2. Antioxidant Activity
2.3. Other Analyses
2.4. Identification and Quantification of Polyphenols by HPLC
2.5. Statistical Analysis
3. Results and Discussion
3.1. Polyphenols Profiles of the Commercial Italian Ciders
3.2. Antioxidant Activity of Ciders
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Blanco-Gomis, D.; Herrero-Sanchez, I.; Alonso, J.J.M. Characterisation of apple cider cultivars by chemometric techniques using data from high-performance liquid chromatography and flow-injection analysis. Analyst 1998, 123, 1187–1191. [Google Scholar] [CrossRef]
- Lea, A. Craft Cider Making; The Crowood Press: Marlborough, UK, 2015. [Google Scholar]
- Alberti, A.; Vieira, R.G.; Drilleau, J.F.; Wosiacki, G.; Nogueira, A. Apple wine processing with different nitrogen contents. Braz. Arch. Biol. Technol. 2011, 54, 551–558. [Google Scholar] [CrossRef]
- Lomolino, G.; Marangon, M.; Vincenzi, S.; De Iseppi, A. Sparkling Cider Paired with Italian Cheese: Sensory Analysis and Consumer Assessment. Beverages 2022, 8, 82. [Google Scholar] [CrossRef]
- Fratianni, F.; De Giulio, A.; Sada, A.; Nazzaro, F. Biochemical characteristics and biological properties of annurca apple cider. J. Med. Food 2012, 15, 18–23. [Google Scholar] [CrossRef]
- Regio, D. Regio decreto n. 497, Disposizioni per la difesa dei vini tipici, pubblicato sulla Gazzetta Ufficiale n. 97 del 24 Aprile 1924. Gazz. Uff. 1924, 97, 1559–1570. [Google Scholar]
- Regio, D. Regio decreto n. 62 del 11 Gennaio 1930 convertito in legge n. 1164 il 10 Luglio 1930. Gazz. Uff. 1930, 204, 3433–3444. [Google Scholar]
- Stara, P. Il discorso del vino in Italia. In Origine, Identità e Qualità Come Problemi Storico-Sociali; Zero in Condotta: Milano, Italy, 2013. [Google Scholar]
- Li, W.; Zhao, Y.; Liu, C.; Li, S.; Yue, T.; Yuan, Y. Effects of secondary fermentation of four in-bottle Saccharomyces cerevisiae strains on sparkling cider sensory quality. Food Biosci. 2022, 48, 101731. [Google Scholar] [CrossRef]
- Riekstina-Dolge, R.; Kruma, Z.; Karklina, D. Aroma composition and polyphenol content of ciders available in Latvian market. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2012, 6, 1063–1067. [Google Scholar]
- Tufariello, M.; Palombi, L.; Rizzuti, A.; Musio, B.; Capozzi, V.; Gallo, V.; Mastrorilli, P.; Grieco, F. Volatile and chemical profiles of Bombino sparkling wines produced with autochthonous yeast strains. Food Control 2023, 145, 109462. [Google Scholar] [CrossRef]
- Farina, V.; Mineo, V.; Francesca, N.; Moscheti, G.; Settani, L.; Planeta, D. Attitudine alla trasformazione in sidro di varietà di mele autoctone siciliane. Ind. Delle Bevande 2011, 231, 11–17. [Google Scholar]
- Fratianni, F.; Coppola, R.; Nazzaro, F. Phenolic composition and antimicrobial and antiquorum sensing activity of an ethanolic extract of peels from the apple cultivar Annurca. J. Med. Food 2011, 14, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Mainente, F.; Rizzi, C.; Zoccatelli, G.; Chignola, R.; Simonato, B.; Pasini, G. Setup of a procedure for cider proteins recovery and quantification. Eur. Food Res. Technol. 2016, 242, 1803–1811. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Zapparoli, G. Yeast species diversity in apple juice for cider production evidenced by culture-based method. Folia Microbiol. 2018, 63, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Salces, R.M.; Guyot, S.; Herrero, C.; Berrueta, L.A.; Drilleau, J.F.; Gallo, B.; Vicente, F. Chemometric characterisation of Basque and French ciders according to their polyphenolic profiles. Anal. Bioanal. Chem. 2004, 379, 464–475. [Google Scholar] [CrossRef]
- Hathway, D.E.; Seakins, J.W.T. The influence of tannins on the degradation of pectin by pectinase enzymes. Biochem. J. 1958, 70, 158–163. [Google Scholar] [CrossRef]
- Commisso, M.; Bianconi, M.; Di Carlo, F.; Poletti, S.; Bulgarini, A.; Munari, F.; Negri, S.; Stocchero, M.; Ceoldo, S.; Avesani, L.; et al. Multi-approach metabolomics analysis and artificial simplified phytocomplexes reveal cultivar-dependent synergy between polyphenols and ascorbic acid in fruits of the sweet cherry (Prunus avium L.). PLoS ONE 2017, 12, e0180889. [Google Scholar] [CrossRef]
- Hidalgo, M.; Sánchez-Moreno, C.; de Pascual-Teresa, S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Zagorac, D.Č.D.; Ćirić, I.Ž.; Pergal, M.V.; Relić, D.J.; Todić, S.R.; Natić, M.M. Phenolic profiles, antioxidant activity and minerals in leaves of different grapevine varieties grown in Serbia. J. Food Compos. Anal. 2017, 62, 76–83. [Google Scholar] [CrossRef]
- Vilanova, M.; Rodríguez, I.; Canosa, P.; Otero, I.; Gamero, E.; Moreno, D.; Talaverano, I.; Valdés, E. Variability in chemical composition of Vitis vinifera cv Mencía from different geographic areas and vintages in Ribeira Sacra (NW Spain). Food Chem. 2015, 169, 187–196. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Vakarelova, M.; Zanoni, F.; Lardo, P.; Rossin, G.; Mainente, F.; Chignola, R.; Menin, A.; Rizzi, C.; Zoccatelli, G. Production of stable food-grade microencapsulated astaxanthin by vibrating nozzle technology. Food Chem. 2017, 221, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Picinelli-Lobo, A.; García, Y.D.; Sánchez, J.M.; Madrera, R.R.; Valles, B.S. Phenolic and antioxidant composition of cider. J. Food Compos. Anal. 2009, 22, 644–648. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Commission, E.U. Commission Regulation (EEC) No. 2676/90 determining Community methods for the analysis of wines. Off. J. L 1990, 272, 1–192. [Google Scholar]
- Abdi, H. Partial least squares regression and projection on latent structure regression (PLS Regression). WIREs Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Partial least squares methods: Partial least squares correlation and partial least square regression. In Computational Toxicology: Volume II, Methods in Molecular Biology; Reisfeld, B., Mayeno, A.N., Eds.; Springer Science & Business Media: Fort Collins, CO, USA, 2013; Volume 930, pp. 549–579. [Google Scholar]
- Wehrens, R.; Van der Linden, W.E. Bootstrapping principal component regression models. J. Chemom. 1997, 11, 157–171. [Google Scholar] [CrossRef]
- Alberti, A.; Machado dos Santos, T.P.; Ferreira Zielinski, A.A.; Eleutério dos Santos, C.M.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. LWT-Food Sci. Technol. 2016, 65, 436–443. [Google Scholar] [CrossRef]
- Budak, N.H.; Ozçelik, F.; Güzel-Seydim, Z.B. Antioxidant activity and phenolic content of apple cider. Turk. J. Agric.-Food Sci. Technol. 2015, 3, 356–360. [Google Scholar] [CrossRef]
- Picinelli, A.; Suárez, B.; Moreno, J.; Rodríguez, R.; Caso-García, L.M.; Mangas, J.J. Chemical characterization of Asturian cider. J. Agric. Food Chem. 2000, 48, 3997–4002. [Google Scholar] [CrossRef]
- Reuss, R.M.; Stratton, J.E.; Smith, D.A.; Read, P.E.; Cuppett, S.L.; Parkhurst, A.M. Malolactic fermentation as a technique for the deacidification of hard apple cider. J. Food Sci. 2010, 75, C74–C78. [Google Scholar] [CrossRef]
- Rumpunen, K.; Ekholm, A.; Nybom, H. Swedish apple cultivars vary in traits for juice and cider making. Acta Hortic. 2017, 1172, 255–258. [Google Scholar] [CrossRef]
- Girschik, L.; Jones, J.E.; Kerslake, F.L.; Robertson, M.; Dambergs, R.G.; Swarts, N.D. Apple variety and maturity profiling of base ciders using UV spectroscopy. Food Chem. 2017, 228, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Witrick, K.A.; Goodrich, K.M.; Neilson, A.P.; Hurley, E.K.; Peck, G.M.; Stewart, A.C. Characterization of the polyphenol composition of 20 cultivars of cider, processing, and dessert apples (Malus x domestica Borkh.) grown in Virginia. J. Agric. Food Chem. 2014, 62, 10181–10191. [Google Scholar] [CrossRef] [PubMed]
- Quendler, S.; Martinello, L. Succo, sidro e aceto di mela: Appunti da esperienze frontaliere. [Essig- und Obstweinbereitung: Theorie, Verfahren, Bestimmungen]. Veneto Agric. 2011, E439, 1–72. [Google Scholar]
- Mainente, F.; Simonato, B.; Pasini, G.; Franchin, C.; Arrigoni, G.; Rizzi, C. Hen egg white lysozyme is a hidden allergen in Italian commercial ciders. Food Addit. Contam.-Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 145–151. [Google Scholar] [CrossRef]
- Del Campo, G.; Berregi, I.; Santos, J.I.; Dueñas, M.; Irastorza, A. Development of alcoholic and malolactic fermentations in highly acidic and phenolic apple musts. Bioresour. Technol. 2008, 99, 2857–2863. [Google Scholar] [CrossRef]
- Sánchez, A.; Rodríguez, R.; Coton, M.; Coton, E.; Herrero, M.; García, L.A.; Díaz, M. Population dynamics of lactic acid bacteria during spontaneous malolactic fermentation in industrial cider. Food Res. Int. 2010, 43, 2101–2107. [Google Scholar] [CrossRef]
- Madrera, R.R.; Lobo, A.P.; Valles, B.S. Phenolic profile of Asturian (Spain) natural cider. J. Agric. Food Chem. 2006, 54, 120–124. [Google Scholar] [CrossRef]
- Jeantet, R.; Croguennec, T.; Schuck, P. Handbook of Food Science and Technology 3: Food Biochemistry and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 3. [Google Scholar]
- Beech, F.W.; Carr, J.G. Cider and perry. Alcohol. Beverages 1977, 1, 139–313. [Google Scholar]
- Kosseva, M.; Joshi, V.K.; Panesar, P.S. Science and Technology of Fruit Wine Production; Academic Press: London, UK, 2016. [Google Scholar]
- Coton, E.; Coton, M.; Levert, D.; Casaregola, S.; Sohier, D. Yeast ecology in French cider and black olive natural fermentations. Int. J. Food Microbiol. 2006, 108, 130–135. [Google Scholar] [CrossRef]
- Cousin, F.J.; Le Guellec, R.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.-M.; Cretenet, M. Microorganisms in fermented apple beverages: Current knowledge and future directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, W.; Davenport, B.; Querol, A.; Dobson, A. The role of indigenous yeasts in traditional Irish cider fermentations. J. Appl. Microbiol. 2004, 97, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, M.; Cappello, M.S.; Andreolli, M.; Zapparoli, G. Characterization of selected species of Pichia and Candida for their growth capacity in apple and grape must and their biofilm parameters. Lett. Appl. Microbiol. 2023, 76, ovac028. [Google Scholar]
- Simonato, B.; Lorenzini, M.; Zapparoli, G. Effects of post-harvest fungal infection of apples on chemical characteristics of cider. LWT 2021, 138, 110620. [Google Scholar] [CrossRef]
- Cusano, E.; Cagliani, L.R.; Consonni, R.; Simonato, B.; Zapparoli, G. NMR-based metabolic profiling of different yeast fermented apple juices. LWT 2020, 118, 108771. [Google Scholar] [CrossRef]
- Macheix, J.J. Fruit Phenolics: 0; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Laaksonen, O.; Kuldjarv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef]
- Nykänen, L.; Suomalainen, H. Aroma of Beer, Wine and Distilled Alcoholic Beverages; Reidel Publishing Company, Ed.; Springer Science & Business Media: London, UK, 1983; Volume 3. [Google Scholar]
- Zuriarrain, A.; Zuriarrain, J.; Puertas, A.I.; Duenas, M.T.; Ostra, M.; Berregi, I. Polyphenolic profile in cider and antioxidant power. J. Sci. Food Agric. 2015, 95, 2931–2943. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Kiralj, R.; Ferreira, M. Basic validation procedures for regression models in QSAR and QSPR studies: Theory and application. J. Braz. Chem. Soc. 2009, 20, 770–787. [Google Scholar] [CrossRef]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Leon-Gonzalez, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Duarte, A.P.; Pereira, L.; Domingues, F. Interactions between the major bioactive polyphenols of berries: Effects on antioxidant properties. Eur. Food Res. Technol. 2017, 244, 175–185. [Google Scholar] [CrossRef]
- Nicolini, G.; Román, T.; Carlin, S.; Malacarne, M.; Nardin, T.; Bertoldi, D.; Larcher, R. Characterisation of single-variety still ciders produced with dessert apples in the Italian Alps. J. Inst. Brew. 2018, 124, 457–466. [Google Scholar] [CrossRef]
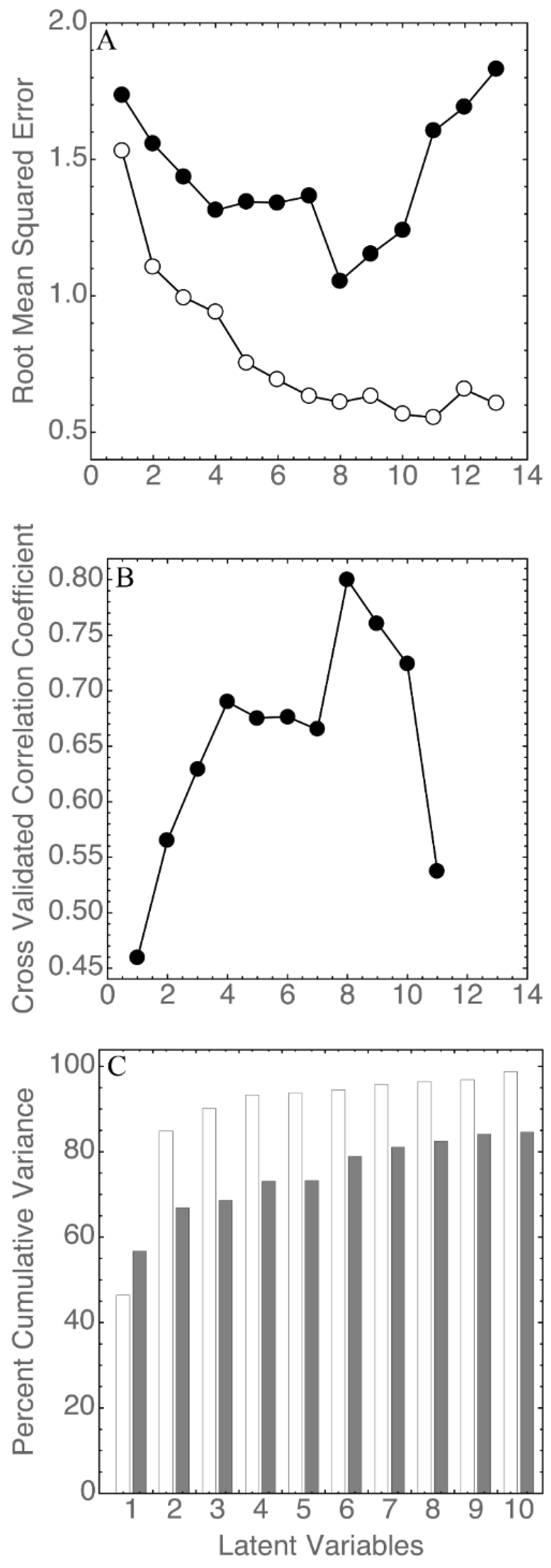
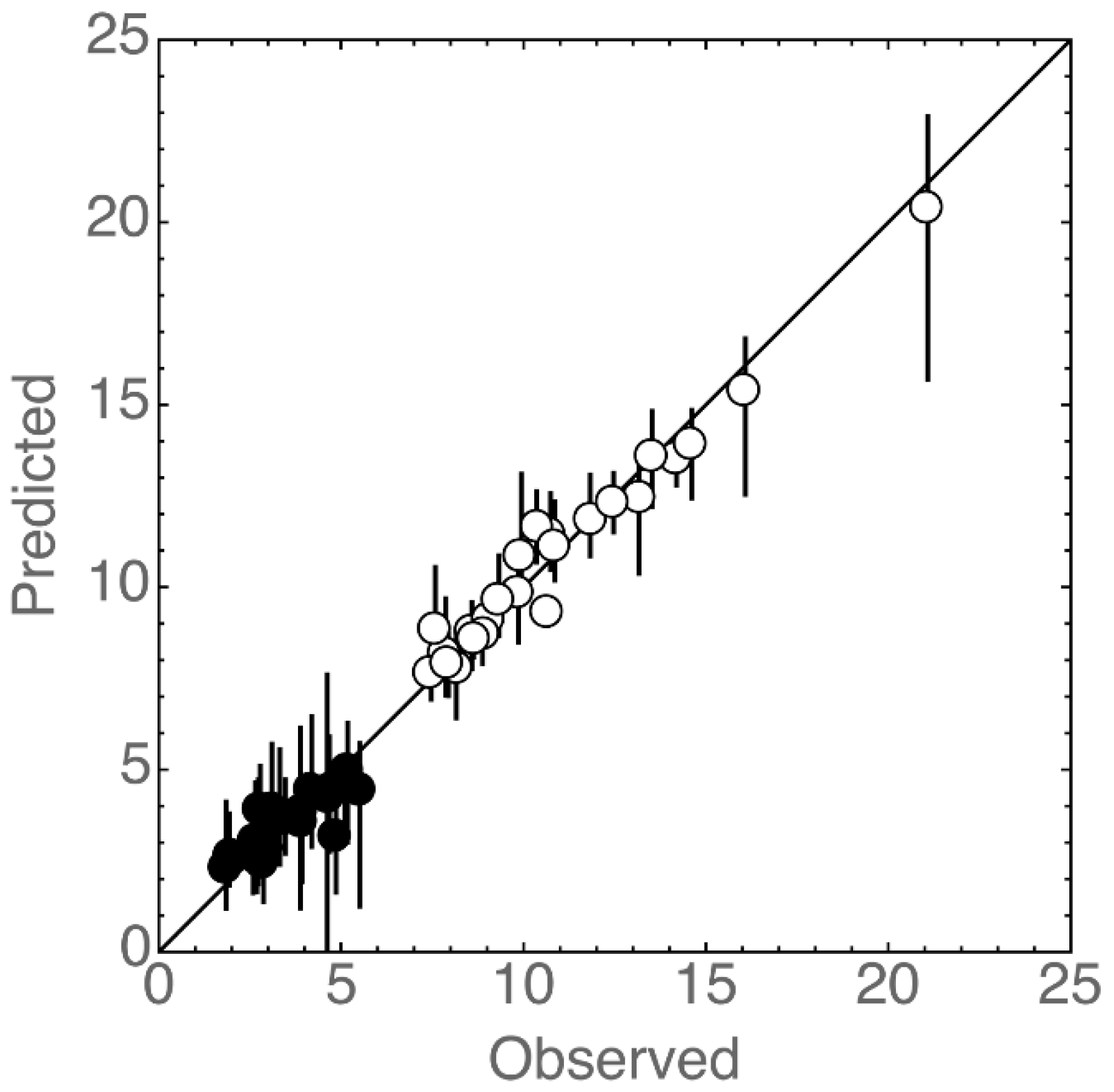
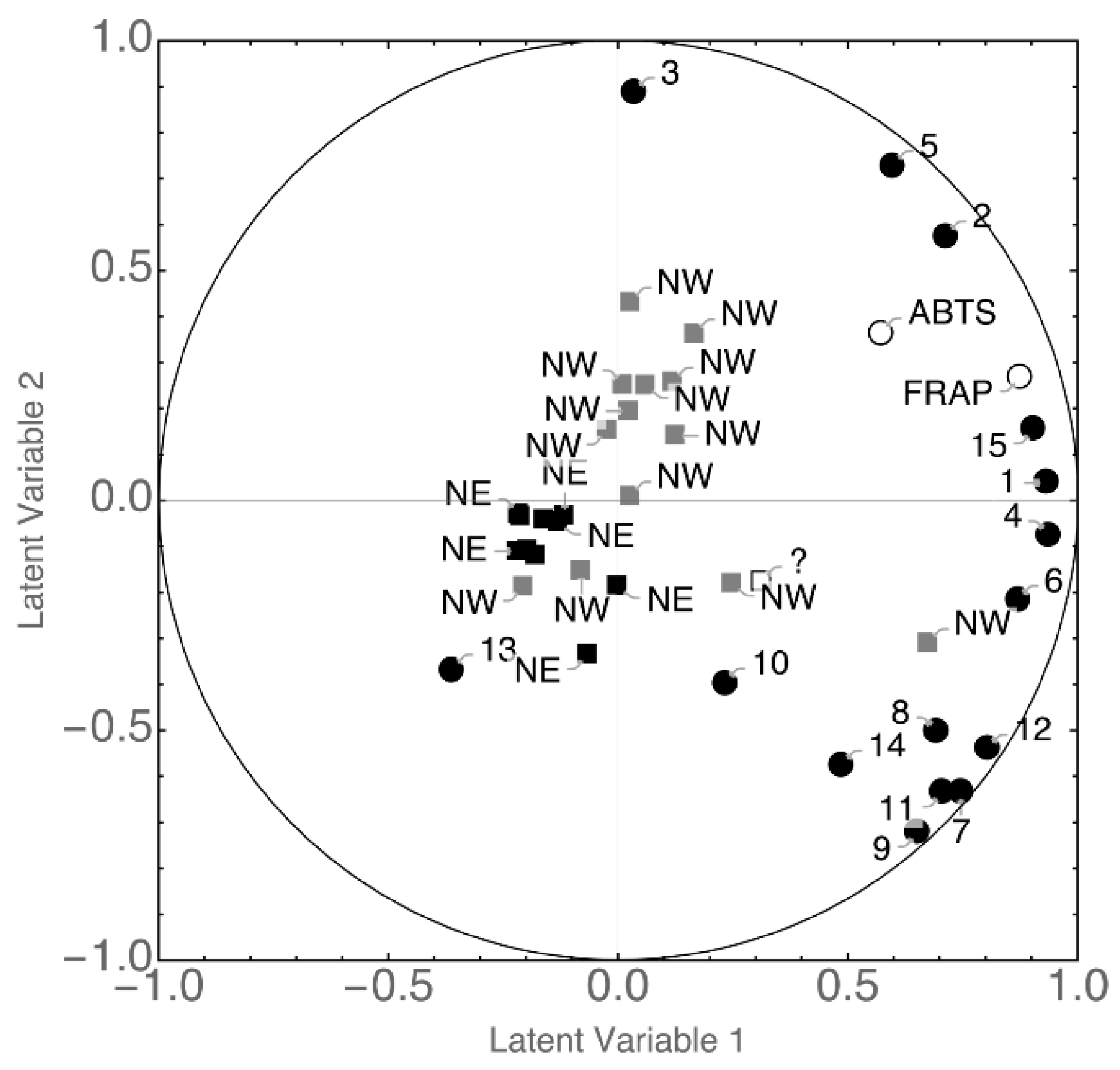
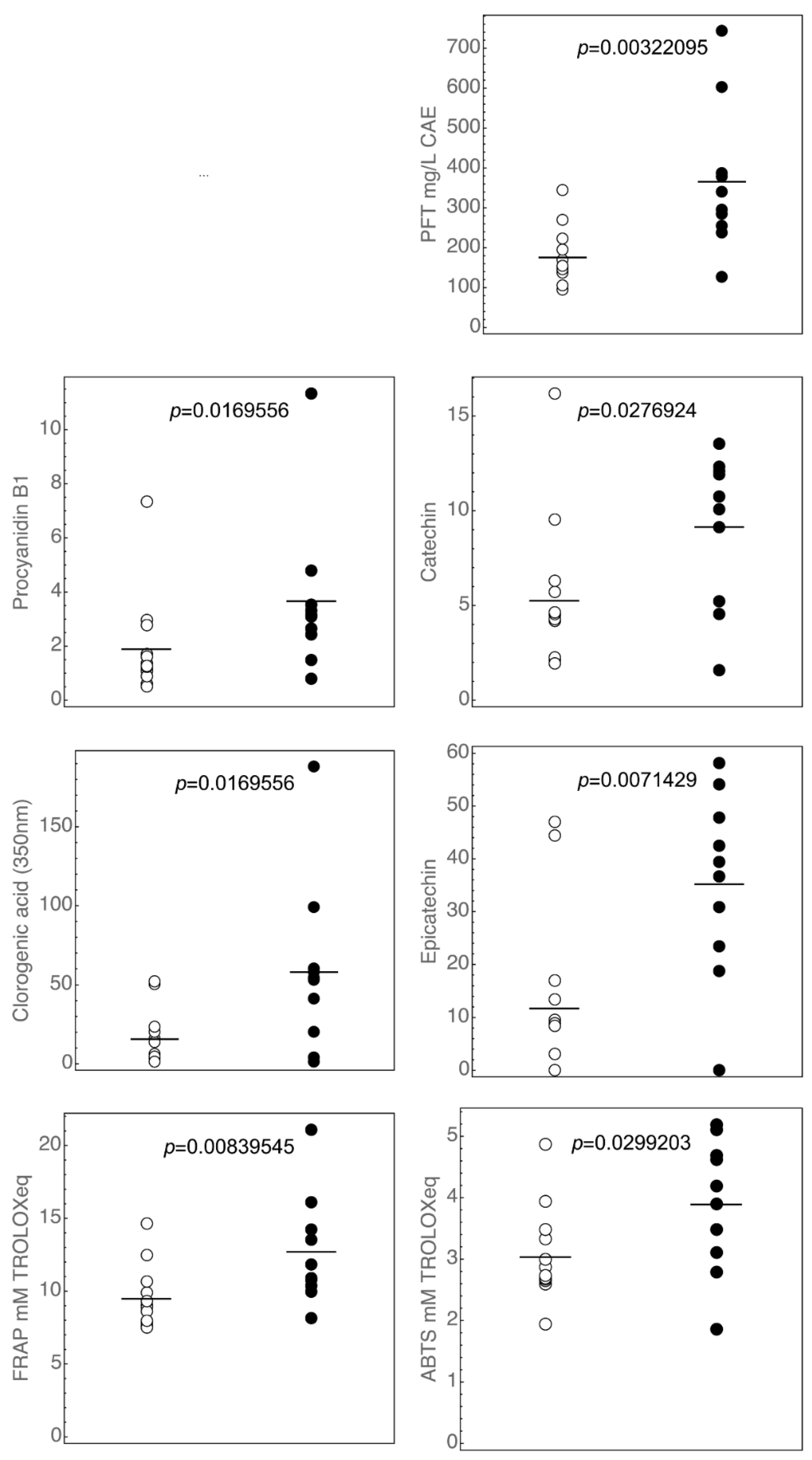
| Sample | Ethanol % vol | pH | Titratable Acidity a | Geographical Origin | Cidermaking Procedure |
|---|---|---|---|---|---|
| S1 | 4.8 | 3.43 | 3.44 | North-East | Charmat |
| S2 | 7 | 3.3 | 4.89 | North-East | Charmat |
| S3 | 5 | 3.55 | 3.01 | North-East | Charmat |
| S4 | 8 | 3.75 | 3.49 | North-East | Charmat |
| S5 | 8 | 3.79 | 2.88 | North-West | Champenoise |
| S6 | 3 | 3.62 | 4.89 | North-West | Charmat |
| S7 | 4.5 | 3.75 | 3.06 | North-West | Champenoise |
| S8 | 6 | 3.61 | 4.57 | North-West | Charmat |
| S9 | 3.5 | 3.33 | 4.87 | Unknown | Charmat |
| S10 | 8 | 3.48 | 5.42 | North-East | - |
| S11 | 8 | 3.80 | 3.13 | North-West | Champenoise |
| S12 | 8 | 3.18 | 4.92 | North-East | Charmat |
| S13 | 8 | 3.31 | 6.33 | North-West | Champenoise |
| S14 | 8 | 3.76 | 3.11 | North-East | Charmat |
| S15 | 6 | 3.65 | 4.81 | North-East | Charmat |
| S16 | 3.5 | 3.57 | 5.45 | North-East | Charmat |
| S17 | 3 | 3.22 | 3.51 | North-West | Charmat |
| S18 | 5 | 3.14 | 3.80 | North-West | Charmat |
| S19 | 5 | 3.63 | 5.55 | North-East | Charmat |
| S20 | 7 | 3.59 | 5.46 | North-West | Champenoise |
| S21 | 7 | 3.59 | 4.86 | North-East | Charmat |
| S22 | 7 | 3.78 | 3.22 | North-East | Charmat |
| S23 | 6 | 3.82 | 4.38 | North-East | Charmat |
| S24 | 8.5 | 3.79 | 3.99 | North-West | Champenoise |
| mean | 6 | 3.56 | 4.29 | ||
| dev.st | 2 | 0.21 | 0.98 | ||
| min | 3.5 | 3.14 | 2.88 | ||
| max | 8.5 | 3.80 | 6.33 |
| Min | Max | Mean | Dev.st | |
|---|---|---|---|---|
| Phenolic acids | ||||
| Clorogenic acid (350 nm) | 0.93 | 188.04 | 37.62 | 45.81 |
| Flavan-3-ols | ||||
| Procyanidin B1 | 0.49 | 11.34 | 2.76 | 2.47 |
| Catechin | 1.59 | 16.19 | 7.14 | 4.36 |
| Procyanidin B2 | 0.03 | 29.15 | 11.54 | 10.51 |
| Epicatechin | 0.02 | 58.16 | 21.88 | 19.79 |
| Dihydrochalcone | ||||
| Phloridzin (280 nm) | 0 | 39.17 | 12.76 | 10.83 |
| Flavonols | ||||
| Quercetin-3-arabinofuranoside | 0 | 4.33 | 0.78 | 1.05 |
| Quercetin-3-xyloside | 0 | 2.70 | 0.86 | 0.64 |
| Quercetin-3-arabinopiranoside | 0 | 4.77 | 0.97 | 1.04 |
| Quercetin-3-glucoside (350 nm) | 0 | 4.90 | 1.05 | 1.02 |
| Quercetin | 0.61 | 4.57 | 1.73 | 0.91 |
| Quercetin-3-rhamnoside (350 nm) | 0 | 12.70 | 3.30 | 2.46 |
| Hyperoside (quercetin-3-galactoside) | 0 | 30.52 | 8.90 | 7.47 |
| Volatile phenols | ||||
| Tyrosol | 0 | 14.16 | 5.51 | 3.40 |
| Sample | FRAP a | DPPH a | ABTS a | PFT b |
|---|---|---|---|---|
| S1 | 8.58 | 8.75 | 2.66 | 149.05 |
| S2 | 9.02 | 9.13 | 2.59 | 141.35 |
| S3 | 7.86 | 8.13 | 2.65 | 150.67 |
| S4 | 8.88 | 9.01 | 2.87 | 136.36 |
| S5 | 14.19 | 13.56 | 5.10 | 377.16 |
| S6 | 11.83 | 11.54 | 3.10 | 338.69 |
| S7 | 21.08 | 19.47 | 4.62 | 743.07 |
| S8 | 10.74 | 10.61 | 3.47 | 254.87 |
| S9 | 13.16 | 12.68 | 5.51 | 475.42 |
| S10 | 8.63 | 8.8 | 2.99 | 144.73 |
| S11 | 13.52 | 12.99 | 5.18 | 387.01 |
| S12 | 9.86 | 9.85 | 3.32 | 169.16 |
| S13 | 8.16 | 8.40 | 1.85 | 126.78 |
| S14 | 10.66 | 10.54 | 2.67 | 194.67 |
| S15 | 12.47 | 12.09 | 3.47 | 268.64 |
| S16 | 14.61 | 13.92 | 3.93 | 344.36 |
| S17 | 10.36 | 10.28 | 2.78 | 294.82 |
| S18 | 16.08 | 15.18 | 4.19 | 602.29 |
| S19 | 7.58 | 7.90 | 2.69 | 154.18 |
| S20 | 9.94 | 9.92 | 3.89 | 284.29 |
| S21 | 7.47 | 7.80 | 1.94 | 93.17 |
| S22 | 7.94 | 8.20 | 4.86 | 104.37 |
| S23 | 9.33 | 9.40 | 2.73 | 221.39 |
| S24 | 10.86 | 10.70 | 4.68 | 236.51 |
| mean | 10.95 | 10.79 | 3.49 | 266.38 |
| dev.st | 3.24 | 2.78 | 1.10 | 161.98 |
| min | 7.47 | 7.80 | 1.88 | 93.17 |
| max | 21.08 | 19.47 | 5.51 | 743.07 |
| Var. # | Predictors | a FRAP | a ABTS |
|---|---|---|---|
| b Intercept | 0.113 ± 1.0·10−3 | 0.041 ± 4.3·10−4 | |
| 1 | Procyanidin B1 | 0.137 ± 1.5·10−3 | 0.003 ± 7.9·10−4 |
| 2 | Catechin | 0.277 ± 2.5·10−3 | 0.048 ± 1.6·10−3 |
| 3 | Procyanidin B2 | 0.162 ± 7.9·10−4 | 0.042 ± 4.9·10−4 |
| 4 | Clorogenic acid | −0.039 ± 4.1·10−4 | −0.013 ± 2.3·10−4 |
| 5 | Epicatechin | −0.043 ± 5.4·10−4 | 0.017 ± 3.5·10−4 |
| 6 | Phloridzin | −0.204 ± 1.7·10−3 | −0.053 ± 1.0·10−3 |
| 7 | Quercitin-3-glucoside | 0.080 ± 8.1·10−4 | 0.023 ± 4.0·10−4 |
| 8 | Quercitin-3-rhamnoside | 0.416 ± 2.4·10−3 | 0.110 ± 1.6·10−3 |
| 9 | Quercitin-3-xyloside | 0.072 ± 4.0·10−4 | 0.026 ± 2.2·10−4 |
| 10 | Quercitin-3-arabinofuranoside | 0.005 ± 1.5·10−3 | 0.038 ± 7.8·10−4 |
| 11 | Quercitin-3-arabinopiranoside | 0.060 ± 7.3·10−4 | 0.011 ± 4.2·10−4 |
| 12 | Hyperoside | 0.290 ± 2.2·10−3 | 0.096 ± 1.2·10−3 |
| 13 | Tyrosol | 0.039 ± 1.5·10−3 | 0.069 ± 9.3·10−4 |
| 14 | Quercitin | 0.175 ± 2.8·10−3 | 0.058 ± 1.1·10−3 |
| 15 | PFT | 0.022 ± 1.3·10−4 | 0.004 ± 6.4·10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mainente, F.; Vincenzi, S.; Rizzi, C.; Pasini, G. Polyphenols Profile and Antioxidant Activity Characterization of Commercial North Italian Ciders in Relation to Their Geographical Area of Production and Cidermaking Procedures. Beverages 2023, 9, 54. https://doi.org/10.3390/beverages9020054
Mainente F, Vincenzi S, Rizzi C, Pasini G. Polyphenols Profile and Antioxidant Activity Characterization of Commercial North Italian Ciders in Relation to Their Geographical Area of Production and Cidermaking Procedures. Beverages. 2023; 9(2):54. https://doi.org/10.3390/beverages9020054
Chicago/Turabian StyleMainente, Federica, Simone Vincenzi, Corrado Rizzi, and Gabriella Pasini. 2023. "Polyphenols Profile and Antioxidant Activity Characterization of Commercial North Italian Ciders in Relation to Their Geographical Area of Production and Cidermaking Procedures" Beverages 9, no. 2: 54. https://doi.org/10.3390/beverages9020054
APA StyleMainente, F., Vincenzi, S., Rizzi, C., & Pasini, G. (2023). Polyphenols Profile and Antioxidant Activity Characterization of Commercial North Italian Ciders in Relation to Their Geographical Area of Production and Cidermaking Procedures. Beverages, 9(2), 54. https://doi.org/10.3390/beverages9020054










