Role of p-Coumaric Acid and Micronutrients in Sulfur Dioxide Tolerance in Brettanomyces bruxellensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strain and Maintenance
2.2. Culture Conditions
2.3. Sulfite Stress
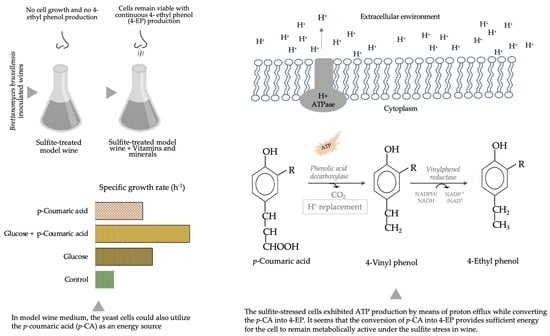
2.4. H+ Translocation
2.5. Chemical Analysis
3. Results
3.1. Effect of Sulfite on Cell Viability and 4-EP Production
3.2. Effect of Micronutrients on Cell Activity
3.3. Effect of p-Coumaric Acid on Growth and Proton Efflux
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malfeito-Ferreira, M. Two Decades of “Horse Sweat” Taint and Brettanomyces Yeasts in Wine: Where Do We Stand Now? Beverages 2018, 4, 32. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdie, D.; Boidron, J.; Pons, M. The Origin of Ethylphenols in Wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Schopp, L.M.; Lee, J.; Osborne, J.P.; Chescheir, S.C.; Edwards, C.G. Metabolism of nonesterified and esterified hydroxycinnamic acids in red wines by Brettanomyces bruxellensis. J. Agric. Food Chem. 2013, 61, 11610–11617. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 1, ISBN 0-470-01035-5. [Google Scholar]
- Agnolucci, M.; Cristani, C.; Maggini, S.; Rea, F.; Cossu, A.; Tirelli, A.; Nuti, M. Impact of Sulphur Dioxide on the Viability, Culturability, and Volatile Phenol Production of Dekkera bruxellensis in Wine. Ann. Microbiol. 2014, 64, 653–659. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.N. The Influence of Brettanomyces/Dekkera Sp. Yeasts and Lactic Acid Bacteria on the Ethylphenol Content of Red Wines. Am. J. Enol. Vitic. 1995, 46, 463–468. [Google Scholar] [CrossRef]
- Du Toit, W.; Pretorius, I.; Lonvaud-Funel, A. The Effect of Sulphur Dioxide and Oxygen on the Viability and Culturability of a Strain of Acetobacter Pasteurianus and a Strain of Brettanomyces bruxellensis Isolated from Wine. J. Appl. Microbiol. 2005, 98, 862–871. [Google Scholar] [CrossRef]
- Duckitt, E. Investigating the Impact of Sulphur Dioxide on Brettanomyces bruxellensis at a Molecular and Cellular Level. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2012. [Google Scholar]
- Serpaggi, V.; Remize, F.; Recorbet, G.; Gaudot-Dumas, E.; Sequeira-Le Grand, A.; Alexandre, H. Characterization of the “Viable but Nonculturable” (VBNC) State in the Wine Spoilage Yeast Brettanomyces. Food Microbiol. 2012, 30, 438–447. [Google Scholar] [CrossRef]
- Hinze, H.; Holzer, H. Analysis of the Energy Metabolism after Incubation of Saccharomyces cerevisiae with Sulfite or Nitrite. Arch. Microbiol. 1986, 145, 27–31. [Google Scholar] [CrossRef]
- Macris, B.J.; Markakis, P. Transport and Toxicity of Sulphur Dioxide in Saccharomyces cerevisiae Var Ellipsoideus. J. Sci. Food Agric. 1974, 25, 21–29. [Google Scholar] [CrossRef]
- Pagano, D.A.; Zeiger, E.; Stark, A.-A. Autoxidation and Mutagenicity of Sodium Bisulfite. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1990, 228, 89–96. [Google Scholar] [CrossRef]
- Abramovič, H. Antioxidant Properties of Hydroxycinnamic Acid Derivatives: A Focus on Biochemistry, Physicochemical Parameters, Reactive Species, and Biomolecular Interactions. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 843–852. [Google Scholar]
- Campolongo, S.; Siegumfeldt, H.; Aabo, T.; Cocolin, L.; Arneborg, N. The Effects of Extracellular pH and Hydroxycinnamic Acids Influence the Intracellular PH of Brettanomyces bruxellensis DSM 7001. LWT-Food Sci. Technol. 2014, 59, 1088–1092. [Google Scholar] [CrossRef]
- Laforgue, R.; Lonvaud-Funel, A. Hydroxycinnamic Acid Decarboxylase Activity of Brettanomyces bruxellensis Involved in Volatile Phenol Production: Relationship with Cell Viability. Food Microbiol. 2012, 32, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, M.R.; Alberto, M.R.; de Nadra, M.M. Antibacterial Effect of Phenolic Compounds from Different Wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Edlin, D.A.; Narbad, A.; Dickinson, J.R.; Lloyd, D. The Biotransformation of Simple Phenolic Compounds by Brettanomyces Anomalus. FEMS Microbiol. Lett. 1995, 125, 311–315. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-Vinyl and 4-Ethyl Derivatives from Hydroxycinnamic Acids: Occurrence of Volatile Phenolic Flavour Compounds in Beer and Distribution of Pad1-Activity among Brewing Yeasts. Food Chem. 2008, 107, 221–230. [Google Scholar] [CrossRef]
- Baranowski, J.; Davidson, P.; Nagel, C.; Branen, A. Inhibition of Saccharomyces cerevisiae by Naturally Occurring Hydroxycinnamates. J. Food Sci. 1980, 45, 592–594. [Google Scholar] [CrossRef]
- Goodey, A.R.; Tubb, R.S. Genetic and Biochemical Analysis of the Ability of Saccharomyces cerevisiae to Decarboxylate Cinnamic Acids. Microbiology 1982, 128, 2615–2620. [Google Scholar] [CrossRef]
- Chandra, M.; Barata, A.; Ferreira-Dias, S.; Malfeito-Ferreira, M.; Loureiro, V. A Response Surface Methodology Study on the Role of Factors Affecting Growth and Volatile Phenol Production by Brettanomyces bruxellensis ISA 2211 in Wine. Food Microbiol. 2014, 42, 40–46. [Google Scholar] [CrossRef]
- Harris, V.; Jiranek, V.; Ford, C.M.; Grbin, P.R. Inhibitory Effect of Hydroxycinnamic Acids on Dekkera Spp. Appl. Microbiol. Biotechnol. 2010, 86, 721–729. [Google Scholar] [CrossRef]
- Piper, P.; Calderon, C.O.; Hatzixanthis, K.; Mollapour, M. Weak Acid Adaptation: The Stress Response That Confers Yeasts with Resistance to Organic Acid Food Preservatives. Microbiology 2001, 147, 2635–2642. [Google Scholar] [CrossRef]
- Stratford, M.; Nebe-von-Caron, G.; Steels, H.; Novodvorska, M.; Ueckert, J.; Archer, D.B. Weak-Acid Preservatives: pH and Proton Movements in the Yeast Saccharomyces cerevisiae. Int. J. Food Microbiol. 2013, 161, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Conterno, L.; Joseph, C.M.L.; Arvik, T.J.; Henick-kling, T.; Bisson, L.F. Genetic and Physiological Characterization of Brettanomyces bruxellensis Strains Isolated from Wines. Am. J. Enol. Vitic. 2006, 57, 139–147. [Google Scholar] [CrossRef]
- Van der Walt, J.; Van Kerken, A.E. The Wine Yeasts of the Cape: Part II.—The Occurrence of Brettanomyces intermedius and Brettanomyces schanderlii in South African Table Wines. Antonie Van Leeuwenhoek 1959, 25, 145–151. [Google Scholar] [CrossRef]
- Burkholder, P.R.; McVeigh, I.; Moyer, D. Studies on Some Growth Factors of Yeasts. J. Bacteriol. 1944, 48, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Caldeira, J.; Botelheiro, R.; Pagliara, D.; Malfeito-Ferreira, M.; Loureiro, V. Survival Patterns of Dekkera bruxellensis in Wines and Inhibitory Effect of Sulphur Dioxide. Int. J. Food Microbiol. 2008, 121, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Van Uden, N. Transport-Limited Fermentation and Growth of Saccharomyces cerevisiae and Its Competitive Inhibition. Arch. Für Mikrobiol. 1967, 58, 155–168. [Google Scholar] [CrossRef]
- Madeira, A.; Leitão, L.; Soveral, G.; Dias, P.; Prista, C.; Moura, T.; Loureiro-Dias, M.C. Effect of Ethanol on Fluxes of Water and Protons across the Plasma Membrane of Saccharomyces cerevisiae. FEMS Yeast Res. 2010, 10, 252–258. [Google Scholar] [CrossRef]
- Bertrand, A. Formation des Substances Volatiles Au Cours de La Fermentation Alcoolique. Incidence Sur La Qualité Du Vin; Colloque Soc Fr Microbiol: Reims, France, 1981; pp. 251–267. [Google Scholar]
- Agnolucci, M.; Rea, F.; Sbrana, C.; Cristani, C.; Fracassetti, D.; Tirelli, A.; Nuti, M. Sulphur Dioxide Affects Culturability and Volatile Phenol Production by Brettanomyces/Dekkera bruxellensis. Int. J. Food Microbiol. 2010, 143, 76–80. [Google Scholar] [CrossRef]
- Zuehlke, J.M.; Edwards, C.G. Impact of Sulfur Dioxide and Temperature on Culturability and Viability of Brettanomyces bruxellensis in Wine. J. Food Prot. 2013, 76, 2024–2030. [Google Scholar] [CrossRef]
- Longin, C.; Degueurce, C.; Julliat, F.; Guilloux-Benatier, M.; Rousseaux, S.; Alexandre, H. Efficiency of Population-Dependent Sulfite against Brettanomyces bruxellensis in Red Wine. Food Res. Int. 2016, 89, 620–630. [Google Scholar] [CrossRef]
- Valdetara, F.; Skalic, M.; Fracassetti, D.; Louw, M.; Compagno, C.; du Toit, M.; Foschino, R.; Petrovič, U.; Divol, B.; Vigentini, I. Transcriptomics Unravels the Adaptive Molecular Mechanisms of Brettanomyces bruxellensis under SO2 Stress in Wine Condition. Food Microbiol. 2020, 90, 103483. [Google Scholar] [CrossRef]
- Von Cosmos, N.H.; Edwards, C.G. Use of Nutritional Requirements for Brettanomyces bruxellensis to Limit Infections in Wine. Fermentation 2016, 2, 17. [Google Scholar] [CrossRef]
- Tangolar, S.G.; Özoğul, Y.; Tangolar, S.; Torun, A. Evaluation of Fatty Acid Profiles and Mineral Content of Grape Seed Oil of Some Grape Genotypes. Int. J. Food Sci. Nutr. 2009, 60, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.S.; Alexandre, H.; Morge, C.; Sparrow, C.; Gobert, A.; Roullier-Gall, C. Exploring the Unexplored: A Characterization of Vitamins and Vitamers in White Grape Musts by High-Performance Liquid Chromatography. Food Chem. 2023, 398, 133860. [Google Scholar] [CrossRef]
- Benito, S.; Palomero, F.; Morata, A.; Calderón, F.; Suárez-Lepe, J. Factors Affecting the Hydroxycinnamate Decarboxylase/Vinylphenol Reductase Activity of Dekkera/Brettanomyces: Application for Dekkera/Brettanomyces Control in Red Wine Making. J. Food Sci. 2009, 74, M15–M22. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.; Ford, C.M.; Jiranek, V.; Grbin, P.R. Dekkera and Brettanomyces Growth and Utilisation of Hydroxycinnamic Acids in Synthetic Media. Appl. Microbiol. Biotechnol. 2008, 78, 997–1006. [Google Scholar] [CrossRef]
- Godoy, L.; Garrido, D.; Martínez, C.; Saavedra, J.; Combina, M.; Ganga, M. Study of the Coumarate Decarboxylase and Vinylphenol Reductase Activities of Dekkera bruxellensis (Anamorph Brettanomyces bruxellensis) Isolates. Lett. Appl. Microbiol. 2009, 48, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Poolman, B. Energy Transduction in Lactic Acid Bacteria. FEMS Microbiol. Rev. 1993, 12, 125–147. [Google Scholar] [CrossRef]
- Godoy, L.; Varela, J.; Martínez, C.; Ganga, M. The Effect of Hydroxycinnamic Acids on Growth and H-ATPase Activity of the Wine Spoilage Yeast, Dekkera bruxellensis. Afr. J. Microbiol. Res. 2013, 7, 5300–5305. [Google Scholar] [CrossRef]
- Godoy, L.; Vera-Wolf, P.; Martinez, C.; Ugalde, J.A.; Ganga, M.A. Comparative Transcriptome Assembly and Genome-Guided Profiling for Brettanomyces bruxellensis LAMAP2480 during p-Coumaric Acid Stress. Sci. Rep. 2016, 6, 34304. [Google Scholar] [CrossRef]
- Catrileo, D.; Moreira, S.; Ganga, M.A.; Godoy, L. Effect of Light and P-Coumaric Acid on the Growth and Expression of Genes Related to Oxidative Stress in Brettanomyces bruxellensis LAMAP2480. Front. Microbiol. 2021, 12, 747868. [Google Scholar] [CrossRef] [PubMed]





| Carbon Source | Specific Growth Rate (h−1) | Doubling Time (h) |
|---|---|---|
| Control | 0.0056 | 53.76 |
| Glu* | 0.0171 | 17.60 |
| Glu*+ 0.1 mM p-CA | 0.0282 | 10.67 |
| Glu* + 1.0 mM p-CA | 0.0271 | 11.11 |
| 0.1 mM p-CA | 0.0134 | 22.46 |
| 1.0 mM p-CA | 0.0142 | 21.20 |
| *2 g L−1 Glucose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra, M.; Branco, P.; Prista, C.; Malfeito-Ferreira, M. Role of p-Coumaric Acid and Micronutrients in Sulfur Dioxide Tolerance in Brettanomyces bruxellensis. Beverages 2023, 9, 69. https://doi.org/10.3390/beverages9030069
Chandra M, Branco P, Prista C, Malfeito-Ferreira M. Role of p-Coumaric Acid and Micronutrients in Sulfur Dioxide Tolerance in Brettanomyces bruxellensis. Beverages. 2023; 9(3):69. https://doi.org/10.3390/beverages9030069
Chicago/Turabian StyleChandra, Mahesh, Patrícia Branco, Catarina Prista, and Manuel Malfeito-Ferreira. 2023. "Role of p-Coumaric Acid and Micronutrients in Sulfur Dioxide Tolerance in Brettanomyces bruxellensis" Beverages 9, no. 3: 69. https://doi.org/10.3390/beverages9030069
APA StyleChandra, M., Branco, P., Prista, C., & Malfeito-Ferreira, M. (2023). Role of p-Coumaric Acid and Micronutrients in Sulfur Dioxide Tolerance in Brettanomyces bruxellensis. Beverages, 9(3), 69. https://doi.org/10.3390/beverages9030069