The Impact of Rye and Barley Malt and Different Strains of Saccharomyces cerevisiae on Beer Volatilome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Experimental Setup
2.2. Beer Production
2.2.1. Milling
2.2.2. Mashing
2.2.3. Wort Boiling
2.2.4. Fermentation
2.3. Physicochemical Parameters
2.4. Volatile Component Analysis
2.5. Sensory Analysis
2.6. Statistical Methods
3. Results and Discussion
3.1. Fermentation Monitoring—CO2 Production
3.2. Physicochemical Parameters
3.2.1. Sugars and Alcohol
3.2.2. Free Amino Nitrogen (FAN) and Ammonium (NH4+)
3.2.3. Acetic Acid
3.3. Volatile Compounds
3.3.1. Terpenes
3.3.2. Alcohols
3.3.3. Esters
3.3.4. Acids
3.3.5. Other Compounds
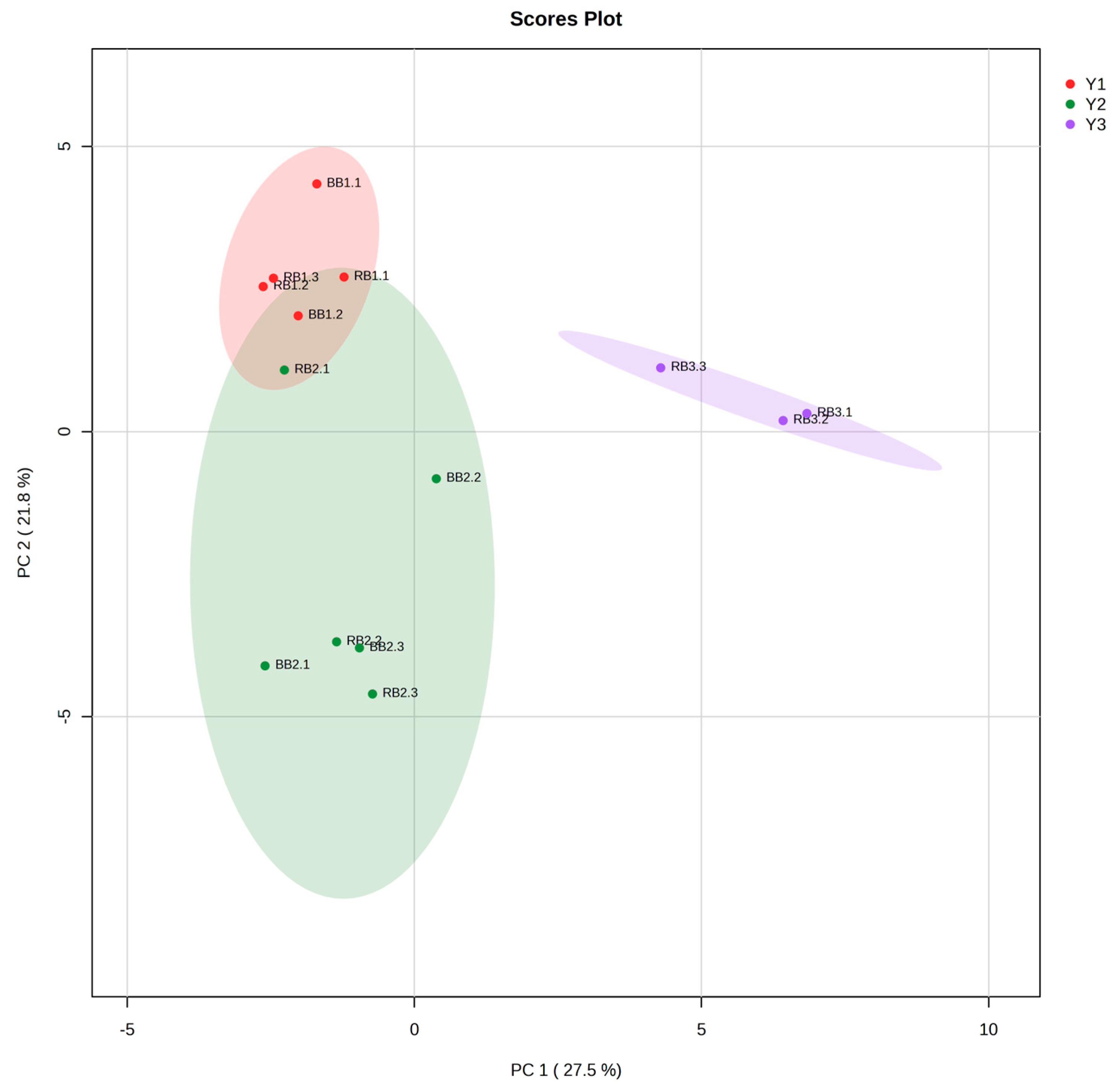
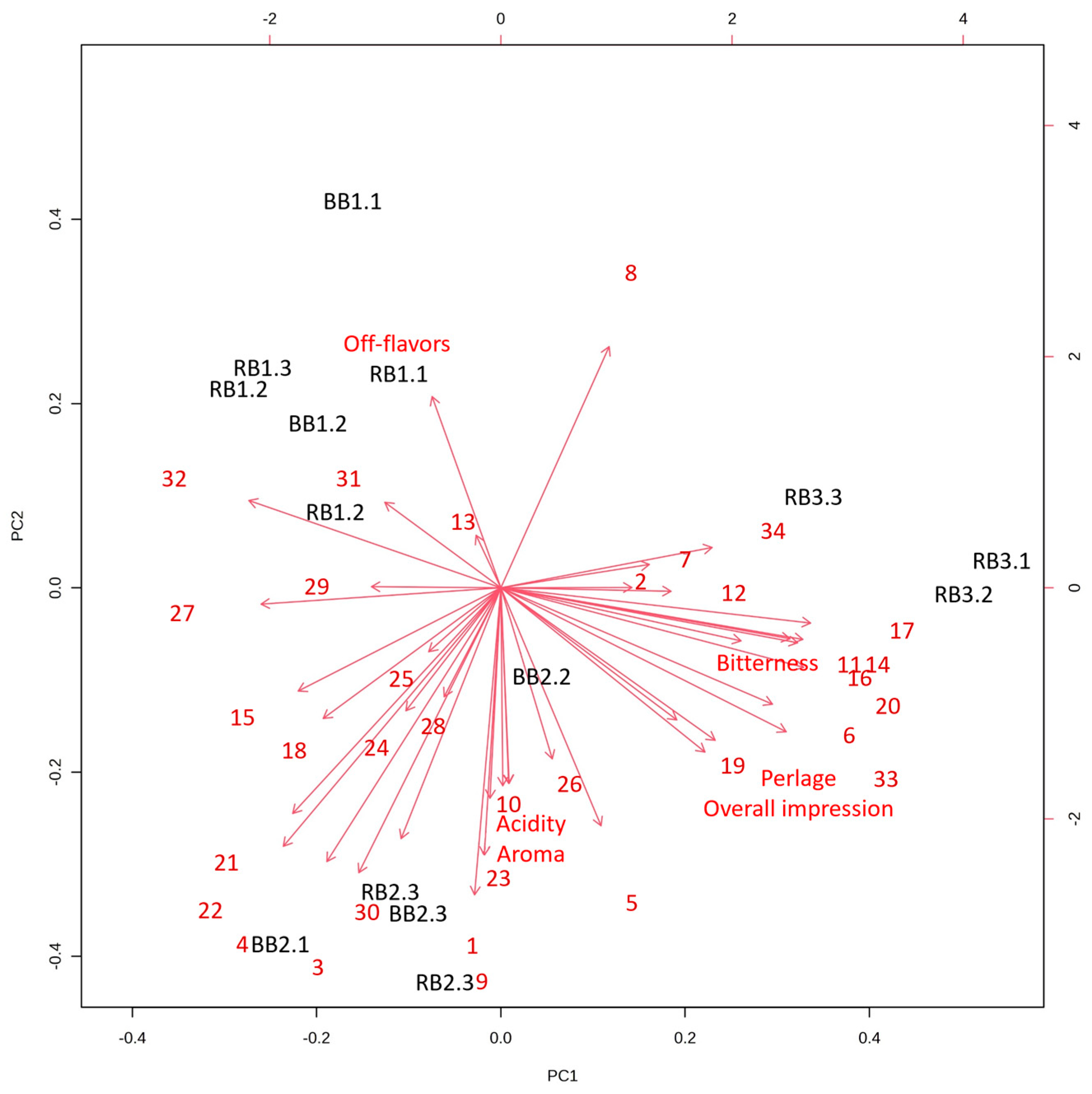
3.4. Sensory Evaluation and VOC Principal Component Analysis (PCA)
3.5. Influence of Malt and Yeast on Beer Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garavaglia, C.; Swinnen, J. The craft beer revolution: An international perspective. Choices 2017, 32, 1–8. [Google Scholar]
- The European Brewers. European Beer Trends; Statistics Report 2022 Edition. Available online: https://brewersofeurope.org/uploads/mycms-files/documents/publications/2022/european-beer-trends-2022.pdf (accessed on 22 October 2023).
- TechNavio. Europe—Craft Beer Market by Distribution Channel, Product and Geography—Forecast and Analysis 2023–2027. 2023, pp. 1–11. Available online: https://www.technavio.com/report/craft-beer-market-in-europe-industry-analysis (accessed on 22 October 2023).
- Garavaglia, C.; Borgoni, R. The local dimension of legitimation: An empirical analysis of firms’ entry in the Italian craft beer market. Reg. Stud. 2022, 57, 1909–1923. [Google Scholar] [CrossRef]
- Baiano, A. Craft beer: An overview. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1829–1856. [Google Scholar] [CrossRef] [PubMed]
- Sayre-Chavez, B.; Bettenhausen, H.; Windes, S.; Aron, P.; Cistué, L.; Fisk, S.; Helgerson, L.; Heuberger, A.L.; Tynan, S.; Hayes, P.; et al. Genetic basis of barley contributions to beer flavor. J. Cereal Sci. 2022, 104, 103430. [Google Scholar] [CrossRef]
- Bogdan, P.; Kordialik-Bogacka, E. Alternatives to malt in brewing. Trends Food Sci. Technol. 2017, 65, 1–9. [Google Scholar] [CrossRef]
- Muñoz, S.; Sheyling, A. Evaluation of the addition of rye (Secale cereale) in the formulation of belgian pale ale craft beer. Enfoque UTE 2022, 13, 14–28. [Google Scholar]
- Villacreces, S.; Blanco, C.A.; Caballero, I. Developments and characteristics of craft beer production processes. Food Biosci. 2022, 45, 101495. [Google Scholar] [CrossRef]
- Calvi, A.; Preiti, G.; Poiana, M.; Marconi, O.; Gastl, M.; Zarnkow, M. Multi-Response Optimization of the Malting Process of an Italian Landrace of Rye (Secale cereale L.) Using Response Surface Methodology and Desirability Function Coupled with Genetic Algorithm. Foods 2022, 11, 3561. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Z.; Barr, J.; Gillespie, J.; Simsek, S.; Horsley, R.D.; Schwarz, P.B. Micro-malting for the quality evaluation of rye (Secale cereale) genotypes. Fermentation 2018, 4, 50. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef]
- Tokpohozin, S.E.; Fischer, S.; Becker, T. Selection of a new Saccharomyces yeast to enhance relevant sorghum beer aroma components, higher alcohols and esters. Food Microbiol. 2019, 83, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Bonatto, D. The diversity of commercially available ale and lager yeast strains and the impact of brewer’s preferential yeast choice on the fermentative beer profiles. Food Res. Int. 2021, 141, 110125. [Google Scholar] [CrossRef] [PubMed]
- Meier-Dörnberg, T.; Kory, O.I.; Jacob, F.; Michel, M.; Hutzler, M. Saccharomyces cerevisiae variety diastaticus friend or foe? spoilage potential and brewing ability of different Saccharomyces cerevisiae variety diastaticus yeast isolates by genetic, phenotypic and physiological characterization. FEMS Yeast Res. 2018, 18, foy023. [Google Scholar] [CrossRef] [PubMed]
- Abbott, E.; Villegas, D.; Van Zandycke, S.; Fischborn, T. Strain specific performance of active dry yeast for fermentation of very high gravity wort. Brew. Sci. 2019, 72, 89–93. [Google Scholar]
- Krogerus, K.; Gibson, B. A re-evaluation of diastatic Saccharomyces cerevisiae strains and their role in brewing. Appl. Microbiol. Biotechnol. 2020, 104, 3745–3756. [Google Scholar] [CrossRef]
- Rate Beer. Available online: https://www.ratebeer.com/ratebeerbest/2020/best-brewers-top-100#! (accessed on 22 October 2023).
- Ravasio, D.; Carlin, S.; Boekhout, T.; Groenewald, M.; Vrhovsek, U.; Walther, A.; Wendland, J. Adding flavor to beverages with non-conventional yeasts. Fermentation 2018, 4, 15. [Google Scholar] [CrossRef]
- Goncalves, J.L.; Figueira, J.A.; Rodrigues, F.P.; Ornelas, L.P.; Branco, R.N.; Silva, C.L.; Camara, J.S. A powerful methodological approach combining headspace solid phase microextraction, mass spectrometry and multivariate analysis for profiling the volatile metabolomic pattern of beer starting raw materials. Food Chem. 2014, 160, 266–280. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Guido, L.F. Brewing, and craft beer. Beverages 2019, 5, 51. [Google Scholar] [CrossRef]
- Montanari, L.; Floridi, S.; Marconi, O.; Tironzelli, M.; Fantozzi, P. Effect of mashing procedures on brewing. Eur. Food Res. Technol. 2005, 221, 175–179. [Google Scholar] [CrossRef]
- Durand, G.A.; Corazza, M.L.; Blanco, A.M.; Corazza, F.C. Dynamic optimization of the mashing process. Food Control 2009, 20, 1127–1140. [Google Scholar] [CrossRef]
- Ivanov, K.; Petelkov, I.; Shopska, V.; Denkova, R.; Gochev, V.; Kostov, G. Investigation of mashing regimes for low-alcohol beer production. J. Inst. Brew. 2016, 122, 508–516. [Google Scholar] [CrossRef]
- Rautio, J.; Londesborough, J. Maltose transport by brewer’s yeasts in brewer’s wort. J. Inst. Brew. 2003, 109, 251–261. [Google Scholar] [CrossRef]
- Zastrow, C.R.; Hollatz, C.; De Araujo, P.S.; Stambuk, B.U. Maltotriose fermentation by Saccharomyces cerevisiae. J. Inst. Brew. 2001, 27, 34–38. [Google Scholar] [CrossRef]
- Hill, A.E.; Stewart, G.G. Free amino nitrogen in brewing. Fermentation 2019, 5, 22. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, S.; Zhang, W. Predicting acetic acid content in the final beer using neural networks and support vector machine. J. Inst. Brew. 2012, 118, 361–367. [Google Scholar] [CrossRef]
- Langos, D.; Granvogl, M.; Schieberle, P. Characterization of the key aroma compounds in two Bavarian wheat beers by means of the sensomics approach. J. Agric. Food Chem. 2013, 61, 11303–11311. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, C.; Wang, L.; Hong, K.; Ma, C.; Lv, C. Potential enzymes involved in beer monoterpenoids transformation: Structures, functions and challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 2082–2092. [Google Scholar] [CrossRef]
- Coelho, E.; Magalhães, J.; Pereira, F.B.; Macieira, F.; Domingues, L.; Oliveira, J.M. Volatile fingerprinting differentiates diverse-aged craft beers. LWT 2019, 108, 129–136. [Google Scholar] [CrossRef]
- Rutnik, K.; Knez Hrnčič, M.; Jože Košir, I. Hop essential oil: Chemical composition, extraction, analysis, and applications. Food Rev. Int. 2022, 38 (Suppl. S1), 529–551. [Google Scholar] [CrossRef]
- Simões, J.; Coelho, E.; Magalhães, P.; Brandão, T.; Rodrigues, P.; Teixeira, J.A.; Domingues, L. Exploiting non-conventional yeasts for low-alcohol beer production. Microorganisms 2023, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Olaniran, A.O.; Hiralal, L.; Mokoena, M.P.; Pillay, B. Flavour-active volatile compounds in beer: Production, regulation and control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef]
- Alves, V.; Gonçalves, J.; Figueira, J.A.; Ornelas, L.P.; Branco, R.N.; Câmara, J.S.; Pereira, J.A. Beer volatile fingerprinting at different brewing steps. Food Chem. 2020, 326, 126856. [Google Scholar] [CrossRef] [PubMed]
- Saerens, S.M.; Verstrepen, K.J.; Thevelein, J.M.; Delvaux, F.R. Ethyl ester production during brewery fermentation: A review. Cerevisia 2008, 33, 82. [Google Scholar]
- Neiens, S.D.; Steinhaus, M. Odor-active compounds in the special flavor hops hull melon and polaris. J. Agric. Food Chem. 2018, 66, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Bravi, E.; Marconi, O.; Sileoni, V.; Perretti, G. Determination of free fatty acids in beer. Food Chem. 2017, 215, 341–346. [Google Scholar] [CrossRef]
- Romero-Guido, C.; Belo, I.; Ta, T.M.N.; Cao-Hoang, L.; Alchihab, M.; Gomes, N.; Thonart, P.; Teixeira, J.A.; Destain, J.; Waché, Y. Biochemistry of lactone formation in yeast and fungi and its utilisation for the production of flavour and fragrance compounds. Appl. Microbiol. Biotechnol. 2011, 89, 535–547. [Google Scholar] [CrossRef]
- Lee, S.M.; Lim, H.J.; Chang, J.W.; Hurh, B.S.; Kim, Y.S. Investigation on the formations of volatile compounds, fatty acids, and γ-lactones in white and brown rice during fermentation. Food Chem. 2018, 269, 347–354. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Miró, P.; Serra-Cayuela, A.; Buxaderas, S.; López-Tamames, E. Assessment of the aroma profiles of low-alcohol beers using HS-SPME–GC-MS. Food Res. Int. 2014, 57, 196–202. [Google Scholar] [CrossRef]
- Štulíková, K.; Vrzal, T.; Kubizniaková, P.; Enge, J.; Matoulková, D.; Brányik, T. Spoilage of bottled lager beer contaminated with Saccharomyces cerevisiae var. diastaticus. J. Inst. Brew. 2021, 127, 256–261. [Google Scholar] [CrossRef]
- Wanikawa, A. Flavors in Malt Whisky: A Review. J. Am. Soc. Brew. Chem. 2020, 78, 260–278. [Google Scholar] [CrossRef]
- Iorizzo, M.; Coppola, F.; Letizia, F.; Testa, B.; Sorrentino, E. Role of yeasts in the brewing process: Tradition and innovation. Processes 2021, 9, 839. [Google Scholar] [CrossRef]

| Profile | Mashing | β-Glucanase Activity | Protein Rest | β-Amylase Rest | α-Amylase Rest | Lautering | Boiling |
|---|---|---|---|---|---|---|---|
| BB | 60 °C | 63 °C 25 min | none | 73 °C 40 min | none | 78 °C 10 min | 100 °C 80 min |
| RB | 35 °C | 45 °C 15 min | 55 °C 25 min | 63 °C 25 min | 73 °C 40 min | 78 °C 10 min | 100 °C 80 min |
| Recipe | Ingredient | Amount |
|---|---|---|
| BB1, BB2 | Pilsner malt | 1000 g |
| Munich malt | 1200 g | |
| Barley caramel malt | 300 g | |
| Hop pellet | 13.6 g | |
| RB1, RB2, RB3 | Pilsner malt | 1125 g |
| Rye malt | 1250 g | |
| Rye caramel malt | 125 g | |
| Hop pellet | 13.6 g | |
| Recipe | Yeast Strain | Amount |
| BB1 | Active dry yeast Y1 | 0.5 (g/L) |
| BB2 | Active dry yeast Y2 | 0.5 (g/L) |
| RB1 | Active dry yeast Y1 | 0.5 (g/L) |
| RB2 | Active dry yeast Y2 | 0.5 (g/L) |
| RB3 | Active dry yeast Y3 | 0.5 (g/L) |
| (a) Wort | BB1 | RB1 | BB2 | RB2 | RB3 |
|---|---|---|---|---|---|
| pH | 5.96 ± 0.04 | 6.17 ± 0.46 | 5.89 ± 0.08 | 5.89 ± 0.05 | 5.93 ± 0.05 |
| Plato | 12.6 ± 0.01 | 12.6 ± 0.01 | 12.47 ± 0.12 | 12.27 ± 0.58 | 12.43 ± 0.40 |
| Maltose (g/L) | 68.00 ± 1.00 a | 68.33 ± 4.16 ab | 65.00 ± 7.00 ab | 58.00 ± 15.39 ab | 64.11 ± 3.39 b |
| Glu-Fru (g/L) | 33.48 ± 3.42 a | 12.76 ± 15.23 a | 6.91 ± 0.61 d | 9.30 ± 0.60 c | 12.14 ± 0.44 b |
| FAN (mg/L) | 246.00 ± 11.53 | 173.67 ± 20.50 | 230.33 ± 18.01 | 227.00 ± 8.72 | 230.33 ± 2.52 |
| NH4+ (mg/L) | 67.33 ± 5.03 c | 81.50 ± 5.50 b | 60.33 ± 7.02 c | 68.00 ± 7.21 c | 97.00 ± 4.36 a |
| (b) first fermentation | BB1 | RB1 | BB2 | RB2 | RB3 |
| pH | 4.60 ± 0.02 b | 4.71 ± 0.01 a | 4.28 ± 0.0 c | 4.29 ± 0.04 c | 4.33 ± 0.04 b |
| Alcohol (% v/v) | 3.57 ± 0.09 b | 3.67 ± 0.14 b | 5.23 ± 0.40 a | 5.20 ± 0.63 a | 4.37 ± 0.31 a |
| Maltose (g/L) | 5.70 ± 9.44 | 2.90 ± 4.95 | 5.07 ± 8.60 | 5.06 ± 8.60 | 0.2 ± 0.17 |
| Glu-Fru (g/L) | 0.01 ± 0.01 | 0.05 ± 0.02 | 0.89 ± 0.71 | 0.55 ± 0.79 | 0.01 ± 0.01 |
| FAN (mg/L) | 58.00 ± 14.14 | 57.00 ± 24.04 | 54.67 ± 16.07 | 49.00 ± 6.93 | 61.33 ± 1.15 |
| NH4+ (mg/L) | 22.50 ± 0.71 b | 22.50 ± 2.12 b | 26.33 ± 4.5 b | 27.33 ± 4.50 b | 84.33 ± 7.57 a |
| Acetic acid (g/L) | 0.05 ± 0.05 c | 0.07 ± 0.03 c | 0.29 ± 0.01 a | 0.17 ± 0.02 b | 0.06 ± 0.01 c |
| (c) bottle fermentation | BB1 | RB | BB2 | RB2 | RB3 |
| pH | 4.64 ± 0.09 a | 4.63 ± 0.10 a | 4.30 ± 0.05 b | 4.38 ± 0.05 b | 4.42 ± 0.03 b |
| Alcohol (% v/v) | 4.36 ± 0.19 c | 4.31 ± 0.14 c | 7.17 ± 0.05 a | 7.04 ± 0.31 a | 5.07 ± 0.07 b |
| Maltose (g/L) | 1.30 ± 0.40 a | 1.30 ± 0.17 a | 0.00 b | 0.00 b | 0.17 ± 0.15 b |
| Glu-Fru (g/L) | 0.01 ± 0.03 | 0.07 ± 0.09 | 0.02 ± 0.01 | 0.05 ± 0.05 | 0.04 ± 0.03 |
| FAN (mg/L) | 115.00 ± 35.38 | 101.33 ± 67.80 | 58.00 ± 5.20 | 55.33 ± 2.89 | 83.00 ± 5.29 |
| NH4+ (mg/L) | 53.67 ± 28.74 | 56.67 ± 23.86 | 84.33 ± 8.96 | 74.00 ± 3.46 | 61.33 ± 16.74 |
| Acetic acid (g/L) | 0.07 ± 0.03 b | 0.11 ± 0.05 b | 0.24 ± 0.00 a | 0.13 ± 0.02 b | 0.075 ± 0.00 b |
| Class | Compound | BB1 | RB1 | BB2 | RB2 | RB3 |
|---|---|---|---|---|---|---|
| Terpenes | Linalool | 0.056 ± 0.003 b | 0.056 ± 0.007 b | 0.069 ± 0.010 ab | 0.052 ± 0.018 b | 0.111 ± 0.011 a |
| Citronellol | 0.009 ± 0.002 b | 0.011 ± 0.001 b | 0.012 ± 0.003 b | 0.011 ± 0.002 b | 0.020 ± 0.001 a | |
| Humulene | 0.002 ± 0.001 b | 0.004 ± 0.001 b | 0.001 ± 0.001 b | 0.003 ± 0.001 b | 0.020 ± 0.003 a | |
| Humulene epoxide I | 0.002 ± 0.001 | 0.004 ± 0.002 | 0.005 ± 0.002 | 0.004 ± 0.001 | 0.003 ± 0.001 | |
| Methyl geraniate | 0.007 ± 0.002 b | 0.008 ± 0.002 b | 0.012 ± 0.002 b | 0.010 ± 0.001 b | 0.020 ± 0.001 a | |
| α-Terpineol | 0.004 ± 0.001 | 0.003 ± 0.001 | 0.003 ± 0.001 | 0.003 ± 0.001 | 0.004 ± 0.001 | |
| Sum | 0.077 ± 0.006 b | 0.077 ± 0.012 b | 0.093 ± 0.012 b | 0.073 ± 0.021 b | 0.170 ± 0.010 a | |
| Alcohols | 2-Methyl- 1-propanol | 0.326 ± 0.075 b | 0.314 ± 0.030 b | 0.280 ± 0.018 b | 0.280 ± 0.053 b | 0.545 ± 0.036 a |
| 3-Methyl- 1-butanol | 3.200 ± 0.681 ab | 3.085 ± 0.333 ab | 4.433 ± 1.275 ab | 5.142 ± 1.080 a | 2.384 ± 0.07 b | |
| 1-Hexanol | 0.017 ± 0.002 a | 0.018 ± 0.002 a | 0.013 ± 0.002 b | 0.016 ± 0.004 ab | 0.021 ± 0.004 a | |
| 2,3-Butanediol | 0.013 ± 0.005 | 0.013 ± 0.002 | 0.013 ± 0.006 | 0.009 ± 0.002 | 0.016 ± 0.005 | |
| 2-Decanol | 0.012 ± 0.001 bc | 0.015 ± 0.002 bc | 0.019 ± 0.005 ab | 0.012 ± 0.004 bc | 0.029 ± 0.005 a | |
| Phenylethyl alcohol | 5.075 ± 0.922 | 4.827 ± 0.573 | 5.232 ± 0.459 | 4.641 ± 1.024 | 3.564 ± 0.143 | |
| 2-Methoxy-4-vinylphenol | 0.004 ± 0.001 b | 0.006 ± 0.001 b | 0.017 ± 0.009 b | 0.047 ± 0.013 a | 0.006 ± 0.002 b | |
| Sum | 8.647 ± 1.674 | 8.277 ± 0.923 | 10.008 ± 1.423 | 10.148 ± 2.173 | 6.565 ± 0.221 | |
| Esters | Ethyl acetate | 0.627 ± 0.165 b | 0.688 ± 0.123 b | 1.235 ± 0.412 a | 1.222 ± 0.211 a | 0.885 ± 0.045 ab |
| 1-Butanol, 3-methyl-, acetate | 0.674 ± 0.371 b | 1.363 ± 0.787 b | 3.295 ± 1.873 a | 4.520 ± 0.762 a | 0.813 ± 0.082 b | |
| Hexanoic acid, ethyl ester | 0.449 ± 0.139 b | 0.539 ± 0.140 b | 0.834 ± 0.177 a | 0.854 ± 0.168 a | 0.767 ± 0.098 a | |
| Heptanoic acid, ethyl ester | 0.042 ± 0.015 b | 0.044 ± 0.011 b | 0.057 ± 0.011 b | 0.066 ± 0.012 ab | 0.101 ± 0.012 a | |
| Octanoic acid, ethyl ester | 8.612 ± 2.515 | 8.610 ± 2.135 | 13.137 ± 2.204 | 12.445 ± 2.322 | 8.776 ± 0.137 | |
| Isopentyl hexanoate | 0.033 ± 0.016 b | 0.032 ± 0.013 b | 0.079 ± 0.027 ab | 0.105 ± 0.030 a | 0.047 ± 0.003 b | |
| Nonanoic acid, ethyl ester | 0.269 ± 0.077 | 0.353 ± 0.111 | 0.535 ± 0.156 | 0.400 ± 0.117 | 0.384 ± 0.049 | |
| Decanoic acid, ethyl ester | 4.679 ± 1.597 | 4.315 ± 2.021 | 6.191 ± 1.734 | 4.358 ± 0.576 | 2.296 ± 0.103 | |
| Ethyl (Z)-4-decenoate | 0.134 ± 0.029 | 0.274 ± 0.157 | 0.483 ± 0.230 | 0.401 ± 175 | 0.077 ± 0.017 | |
| 2-phenylethyl acetate | 0.714 ± 0.332 b | 1.175 ± 0.647 a | 1.621 ± 0.318 a | 1.497 ± 0.402 a | 0.439 ± 0.060 b | |
| Ethyl hexadecanoate | 0.027 ± 0.006 | 0.022 ± 0.006 | 0.031 ± 0.006 | 0.025 ± 0.004 | 0.015 ± 0.001 | |
| Butyl hexadecanoate | 0.005 ± 0.002 | 0.005 ± 0.002 | 0.007 ± 0.003 | 0.007 ± 0.003 | 0.010 ± 0.002 | |
| Sum | 16.256 ± 3.725 b | 17.427 ± 5.939 b | 27.513 ± 4.959 a | 25.900 ± 4.411 a | 14.600 ± 0.283 b | |
| Acids | 3-Decenoic acid | 0.006 ± 0.002 b | 0.005 ± 0.002 b | 0.008 ± 0.002 b | 0.007 ± 0.001 b | 0.015 ± 0.002 a |
| Heptanoic acid | 0.284 ± 0.036 ab | 0.317 ± 0.051 ab | 0.477 ± 0.105 a | 0.447 ± 0.124 a | 0.166 ± 0.010 b | |
| Octanoic acid | 1.208 ± 0.009 ab | 1.385 ± 0.305 b | 1.705 ± 0.227 a | 1.333 ± 0.336 b | 0.412 ± 0.039 b | |
| Nonanoic Acid | 0.012 ± 0.001 | 0.018 ± 0.003 | 0.014 ± 002 | 0.010 ± 0.002 | 0.005 ± 0.001 | |
| n-Decanoic acid | 0.207 ± 0.017 a | 0.164 ± 0.072 ab | 0.172 ± 0.057 ab | 0.105 ± 0.017 ab | 0.030 ± 0.001 b | |
| Sum | 1.717 ± 0.011 b | 1.889 ± 0.423 ab | 2.376 ± 0.282 a | 1.901 ± 0.479 ab | 0.629 ± 0.051 c | |
| Others | 2-Decanone | 0.003 ± 0.001 | 0.007 ± 0.002 | 0.004 ± 0.001 | 0.003 ± 0.001 | 0.002 ± 0.001 |
| γ-Butylbutyrolactone | 0.029 ± 0.003 | 0.029 ± 0.010 | 0.027 ± 0.003 | 0.033 ± 0.007 | 0.029 ± 0.002 | |
| γ-Decalactone | 0.004 ± 0.001 | 0.004 ± 0.001 | 0.004 ± 0.001 | 0.003 ± 0.001 | 0.002 ± 0.001 | |
| 2-Acetylpyrrole | 0.006 ± 0.001 ab | 0.004 ± 0.001 b | 0.009 ± 0.001 a | 0.003 ± 0.001 b | 0.004 ± 0.001 b | |
| Sum | 0.042 ± 0.005 | 0.044 ± 0.012 | 0.044 ± 0.003 | 0.042 ± 0.008 | 0.037 ± 0.002 |
| Yeast | Malt | YxM | |
|---|---|---|---|
| Physicochemical parameters | |||
| Ethyl alcohol | 378.56 *** | 0.803 | 0.166 |
| Brix | 91.43 *** | 0.242 | 0.544 |
| pH | 27.60 ** | 0.73 | 1.207 |
| Acetic acid | 23.82 ** | 4.201 | 26.945 * |
| Volatile compounds | |||
| Humulene | 77.00 ** | 0.804 | 1.65 × 10−4 |
| Maltose | 64.89 ** | 0 | 0 |
| 2-Methyl-1 propanol | 38.71 ** | 0.179 | 0.199 |
| Terpene Sum | 35.46 ** | 1.407 | 1.103 |
| Methyl geraniate | 32.46 ** | 1.621 | 0.409 |
| Linalool | 25.47 ** | 2.721 | 1.01 |
| 3-Decenoic acid | 23.75 ** | 1.437 | 0.001 |
| 2-Methoxy-4-vinylphenol | 23.01 ** | 9.336 | 13.304 |
| Nonanoic Acid | 21.46 * | 0.289 | 21.379 * |
| Citronellol | 20.04 * | 0.002 | 0.458 |
| Octanoic acid | 18.75 * | 0.548 | 4.359 |
| Acid Sum | 18.10 * | 0.675 | 3.248 |
| 2-Decanol | 16.78 * | 1.981 | 3.206 |
| Heptanoic acid, ethyl ester | 15.33 * | 0.049 | 0.886 |
| Hexanoic acid, butyl ester | 14.69 * | 0.05 | 0.732 |
| 2-Acetylpyrrole | 2.07 | 44.644 ** | 9.691 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tocci, N.; Riccio, G.M.; Ramu Ganesan, A.; Hoellrigl, P.; Robatscher, P.; Conterno, L. The Impact of Rye and Barley Malt and Different Strains of Saccharomyces cerevisiae on Beer Volatilome. Beverages 2023, 9, 93. https://doi.org/10.3390/beverages9040093
Tocci N, Riccio GM, Ramu Ganesan A, Hoellrigl P, Robatscher P, Conterno L. The Impact of Rye and Barley Malt and Different Strains of Saccharomyces cerevisiae on Beer Volatilome. Beverages. 2023; 9(4):93. https://doi.org/10.3390/beverages9040093
Chicago/Turabian StyleTocci, Noemi, Gian Marco Riccio, Abirami Ramu Ganesan, Philipp Hoellrigl, Peter Robatscher, and Lorenza Conterno. 2023. "The Impact of Rye and Barley Malt and Different Strains of Saccharomyces cerevisiae on Beer Volatilome" Beverages 9, no. 4: 93. https://doi.org/10.3390/beverages9040093
APA StyleTocci, N., Riccio, G. M., Ramu Ganesan, A., Hoellrigl, P., Robatscher, P., & Conterno, L. (2023). The Impact of Rye and Barley Malt and Different Strains of Saccharomyces cerevisiae on Beer Volatilome. Beverages, 9(4), 93. https://doi.org/10.3390/beverages9040093








