Comprehensive Landscape of STEAP Family Members Expression in Human Cancers: Unraveling the Potential Usefulness in Clinical Practice Using Integrated Bioinformatics Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oncomine Analysis
2.2. cBioPortal Analysis
3. Results and Discussion
3.1. Bladder Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Infiltrating Bladder Urothelial Carcinoma vs. Normal | |||||||
| STEAP1 | No difference | 1.162 | 41 | Sanchez-Carbayo Bladder 2 | 129 (81/48) | 0.123 | [48] |
| No difference | 1.074 | 56 | Dyrskjot Bladder 3 | 27 (13/14) | 0.379 | [49] | |
| Underexpressed | −1.649 | 14 | Lee Bladder | 130 (62/68) | 3.90 × 10−4 | [50] | |
| STEAP2 | Underexpressed | −1.614 | 17 | Lee Bladder | 130 (62/68) | 0.001 | [50] |
| STEAP3 | Overexpressed | 1.729 | 4 | Dyrskjot Bladder 3 | 27 (13/14) | 5.45 × 10−6 | [49] |
| Overexpressed | 1.667 | 3 | Sanchez-Carbayo Bladder 2 | 129 (81/48) | 1.11 × 10−11 | [48] | |
| Overexpressed | 1.443 | 18 | Lee Bladder | 130 (62/68) | 0.018 | [50] | |
| STEAP4 | No difference | 1.007 | 57 | Dyrskjot Bladder 3 | 27 (13/14) | 0.441 | [49] |
| No difference | −1.288 | 40 | Sanchez-Carbayo Bladder 2 | 129 (81/48) | 0.124 | [48] | |
| Underexpressed | −1.29 | 31 | Lee Bladder | 130 (62/68) | 0.035 | [50] | |
| Superficial Bladder Cancer vs. Normal | |||||||
| STEAP1 | No difference | −1.019 | 50 | Sanchez-Carbayo Bladder 2 | 76 (28/48) | 0.462 | [48] |
| No difference | −1.065 | 52 | Dyrskjot Bladder 3 | 42 (28/14) | 0.378 | [49] | |
| Underexpressed | −2.131 | 5 | Lee Bladder | 256 (126/68) | 3.58 × 10−10 | [50] | |
| STEAP2 | Underexpressed | −1.448 | 25 | Lee Bladder | 194 (126/68) | 0.004 | [50] |
| STEAP3 | Overexpressed | 2.084 | 1 | Dyrskjot Bladder 3 | 42 (28/14) | 1.26 × 10−9 | [49] |
| Overexpressed | 3.125 | 3 | Sanchez-Carbayo Bladder 2 | 76 (28/48) | 3.06 × 10−17 | [48] | |
| Overexpressed | 1.741 | 7 | Lee Bladder | 194 (126/68) | 1.39 × 10−4 | [50] | |
| STEAP4 | Underexpressed | −1.101 | 37 | Dyrskjot Bladder 3 | 42 (28/14) | 0.031 | [49] |
| Underexpressed | −1.942 | 26 | Sanchez-Carbayo Bladder 2 | 76 (28/48) | 0.01 | [48] | |
| No difference | −1.217 | 37 | Lee Bladder | 194 (126/68) | 0.071 | [50] | |
3.2. Brain/CNS Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Glioblastoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.965 | 4 | Lee Brain | 25 (22/3) | 4.54 × 10−5 | [60] |
| No difference | 1.594 | 31 | Liang Brain | 32 (29/3) | 0.148 | [61] | |
| Overexpressed | 1.355 | 17 | Murat Brain | 84 (80/4) | 0.002 | [62] | |
| No difference | −1.308 | 48 | TCGA Brain | 15 (5/10) | 0.102 | [63] | |
| No difference | 1.124 | 40 | Shai Brain | 34 (27/7) | 0.164 | [64] | |
| Overexpressed | 1.332 | 19 | Sun Brain | 104 (81/23) | 2.06 × 10−5 | [65] | |
| Underexpressed | −1.68 | 23 | Bredel Brain 2 | 31 (27/4) | 0.005 | [66] | |
| STEAP2 | Overexpressed | 4.854 | 14 | Lee Brain | 25 (22/3) | 0.021 | [60] |
| No difference | −1.428 | 28 | Liang Brain | 31 (28/3) | 0.138 | [61] | |
| No difference | −1.079 | 45 | Bredel Brain 2 | 31 (27/4) | 0.138 | [66] | |
| Underexpressed | −3.622 | 11 | Sun Brain | 104 (81/23) | 7.58 × 10−12 | [65] | |
| Underexpressed | −3.766 | 2 | Murat Brain | 84 (8/40) | 2.78 × 10−8 | [62] | |
| STEAP3 | Overexpressed | 3.427 | 1 | Sun Brain | 104 (81/23) | 1.65 × 10−22 | [65] |
| Overexpressed | 4.968 | 2 | TCGA Brain | 552 (542/10) | 2.93 × 10−12 | [63] | |
| Overexpressed | 5.978 | 6 | Bredel Brain 2 | 31 (27/4) | 1.11 × 10−5 | [66] | |
| Overexpressed | 2.349 | 9 | Liang Brain | 33 (30/3) | 0.014 | [61] | |
| Overexpressed | 4.311 | 7 | Lee Brain | 25 (22/3) | 8.89 × 10−4 | [60] | |
| Overexpressed | 1.627 | 8 | Murat Brain | 84 (80/4) | 3.29 × 10−5 | [62] | |
| STEAP4 | No difference | 1.381 | 26 | Liang Brain | 33 (30/3) | 0.109 | [61] |
| No difference | 1.898 | 29 | Lee Brain | 25 (22/3) | 0.208 | [60] | |
| No difference | 1.12 | 49 | Sun Brain | 104 (81/23) | 0.169 | [65] | |
| No difference | −1.754 | 38 | Bredel Brain 2 | 28 (24/4) | 0.062 | [66] | |
| Underexpressed | −1.184 | 37 | TCGA Brain | 15 (5/10) | 0.041 | [63] | |
| No difference | 1.117 | 46 | Murat Brain | 84 (80/4) | 0.127 | [62] | |
| Astrocytoma vs. Normal | |||||||
| STEAP1 | No difference | 1.709 | 24 | Liang Brain | 6 (3/3) | 0.124 | [61] |
| No difference | −1.207 | 54 | Shai Brain | 10 (3/7) | 0.188 | [64] | |
| No difference | 1.121 | 41 | Sun Brain | 42 (19/23) | 0.147 | [65] | |
| Underexpressed | −1.289 | 14 | Bredel Brain 2 | 10 (6/4) | 0.004 | [66] | |
| STEAP2 | No difference | −1.341 | 38 | Liang Brain | 6 (3/3) | 0.208 | [61] |
| No difference | 1.041 | 45 | Bredel Brain 2 | 10 (6/4) | 0.311 | [66] | |
| STEAP3 | Overexpressed | 2.299 | 7 | Sun Brain | 42 (19/23) | 2.12 × 10−5 | [65] |
| No difference | −1.58 | 21 | Liang Brain | 6 (3/3) | 0.073 | [61] | |
| STEAP4 | Overexpressed | 1.662 | 12 | Liang Brain | 6 (3/3) | 0.048 | [61] |
| No difference | −1.1 | 53 | Sun Brain | 42 (19/23) | 0.242 | [65] | |
| No difference | −1.34 | 47 | Bredel Brain 2 | 9 (5/4) | 0.179 | [66] | |
| Oligodendroglioma vs. Normal | |||||||
| STEAP1 | No difference | −1.111 | 54 | Shai Brain | 10 (3/7) | 0.188 | [64] |
| Underexpressed | −1.134 | 40 | Sun Brain | 73 (50/23) | 0.035 | [65] | |
| No difference | −1.084 | 47 | French Brain | 29 (23/6) | 0.206 | [67] | |
| Underexpressed | −1.136 | 5 | Bredel Brain 2 | 9 (5/4) | 0.001 | [66] | |
| STEAP2 | No difference | 1.022 | 50 | Bredel Brain 2 | 9 (5/4) | 0.424 | [66] |
| Underexpressed | −1.877 | 28 | French Brain | 29 (23/6) | 0.043 | [67] | |
| Underexpressed | −1.885 | 17 | Sun Brain | 73 (50/23) | 5.13 × 10−6 | [65] | |
| STEAP3 | Overexpressed | 1.364 | 21 | French Brain | 29 (23/6) | 0.004 | [67] |
| STEAP4 | No difference | 1.242 | 45 | Sun Brain | 73 (50/23) | 0.193 | [65] |
| Underexpressed | −1.966 | 29 | Bredel Brain 2 | 9 (5/4) | 0.042 | [66] | |
| No difference | 1.029 | 46 | French Brain | 29 (23/6) | 0.177 | [67] | |
3.3. Breast Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Invasive Ductal Breast Carcinoma vs. Normal | |||||||
| STEAP1 | No difference | −2.025 | 22 | Ma Breast 4 | 23 (9/14) | 0.066 | [75] |
| Overexpressed | 1.549 | 32 | Zhao Breast | 41 (38/3) | 0.025 | [76] | |
| Underexpressed | −2.151 | 15 | Sorlie Breast 2 | 82 (78/4) | 0.024 | [77] | |
| Underexpressed | −2.296 | 12 | Sorlie Breast | 66 (62/4) | 0.013 | [78] | |
| No difference | −2.301 | 17 | Perou Breast | 38 (35/3) | 0.054 | [79] | |
| No difference | −1.26 | 32 | Radvanyi Breast | 36 (28/8) | 0.199 | [80] | |
| Underexpressed | −1.923 | 7 | Curtis Breast | 1700 (1556/144) | 8.32 × 10−40 | [81] | |
| No difference | 1.115 | 55 | Turashvili Breast | 25 (5/20) | 0.413 | [82] | |
| Underexpressed | −3.133 | 5 | TCGA Breast | 450 (389/61) | 4.07 × 10−27 | [63] | |
| Underexpressed | −2.602 | 14 | Richardson Breast 2 | 47 (40/7) | 0.001 | [83] | |
| STEAP2 | No difference | 1.966 | 19 | Radvanyi Breast | 33 (28/5) | 0.068 | [80] |
| Underexpressed | −2.132 | 8 | TCGA Breast | 450 (389/61) | 4.73 × 10−22 | [63] | |
| No difference | −3.814 | 23 | Sorlie Breast 2 | 92 (89/3) | 0.067 | [77] | |
| No difference | −2.738 | 23 | Perou Breast | 39 (36/3) | 0.115 | [79] | |
| Underexpressed | −3.395 | 16 | Sorlie Breast | 68 (64/4) | 0.031 | [78] | |
| No difference | −1.343 | 32 | Zhao Breast | 41 (38/3) | 0.139 | [76] | |
| No difference | −1.057 | 63 | Turashvili Breast | 25 (5/20) | 0.46 | [82] | |
| Underexpressed | −1.859 | 4 | Curtis Breast | 1700 (1556/144) | 7.32 × 10−60 | [81] | |
| No difference | −1.529 | 40 | Ma Breast 4 | 23 (9/14) | 0.22 | [75] | |
| Underexpressed | −5.471 | 3 | Richardson Breast 2 | 47 (40/7) | 1.53 × 10−8 | [83] | |
| STEAP3 | No difference | 1.038 | 62 | Radvanyi Breast | 39 (30/9) | 0.435 | [80] |
| Overexpressed | 1.15 | 41 | Curtis Breast | 1700 (1556/144) | 8.55 × 10−6 | [81] | |
| Overexpressed | 1.309 | 31 | TCGA Breast | 450 (389/61) | 3.19 × 10−6 | [63] | |
| No difference | 1.158 | 52 | Zhao Breast | 38 (35/3) | 0.167 | [76] | |
| Underexpressed | −1.452 | 13 | Ma Breast 4 | 23 (9/14) | 0.019 | [75] | |
| No difference | 1.36 | 50 | Richardson Breast 2 | 47 (40/7) | 0.058 | [83] | |
| Underexpressed | −3.647 | 3 | Turashvili Breast | 25 (5/20) | 0.006 | [82] | |
| STEAP4 | No difference | 2.276 | 24 | Radvanyi Breast | 27 (21/6) | 0.098 | [80] |
| No difference | 1.218 | 27 | Ma Breast 4 | 23 (9/14) | 0.044 | [75] | |
| Underexpressed | −1.198 | 22 | Curtis Breast | 1700 (1556/144) | 1.2 × 10−10 | [81] | |
| Underexpressed | −2.537 | 19 | Zhao Breast | 40 (37/3) | 0.034 | [76] | |
| Underexpressed | −2.845 | 13 | TCGA Breast | 450 (389/61) | 1.7 × 10−16 | [63] | |
| No difference | −2.553 | 17 | Turashvili Breast | 25 (5/20) | 0.077 | [82] | |
| No difference | −1.527 | 89 | Richardson Breast 2 | 47 (40/7) | 0.948 | [83] | |
| Lobular Breast Carcinoma vs. Normal | |||||||
| STEAP1 | No difference | 1.261 | 41 | Zhao Breast | 24 (21/3) | 0.078 | [76] |
| No difference | −1.352 | 31 | Sorlie Breast 2 | 9 (5/4) | 0.203 | [77] | |
| No difference | −1.534 | 19 | Sorlie Breast | 8 (4/4) | 0.129 | [78] | |
| No difference | −1.604 | 23 | Perou Breast | 7 (4/3) | 0.133 | [79] | |
| No difference | 1.57 | 52 | Radvanyi Breast | 8 (5/3) | 0.336 | [80] | |
| Underexpressed | −1.8 | 9 | Curtis Breast | 292 (148/144) | 9.83 × 10−20 | [81] | |
| No difference | 1.086 | 61 | Turashvili Breast | 25 (5/20) | 0.413 | [82] | |
| Underexpressed | −2.211 | 91 | TCGA Breast | 97 (36/61 | 7.89 × 10−6 | [63] | |
| STEAP2 | No difference | 1.423 | 44 | Radvanyi Breast | 12 (7/5) | 0.263 | [80] |
| Overexpressed | 1.325 | 41 | TCGA Breast | 97 (36/61) | 0.031 | [63] | |
| No difference | −2.276 | 24 | Sorlie Breast 2 | 9 (6/3) | 0.141 | [77] | |
| No difference | −1.966 | 28 | Perou Breast | 7 (4/3) | 0.187 | [79] | |
| No difference | −2.768 | 11 | Sorlie Breast | 8 (4/4) | 0.062 | [78] | |
| No difference | 1.02 | 69 | Zhao Breast | 24 (21/3) | 0.481 | [76] | |
| No difference | 1.446 | 49 | Turashvili Breast | 25 (5/20) | 0.307 | [82] | |
| Underexpressed | −1.469 | 15 | Curtis Breast | 292 (148/144) | 4.25 × 10−11 | [81] | |
| STEAP3 | No difference | −1.391 | 25 | Radvanyi Breast | 16 (7/9) | 0.204 | [80] |
| Overexpressed | 1.11 | 45 | Curtis Breast | 292 (148/144) | 0.020 | [81] | |
| Overexpressed | 1.225 | 37 | TCGA Breast | 97 (36/61) | 0.013 | [63] | |
| No difference | 1.066 | 63 | Zhao Breast | 24 (21/3) | 0.338 | [76] | |
| No difference | 1.053 | 65 | Turashvili Breast | 25 (5/20) | 0.452 | [82] | |
| STEAP4 | Overexpressed | 3.969 | 7 | Radvanyi Breast | 11 (5/6) | 0.024 | [80] |
| Underexpressed | −1.108 | 35 | Curtis Breast | 292 (148/144) | 0.006 | [81] | |
| No difference | 1.035 | 68 | Zhao Breast | 23 (20/3) | 0.463 | [76] | |
| Underexpressed | −2.024 | 22 | TCGA Breast | 97 (36/61) | 1.29 × 10−4 | [63] | |
| No difference | −3.8 | 16 | Turashvili Breast | 25 (5/20) | 0.103 | [82] | |
| Fibroadenoma vs. Normal | |||||||
| STEAP1 | Underexpressed | −2.412 | 5 | Sorlie Breast 2 | 6 (2/4) | 0.02 | [77] |
| Underexpressed | −2.95 | 3 | Sorlie Breast | 7 (3/4) | 0.006 | [78] | |
| STEAP2 | No difference | −2.031 | 25 | Sorlie Breast 2 | 5 (2/3) | 0.168 | [77] |
| No difference | −2.581 | 17 | Sorlie Breast | 7 (3/4) | 0.081 | [78] | |
3.4. Cervical Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Cervical Squamous Cell Carcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 1.935 | 13 | Biewenga Cervix | 45 (40/5) | 5.89 × 10−5 | [87] |
| No difference | 1.08 | 48 | Zhai Cervix | 31 (21/10) | 0.299 | [88] | |
| No difference | 1.101 | 47 | Scotto Cervix 2 | 56 (32/24) | 0.298 | [89] | |
| Overexpressed | 1.697 | 41 | Pyeon Multi-cancer | 42 (20/22) | 0.018 | [90] | |
| STEAP2 | No difference | 1.014 | 63 | Pyeon Multi-cancer | 42 (20/22) | 0.464 | [90] |
| No difference | 1.017 | 64 | Biewenga Cervix | 45 (40/5) | 0.452 | [87] | |
| STEAP3 | Overexpressed | 1.438 | 6 | Scotto Cervix 2 | 56 (32/24) | 1.05 × 10−5 | [89] |
| Overexpressed | 2.07 | 13 | Biewenga Cervix | 45 (40/5) | 7.39 × 10−5 | [87] | |
| Overexpressed | 1.466 | 31 | Pyeon Multi-cancer | 42 (20/22) | 0.002 | [90] | |
| STEAP4 | No difference | −1.074 | 52 | Zhai Cervix | 31 (21/10) | 0.342 | [88] |
| No difference | 1.162 | 56 | Biewenga Cervix | 45 (40/5) | 0.170 | [87] | |
| No difference | −2.22 | 92 | Scotto Cervix 2 | 56 (32/24) | 0.998 | [89] | |
| No difference | 1.048 | 61 | Pyeon Multi-cancer | 42 (20/22) | 0.369 | [90] | |
3.5. Colorectal Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Colorectal Carcinoma vs. Normal | |||||||
| STEAP1 | No difference | 1.257 | 26 | Zou Colon | 17 (9/8) | 0.100 | [94] |
| Overexpressed | 1.629 | 13 | Skrzypczak Colorectal | 60 (36/24) | 4.41 × 10−5 | [95] | |
| No difference | 1.003 | 62 | Skrzypczak Colorectal 2 | 15 (5/10) | 0.497 | [95] | |
| No difference | −1.372 | 89 | Hong Colorectal | 82 (70/12) | 0.989 | [96] | |
| STEAP2 | No difference | 1.117 | 43 | Zou Colon | 17 (9/8) | 0.333 | [94] |
| Overexpressed | 1.596 | 28 | Skrzypczak Colorectal 2 | 15 (5/10) | 0.002 | [95] | |
| No difference | −1.203 | 31 | Skrzypczak Colorectal | 60 (36/24) | 0.044 | [95] | |
| Underexpressed | −1.466 | 14 | Hong Colorectal | 82 (70/12) | 1.74 × 10−4 | [96] | |
| STEAP3 | Overexpressed | 1.394 | 7 | Skrzypczak Colorectal 2 | 15 (5/10) | 3.37 × 10−7 | [95] |
| Overexpressed | 1.195 | 33 | Skrzypczak Colorectal | 60 (36/24) | 0.022 | [95] | |
| No difference | −1.018 | 68 | Hong Colorectal | 82 (70/12) | 0.558 | [96] | |
| STEAP4 | No difference | 1.119 | 41 | Skrzypczak Colorectal 2 | 15 (5/10) | 0.058 | [95] |
| No difference | 1.153 | 54 | Skrzypczak Colorectal | 60 (36/24) | 0.242 | [95] | |
| Underexpressed | −2.091 | 20 | Hong Colorectal | 82 (70/12) | 0.005 | [96] | |
| Rectal Adenocarcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 1.729 | 18 | Gaedcke Colorectal | 130 (65/65) | 2.07 × 10−9 | [97] |
| Overexpressed | 1.947 | 28 | Sabates-Bellver Colon | 39 (7/32) | 0.005 | [98] | |
| No difference | 1.053 | 59 | Kaiser Colon | 13 (8/5) | 0.390 | [99] | |
| No difference | 1.019 | 60 | TCGA Colorectal | 82 (60/22) | 0.436 | [63] | |
| STEAP2 | Overexpressed | 1.326 | 29 | Gaedcke Colorectal | 130 (65/65) | 1.23 × 10−5 | [97] |
| No difference | 1.08 | 53 | Kaiser Colon | 13 (8/5) | 0.266 | [99] | |
| No difference | −1.106 | 69 | TCGA Colorectal | 123 (101/22) | 0.809 | [63] | |
| No difference | 1.036 | 68 | Sabates-Bellver Colon | 39 (7/32) | 0.416 | [98] | |
| STEAP3 | Overexpressed | 1.939 | 9 | Sabates-Bellver Colon | 39 (7/32) | 3.66 × 10−5 | [98] |
| Overexpressed | 1.707 | 11 | Gaedcke Colorectal | 130 (65/65) | 2.36 × 10−14 | [97] | |
| No difference | −1.148 | 71 | TCGA Colorectal | 82 (60/22) | 0.876 | [63] | |
| No difference | 1.04 | 60 | Kaiser Colon | 13 (8/5) | 0.423 | [99] | |
| STEAP4 | No difference | 1.131 | 52 | TCGA Colorectal | 82 (60/22) | 0.165 | [63] |
| No difference | 1.094 | 37 | Kaiser Colon | 13 (8/5) | 0.059 | [99] | |
| Overexpressed | 1.556 | 32 | Gaedcke Colorectal | 130 (65/65) | 8.75 × 10−5 | [97] | |
| No difference | 1.061 | 68 | Sabates-Bellver Colon | 39 (7/32) | 0.429 | [98] | |
| Colon Adenocarcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 1.771 | 22 | Sabates-Bellver Colon | 57 (25/32) | 1.49 × 10−5 | [98] |
| No difference | −1.134 | 43 | Kaiser Colon | 46 (41/5) | 0.069 | [99] | |
| No difference | 1.09 | 54 | TCGA Colorectal | 123 (101/22) | 0.218 | [63] | |
| No difference | 1.628 | 41 | Skrzypczak Colorectal 2 | 15 (5/10) | 0.073 | [95] | |
| STEAP2 | Overexpressed | 1.215 | 22 | Ki Colon | 91 (50/41) | 7.99 × 10−4 | [100] |
| Overexpressed | 1.658 | 16 | Skrzypczak Colorectal 2 | 15 (5/10) | 0.001 | [95] | |
| No difference | 1.024 | 61 | Kaiser Colon | 46 (41/5) | 0.379 | [99] | |
| No difference | −1.031 | 64 | TCGA Colorectal | 123 (101/22) | 0.624 | [63] | |
| No difference | 1.006 | 71 | Sabates-Bellver Colon | 39 (7/32) | 0.476 | [98] | |
| STEAP3 | Overexpressed | 1.472 | 8 | Skrzypczak Colorectal 2 | 15 (5/10) | 3.12 × 10−5 | [95] |
| Overexpressed | 1.572 | 18 | Sabates-Bellver Colon | 57 (25/32) | 2.37 × 10−6 | [98] | |
| No difference | −1.02 | 63 | TCGA Colorectal | 123 (101/22) | 0.570 | [63] | |
| No difference | 1.237 | 52 | Kaiser Colon | 46 (41/5) | 0.141 | [99] | |
| STEAP4 | No difference | 1.082 | 49 | Skrzypczak Colorectal 2 | 15 (5/10) | 0.148 | [95] |
| No difference | 1.191 | 49 | TCGA Colorectal | 123 (101/22) | 0.088 | [63] | |
| No difference | 1.037 | 52 | Kaiser Colon | 46 (41/5) | 0.140 | [99] | |
| Underexpressed | −2.042 | 2 | Sabates-Bellver Colon | 39 (7/32) | 7.88 × 10−5 | [98] | |
3.6. Esophageal Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Barrett’s Esophagus vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.922 | 8 | Hao Esophagus | 39 (14/25) | 0.001 | [103] |
| Overexpressed | 2.019 | 4 | Kimchi Esophagus | 16 (8/8) | 0.005 | [104] | |
| No difference | −1.049 | 51 | Kim Esophagus | 43 (15/28) | 0.610 | [105] | |
| STEAP2 | Overexpressed | 2.178 | 6 | Hao Esophagus | 41 (13/28) | 3.98 × 10−4 | [103] |
| Overexpressed | 1.985 | 7 | Kim Esophagus | 43 (15/28) | 8.10 × 10−6 | [105] | |
| STEAP3 | No difference | 1.369 | 28 | Hao Esophagus | 42 (14/28) | 0.066 | [103] |
| No difference | 1.056 | 39 | Kimchi Esophagus | 16 (8/8) | 0.367 | [104] | |
| No difference | 1.019 | 37 | Kim Esophagus | 43 (15/28) | 0.198 | [105] | |
| STEAP4 | No difference | 1.129 | 40 | Kimchi Esophagus | 16 (8/8) | 0.377 | [104] |
| No difference | 1.296 | 40 | Hao Esophagus | 41 (13/28) | 0.160 | [103] | |
| Underexpressed | −1.791 | 12 | Kim Esophagus | 43 (15/28) | 2.36 × 10−7 | [105] | |
| Esophageal Squamous Cell Carcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 1.798 | 7 | Su Esophagus 2 | 106 (53/53) | 1.40 × 10−10 | [106] |
| Overexpressed | 1.577 | 18 | Hu Esophagus | 34 (17/17) | 0.002 | [107] | |
| STEAP2 | Overexpressed | 1.118 | 38 | Su Esophagus 2 | 102 (51/51) | 0.040 | [106] |
| STEAP3 | Overexpressed | 1.278 | 30 | Hu Esophagus | 34 (17/17) | 0.031 | [107] |
| Overexpressed | 1.165 | 28 | Su Esophagus 2 | 106 (53/53) | 0.002 | [106] | |
| STEAP4 | Underexpressed | −1.39 | 25 | Hu Esophagus | 34 (17/17) | 0.012 | [107] |
| Underexpressed | −1.744 | 7 | Su Esophagus 2 | 102 (51/51) | 7.01 × 10−9 | [106] | |
| Esophageal Adenocarcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 14.326 | 1 | Hao Esophagus | 30 (5/25) | 7.24 × 10−9 | [103] |
| Overexpressed | 2.102 | 12 | Kimchi Esophagus | 16 (8/8) | 0.013 | [104] | |
| No difference | 1.034 | 46 | Kim Esophagus | 93 (75/28) | 0.409 | [105] | |
| STEAP2 | Overexpressed | 2.448 | 6 | Hao Esophagus | 31 (5/26) | 1.66 × 10−4 | [103] |
| Overexpressed | 1.672 | 10 | Kim Esophagus | 93 (75/28) | 4.10 × 10−7 | [105] | |
| STEAP3 | Overexpressed | 1.743 | 35 | Hao Esophagus | 33 (5/28) | 0.046 | [103] |
| No difference | −1.122 | 48 | Kimchi Esophagus | 16 (8/8) | 0.247 | [104] | |
| No difference | 1.028 | 30 | Kim Esophagus | 93 (75/28) | 0.057 | [105] | |
| STEAP4 | No difference | −1.895 | 33 | Kimchi Esophagus | 16 (8/8) | 0.086 | [104] |
| No difference | 1.325 | 61 | Hao Esophagus | 33 (5/28) | 0.314 | [103] | |
| Underexpressed | −1.396 | 29 | Kim Esophagus | 93 (75/28) | 0.001 | [105] | |
3.7. Gastric Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Gastric Cancer vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.544 | 5 | Cui Gastric | 160 (80/80) | 2.04 × 10−4 | [111] |
| Overexpressed | 2.193 | 23 | Wang Gastric | 27 (12/15) | 0.020 | [112] | |
| STEAP2 | Overexpressed | 1.478 | 3 | Cui Gastric | 160 (80/80) | 1.95 × 10−5 | [111] |
| No difference | 1.116 | 60 | Wang Gastric | 27 (12/15) | 0.327 | [112] | |
| STEAP3 | No difference | −1.057 | 46 | Cui Gastric | 160 (80/80) | 0.335 | [111] |
| No difference | 1.078 | 62 | Wang Gastric | 27 (12/15) | 0.371 | [112] | |
| STEAP4 | No difference | −1.073 | 43 | Cui Gastric | 160 (80/80) | 0.273 | [111] |
| No difference | −1.94 | 20 | Wang Gastric | 27 (12/15) | 0.059 | [112] | |
| Gastric Intestinal Type Adenocarcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 1.928 | 8 | Cho Gastric | 39 (20/19) | 0.002 | [113] |
| Overexpressed | 1.862 | 8 | Chen Gastric | 93 (66/27) | 1.75 × 10−8 | [114] | |
| Overexpressed | 2.309 | 13 | DErrico Gastric | 57 (26/31) | 2.76 × 10−6 | [115] | |
| STEAP2 | Overexpressed | 1.689 | 8 | Cho Gastric | 39 (20/19) | 0.002 | [113] |
| Overexpressed | 1.252 | 34 | Chen Gastric | 75 (56/19) | 0.013 | [114] | |
| Overexpressed | 1.35 | 30 | DErrico Gastric | 57 (26/31) | 0.002 | [115] | |
| STEAP3 | No difference | 1.315 | 48 | DErrico Gastric | 57 (26/31) | 0.061 | [115] |
| No difference | 1.014 | 52 | Cho Gastric | 39 (29/19) | 0.305 | [113] | |
| STEAP4 | No difference | 1.028 | 71 | DErrico Gastric | 57 (26/31) | 0.459 | [115] |
| No difference | 1.029 | 41 | Cho Gastric | 39 (29/19) | 0.151 | [113] | |
| Diffuse Gastric Adenocarcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.13 | 2 | Cho Gastric | 50 (31/19) | 8.30 × 10−7 | [113] |
| Overexpressed | 1.689 | 5 | Chen Gastric | 39 (12/27) | 1.05 × 10−4 | [114] | |
| Overexpressed | 1.987 | 18 | DErrico Gastric | 37 (6/31) | 0.015 | [115] | |
| STEAP2 | Overexpressed | 1.565 | 10 | Cho Gastric | 50 (31/19) | 7.38 × 10−4 | [113] |
| Overexpressed | 1.262 | 15 | Chen Gastric | 28 (9/19) | 0.004 | [114] | |
| No difference | 1.341 | 33 | DErrico Gastric | 37 (6/31) | 0.064 | [115] | |
| STEAP3 | No difference | −1.052 | 45 | DErrico Gastric | 37 (6/31) | 0.368 | [115] |
| No difference | 1.004 | 59 | Cho Gastric | 23 (4/19) | 0.431 | [113] | |
| STEAP4 | Overexpressed | 1.501 | 27 | DErrico Gastric | 37 (6/31) | 0.037 | [115] |
| No difference | 1.027 | 44 | Cho Gastric | 50 (31/19) | 0.15 | [113] | |
3.8. Head and Neck Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Oral Cavity Squamous Cell Carcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.879 | 2 | Toruner Head-Neck | 20 (16/4) | 8.74 × 10−5 | [118] |
| Overexpressed | 3.657 | 18 | Pyeon Multi-cancer | 26 (4/22) | 0.037 | [90] | |
| Overexpressed | 1.639 | 7 | Peng Head-Neck | 79 (57/22) | 1.84 × 10−8 | [119] | |
| STEAP2 | No difference | 1.406 | 36 | Pyeon Multi-cancer | 26 (4/22) | 0.153 | [90] |
| No difference | 1.047 | 40 | Peng Head-Neck | 79 (57/22) | 0.310 | [119] | |
| STEAP3 | Overexpressed | 1.457 | 5 | Peng Head-Neck | 79 (57/22) | 1.53 × 10−9 | [119] |
| Overexpressed | 1.525 | 17 | Toruner Head-Neck | 20 (16/4) | 0.021 | [118] | |
| No difference | 1.58 | 35 | Pyeon Multi-cancer | 26 (4/22) | 0.139 | [90] | |
| STEAP4 | Underexpressed | −1.14 | 22 | Pyeon Multi-cancer | 26 (4/22) | 0.024 | [90] |
| No difference | −1.087 | 31 | Toruner Head-Neck | 20 (16/4) | 0.103 | [118] | |
| Underexpressed | −1.555 | 23 | Peng Head-Neck | 79 (57/22) | 0.003 | [119] | |
| Tongue Carcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.32 | 16 | Pyeon Multi-cancer | 37 (15/22) | 0.001 | [90] |
| Overexpressed | 2.122 | 13 | Estilo Head-Neck | 57 (31/26) | 2.59 × 10−5 | [120] | |
| Overexpressed | 1.535 | 16 | Talbot Lung | 59 (31/28) | 9.08 × 10−5 | [121] | |
| Overexpressed | 2.483 | 8 | Ye Head-Neck | 38 (26/12) | 0.001 | [122] | |
| No difference | −1.08 | 47 | Kuriakose Head-Neck | 25 (3/22) | 0.42 | [123] | |
| STEAP2 | No difference | −1.038 | 59 | Pyeon Multi-cancer | 37 (15/22) | 0.384 | [90] |
| No difference | 1.019 | 68 | Ye Head-Neck | 38 (26/12) | 0.457 | [122] | |
| STEAP3 | Overexpressed | 1.347 | 18 | Pyeon Multi-cancer | 37 (15/22) | 0.002 | [90] |
| Overexpressed | 1.115 | 29 | Ye Head-Neck | 38 (26/12) | 0.044 | [122] | |
| STEAP4 | No difference | 1.248 | 33 | Ye Head-Neck | 38 (26/12) | 0.063 | [122] |
| No difference | 1.07 | 53 | Pyeon Multi-cancer | 37 (15/22) | 0.294 | [90] | |
3.9. Kidney Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Clear Cell Renal Cell Carcinoma vs. Normal | |||||||
| STEAP1 | Underexpressed | −1.304 | 13 | Higgins Renal | 29 (26/3) | 0.013 | [126] |
| Overexpressed | 1.764 | 21 | Yusenko Renal | 31 (26/5) | 0.014 | [127] | |
| No difference | −1.008 | 52 | Jones Renal | 46 (23/23) | 0.456 | [131] | |
| No difference | 1.055 | 47 | Gumz Renal | 20 (10/10) | 0.330 | [133] | |
| No difference | −1.17 | 26 | Lenburg Renal | 18 (9/9) | 0.052 | [132] | |
| STEAP2 | No difference | −1.27 | 27 | Yusenko Renal | 31 (26/5) | 0.132 | [127] |
| Underexpressed | −1.322 | 21 | Lenburg Renal | 18 (9/9) | 0.027 | [132] | |
| STEAP3 | Overexpressed | 1.629 | 18 | Lenburg Renal | 18 (9/9) | 0.019 | [132] |
| Overexpressed | 1.921 | 31 | Jones Renal | 46 (23/23) | 0.001 | [131] | |
| No difference | 1.833 | 32 | Yusenko Renal | 31 (26/5) | 0.055 | [127] | |
| No difference | −1.005 | 60 | Gumz Renal | 20 (10/10) | 0.491 | [133] | |
| STEAP4 | Underexpressed | −1.629 | 11 | Jones Renal | 46 (23/23) | 4.7 × 10−7 | [131] |
| Overexpressed | 1.899 | 17 | Lenburg Renal | 18 (9/9) | 0.017 | [132] | |
| Overexpressed | 4.584 | 25 | Yusenko Renal | 31 (26/5) | 0.027 | [127] | |
| No difference | −1.942 | 35 | Cutcliffe Renal | 17 (14/3) | 0.259 | [134] | |
| No difference | −2.059 | 33 | Gumz Renal | 20 (10/10) | 0.056 | [133] | |
| Papillary Renal Cell Carcinoma vs. Normal | |||||||
| STEAP1 | No difference | −1.179 | 20 | Higgins Renal | 7 (4/3) | 0.067 | [126] |
| Overexpressed | 1.649 | 22 | Yusenko Renal | 31 (26/5) | 0.033 | [127] | |
| No difference | −1.044 | 47 | Jones Renal | 34(11/23) | 0.359 | [131] | |
| STEAP2 | No difference | 1.196 | 44 | Yusenko Renal | 24 (19/5) | 0.172 | [127] |
| STEAP3 | No difference | −1.011 | 49 | Jones Renal | 34 (11/23) | 0.46 | [131] |
| Overexpressed | 1.957 | 24 | Yusenko Renal | 24 (19/5) | 0.040 | [127] | |
| STEAP4 | Underexpressed | −1.19 | 33 | Jones Renal | 34 (11/23) | 0.043 | [131] |
| No difference | 1.238 | 61 | Yusenko Renal | 24 (19/5) | 0.368 | [127] | |
| Chromophobe Renal Cell Carcinoma vs. Normal | |||||||
| STEAP1 | No difference | −1.162 | 26 | Higgins Renal | 6 (3/3) | 0.19 | [126] |
| Underexpressed | −3.393 | 8 | Yusenko Renal | 9 (4/5) | 0.01 | [127] | |
| No difference | −1.173 | 26 | Jones Renal | 29 (6/23) | 0.051 | [131] | |
| STEAP2 | No difference | −4.435 | 27 | Yusenko Renal | 9 (4/5) | 0.117 | [127] |
| STEAP3 | No difference | 1.055 | 51 | Jones Renal | 29 (6/23) | 0.175 | [131] |
| No difference | 2.02 | 47 | Yusenko Renal | 9 (4/5) | 0.176 | [127] | |
| STEAP4 | Overexpressed | 2.672 | 3 | Jones Renal | 29 (6/23) | 4.23 × 10−9 | [131] |
| No difference | 1.151 | 65 | Yusenko Renal | 9 (4/5) | 0.426 | [127] | |
| Renal Wilms Tumor vs. Normal | |||||||
| STEAP1 | No difference | −1.281 | 46 | Yusenko Renal | 9 (4/5) | 0.361 | [127] |
| No difference | −1.113 | 35 | Cutcliffe Renal | 21 (18/3) | 0.318 | [134] | |
| STEAP2 | Underexpressed | −1.919 | 6 | Yusenko Renal | 9 (4/5) | 0.01 | [127] |
| STEAP3 | Overexpressed | 1.488 | 6 | Cutcliffe Renal | 21 (18/3) | 0.003 | [134] |
| No difference | 1.182 | 59 | Yusenko Renal | 9 (4/5) | 0.347 | [127] | |
| STEAP4 | No difference | 1.472 | 57 | Yusenko Renal | 9 (4/5) | 0.316 | [127] |
| No difference | −1.395 | 38 | Cutcliffe Renal | 21 (18/3) | 0.369 | [134] | |
| Renal Oncocytoma vs. Normal | |||||||
| STEAP1 | No difference | −1.526 | 40 | Yusenko Renal | 9 (4/5) | 0.256 | [127] |
| Underexpressed | −1.237 | 26 | Jones Renal | 35 (12/23) | 0.008 | [131] | |
| STEAP2 | No difference | −1.374 | 44 | Yusenko Renal | 9 (4/5) | 0.317 | [127] |
| STEAP3 | No difference | 1.108 | 53 | Jones Renal | 35 (12/23) | 0.163 | [131] |
| No difference | 2.305 | 41 | Yusenko Renal | 9 (4/5) | 0.104 | [127] | |
| STEAP4 | Overexpressed | 3.041 | 2 | Jones Renal | 35 (12/23) | 2.83 × 10−18 | [127] |
| No difference | 1.477 | 60 | Yusenko Renal | 9 (4/5) | 0.311 | [127] | |
3.10. Leukemia
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| T-Cell Acute Lymphoblastic Leukemia vs. Normal | |||||||
| STEAP1 | Overexpressed | 3.812 | 2 | Andersson Leukemia | 17 (11/6) | 5.62 × 10−9 | [136] |
| No difference | −1.014 | 49 | Haferlach Leukemia | 248 (174/74) | 0.175 | [139] | |
| No difference | −1.315 | 42 | Coustan-Smith Leukemia | 50 (46/4) | 0.239 | [140] | |
| STEAP2 | Overexpressed | 1.027 | 36 | Haferlach Leukemia | 248 (174/74) | 0.001 | [139] |
| Underexpressed | −2.202 | 15 | Andersson Leukemia | 17 (11/6) | 8.18 × 10−5 | [136] | |
| STEAP3 | Underexpressed | −3.525 | 2 | Haferlach Leukemia | 248 (174/74) | 5.53 × 10−44 | [139] |
| No difference | 1.441 | 45 | Coustan-Smith Leukemia | 50 (46/4) | 0.233 | [140] | |
| STEAP4 | Overexpressed | 3.472 | 5 | Coustan-Smith Leukemia | 50 (46/4) | 9.05 × 10−5 | [140] |
| Underexpressed | −2.268 | 10 | Haferlach Leukemia | 248 (174/74) | 4.08 × 10−19 | [139] | |
| Underexpressed | −26.262 | 2 | Andersson Leukemia | 15 (9/6) | 6.45 × 10−9 | [136] | |
| B-Cell Acute Lymphoblastic Leukemia vs. Normal | |||||||
| STEAP1 | Overexpressed | 3.533 | 4 | Andersson Leukemia | 92 (86/6) | 8.25 × 10−12 | [136] |
| No difference | −1.021 | 46 | Haferlach Leukemia | 248 (174/74) | 0.081 | [139] | |
| No difference | −1.189 | 50 | Coustan-Smith Leukemia | 242 (238/4) | 0.317 | [140] | |
| STEAP2 | Overexpressed | 1.019 | 41 | Haferlach Leukemia | 248 (174/74) | 0.018 | [139] |
| Underexpressed | −2.006 | 16 | Andersson Leukemia | 93 (87/6) | 2.94 × 10−5 | [136] | |
| STEAP3 | Underexpressed | −3.483 | 3 | Haferlach Leukemia | 248 (174/74) | 1.78 × 10−42 | [139] |
| No difference | 1.337 | 43 | Coustan-Smith Leukemia | 242 (238/4) | 0.275 | [140] | |
| STEAP4 | Overexpressed | 3.687 | 4 | Coustan-Smith Leukemia | 242 (238/4) | 6.93 × 10−4 | [140] |
| Underexpressed | −2.385 | 9 | Haferlach Leukemia | 248 (174/74) | 1.65 × 10−20 | [139] | |
| Underexpressed | −24.399 | 9 | Andersson Leukemia | 88 (82/6) | 1.47 × 10−7 | [136] | |
| Acute Myeloid Leukemia vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.323 | 3 | Andersson Leukemia | 29 (23/6) | 3.28 × 10−9 | [136] |
| No difference | −1 | 51 | Haferlach Leukemia | 616 (542/74) | 0.496 | [139] | |
| Underexpressed | −2.196 | 13 | Stegmaier Leukemia | 15 (9/6) | 0.007 | [137] | |
| Underexpressed | −1.179 | 11 | Valk Leukemia | 293 (285/8) | 0.035 | [138] | |
| STEAP2 | Overexpressed | 1.013 | 49 | Haferlach Leukemia | 616 (542/74) | 0.042 | [139] |
| Underexpressed | −2.077 | 9 | Andersson Leukemia | 29 (23/6) | 5.86 × 10−6 | [136] | |
| STEAP3 | Underexpressed | −1.483 | 9 | Haferlach Leukemia | 616 (542/74) | 5.07 × 10−11 | [139] |
| No difference | −1.122 | 52 | Stegmaier Leukemia | 15 (9/6) | 0.355 | [137] | |
| No difference | 1.017 | 69 | Valk Leukemia | 293 (285/8) | 0.450 | [138] | |
| STEAP4 | Underexpressed | −2.068 | 5 | Haferlach Leukemia | 616 (542/74) | 1.44 × 10−16 | [139] |
| No difference | −2.02 | 42 | Stegmaier Leukemia | 15 (9/6) | 0.213 | [137] | |
| Underexpressed | −16.371 | 2 | Andersson Leukemia | 29 (23/6) | 6.93 × 10−10 | [136] | |
| No difference | −1.567 | 24 | Valk Leukemia | 293 (285/8) | 0.194 | [138] | |
| Chronic Lymphocytic Leukemia vs. Normal | |||||||
| STEAP1 | Underexpressed | −1.943 | 20 | Basso Lymphoma | 59 (34/25) | 0.007 | [141] |
| No difference | −1.019 | 46 | Haferlach Leukemia | 522 (448/74) | 0.105 | [139] | |
| Underexpressed | −2.151 | 24 | Haslinger Leukemia | 111 (100/11) | 0.01 | [142] | |
| STEAP2 | Overexpressed | 1.014 | 51 | Haferlach Leukemia | 522 (448/74) | 0.043 | [139] |
| STEAP3 | Underexpressed | −3.937 | 4 | Haferlach Leukemia | 522 (448/74) | 1.62 × 10−41 | [139] |
| STEAP4 | Underexpressed | −2.149 | 15 | Haferlach Leukemia | 522 (448/74) | 1.23 × 10−17 | [139] |
3.11. Liver Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Hepatocellular Carcinoma vs. Normal | |||||||
| STEAP1 | No difference | −1.051 | 37 | Chen Liver | 179 (103/76) | 0.124 | [151] |
| Overexpressed | 2.309 | 21 | Roessler Liver | 43 (22/21) | 0.003 | [144] | |
| Overexpressed | 1.87 | 26 | Roessler Liver 2 | 445 (225/220) | 4.34 × 10−12 | [144] | |
| Underexpressed | −2.348 | 18 | Mas Liver | 57 (38/19) | 1.45 × 10−4 | [145] | |
| No difference | −1.924 | 40 | Wurmbach Liver | 45 (35/10) | 0.073 | [152] | |
| STEAP2 | Overexpressed | 1.463 | 21 | Chen Liver | 173 (98/75) | 3.15 × 10−4 | [151] |
| No difference | 1.155 | 49 | Wurmbach Liver | 45 (35/10) | 0.329 | [152] | |
| STEAP3 | Underexpressed | −3.051 | 1 | Chen Liver | 180 (104/76) | 3.55 × 10−24 | [151] |
| Underexpressed | −6.944 | 1 | Wurmbach Liver | 45 (35/10) | 7.99 × 10−12 | [152] | |
| Underexpressed | −3.863 | 1 | Roessler Liver 2 | 445 (225/220) | 3.25 × 10−74 | [144] | |
| Underexpressed | −4.137 | 2 | Roessler Liver | 43 (22/21) | 4.91 × 10−9 | [144] | |
| Underexpressed | −2.295 | 2 | Mas Liver | 57 (38/19) | 5.56 × 10−10 | [145] | |
| STEAP4 | Underexpressed | −5.633 | 4 | Wurmbach Liver | 45 (35/10) | 5.0 × 10−5 | [152] |
| Underexpressed | −1.671 | 34 | Mas Liver | 57 (38/19) | 0.01 | [145] | |
| Underexpressed | −2.845 | 7 | Chen Liver | 159 (88/71) | 1.12 × 10−10 | [151] | |
| Underexpressed | −1.097 | 24 | Roessler Liver | 43 (22/21) | 0.006 | [144] | |
| Underexpressed | −1.141 | 21 | Roessler Liver 2 | 445 (225/220) | 8.27 × 10−9 | [144] | |
3.12. Lung Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Squamous Cell Lung Carcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 4.633 | 2 | Hou Lung | 82 (27/65) | 5.06 × 10−16 | [160] |
| Overexpressed | 3.287 | 4 | Garber Lung | 19 (13/6) | 2.31 × 10−4 | [161] | |
| Overexpressed | 2.358 | 8 | Wachi Lung | 10 (5/5) | 0.005 | [162] | |
| Overexpressed | 1.796 | 11 | Talbot Lung | 62 (34/28) | 2.46 × 10−6 | [121] | |
| Overexpressed | 2.744 | 12 | Bhattacharjee Lung | 38 (21/17) | 0.019 | [163] | |
| STEAP2 | No difference | 1.600 | 29 | Garber Lung | 18 (13/5) | 0.071 | [161] |
| Overexpressed | 1.289 | 49 | Hou Lung | 82 (27/65) | 0.041 | [160] | |
| STEAP3 | No difference | 1.155 | 48 | Garber Lung | 19 (13/6) | 0.292 | [161] |
| Overexpressed | 1.538 | 13 | Wachi Lung | 10 (5/5) | 0.013 | [162] | |
| Overexpressed | 1.242 | 33 | Hou Lung | 82 (27/65) | 0.003 | [160] | |
| STEAP4 | Underexpressed | −12.225 | 1 | Garber Lung | 19 (13/6) | 2.79 × 10−09 | [161] |
| Underexpressed | −1.465 | 7 | Wachi Lung | 10 (5/5) | 0.002 | [162] | |
| Underexpressed | −5.802 | 1 | Hou Lung | 82 (27/65) | 7.36 × 10−24 | [160] | |
| Lung Adenocarcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.451 | 12 | Hou Lung | 110 (45/65) | 1.57 × 10−6 | [160] |
| Overexpressed | 3.033 | 3 | Landi Lung | 107 (58/49) | 8.78 × 10−16 | [164] | |
| Overexpressed | 2.888 | 7 | Stearman Lung | 39 (20/19) | 4.53 × 10−5 | [165] | |
| Overexpressed | 2.612 | 6 | Su Lung | 57 (27/30) | 7.78 × 10−5 | [166] | |
| Overexpressed | 2.970 | 5 | Garber Lung | 46 (40/6) | 3.89 × 10−4 | [161] | |
| No difference | 1.099 | 27 | Bhattacharjee Lung | 149 (123/17) | 0.404 | [163] | |
| Overexpressed | 2.703 | 13 | Okayama Lung | 246 (226/20) | 1.39 × 10−7 | [167] | |
| No difference | 1.135 | 39 | Selamat Lung | 116 (58/58) | 0.075 | [168] | |
| STEAP2 | No difference | 1.555 | 39 | Garber Lung | 44 (39/5) | 0.080 | [161] |
| Overexpressed | 1.498 | 33 | Okayama Lung | 246 (226/20) | 0.002 | [167] | |
| No difference | 1.075 | 46 | Selamat Lung | 116 (58/58) | 0.177 | [168] | |
| No difference | 1.163 | 60 | Hou Lung | 110 (45/65) | 0.140 | [160] | |
| STEAP3 | Overexpressed | 2.512 | 5 | Okayama Lung | 246 (226/20) | 2.39 × 10−11 | [167] |
| Overexpressed | 1.734 | 3 | Su Lung | 57 (27/30) | 9.17 × 10−7 | [166] | |
| Overexpressed | 1.823 | 23 | Garber Lung | 46 (40/6) | 0.017 | [161] | |
| Overexpressed | 1.500 | 6 | Landi Lung | 107 (58/49) | 3.89 × 10−11 | [164] | |
| Overexpressed | 1.826 | 5 | Selamat Lung | 116 (58/58) | 5.83 × 10−13 | [168] | |
| Overexpressed | 1.311 | 16 | Hou Lung | 110 (45/65) | 1.82 × 10−5 | [160] | |
| STEAP4 | No difference | 1.014 | 60 | Landi Lung | 107 (58/49) | 0.424 | [164] |
| Underexpressed | −4.561 | 1 | Garber Lung | 46 (40/6) | 3.33 × 10−07 | [161] | |
| Underexpressed | −1.716 | 25 | Su Lung | 57 (27/30) | 0.031 | [166] | |
| No difference | 1.111 | 63 | Okayama Lung | 246 (226/20) | 0.256 | [167] | |
| Underexpressed | −1.212 | 24 | Selamat Lung | 116 (58/58) | 0.002 | [168] | |
| Underexpressed | −2.259 | 1 | Hou Lung | 84 (19/65) | 3.26 × 10−26 | [160] | |
3.13. Lymphoma
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Follicular Lymphoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.225 | 4 | Basso Lymphoma | 31 (6/25) | 0.004 | [141] |
| No difference | 1.045 | 66 | Brune Lymphoma | 30 (5/25) | 0.309 | [171] | |
| Underexpressed | −1.23 | 50 | Compagno Lymphoma | 58 (38/20) | 0.009 | [170] | |
| No difference | −1.036 | 61 | Storz Lymphoma | 14 (8/6) | 0.401 | [172] | |
| STEAP2 | No difference | 1.074 | 43 | Storz Lymphoma | 14 (8/6) | 0.254 | [172] |
| No difference | 1.075 | 40 | Compagno Lymphoma | 58 (38/20) | 0.104 | [170] | |
| Overexpressed | 1.135 | 29 | Brune Lymphoma | 30 (5/25) | 0.030 | [171] | |
| STEAP3 | Overexpressed | 1.302 | 15 | Compagno Lymphoma | 58 (38/20) | 5.53 × 10−6 | [170] |
| Overexpressed | 1.086 | 16 | Brune Lymphoma | 30 (5/25) | 0.007 | [171] | |
| No difference | 1.369 | 42 | Storz Lymphoma | 9 (3/6) | 0.237 | [172] | |
| STEAP4 | Overexpressed | 2.634 | 3 | Compagno Lymphoma | 58 (38/20) | 4.12 × 10−17 | [170] |
| Overexpressed | 1.148 | 17 | Brune Lymphoma | 30 (5/25) | 0.007 | [171] | |
| No difference | −1.393 | 27 | Storz Lymphoma | 14 (8/6) | 0.057 | [172] | |
| Diffuse Large B-Cell Lymphoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 1.786 | 26 | Basso Lymphoma | 57 (32/25) | 0.024 | [141] |
| Overexpressed | 1.332 | 41 | Brune Lymphoma | 36 (11/25) | 0.035 | [171] | |
| Overexpressed | 2.153 | 19 | Compagno Lymphoma | 64 (44/20) | 1.23 × 10−6 | [170] | |
| No difference | −1.003 | 64 | Storz Lymphoma | 12 (6/6) | 0.495 | [172] | |
| STEAP2 | Overexpressed | 1.199 | 17 | Storz Lymphoma | 12 (6/6) | 0.044 | [172] |
| Overexpressed | 1.71 | 17 | Compagno Lymphoma | 64 (44/20) | 3.07 × 10−7 | [170] | |
| Overexpressed | 1.097 | 34 | Brune Lymphoma | 36 (11/25) | 0.016 | [171] | |
| STEAP3 | Overexpressed | 2.261 | 6 | Compagno Lymphoma | 64 (44/20) | 2.73 × 10−13 | [170] |
| Overexpressed | 1.513 | 16 | Brune Lymphoma | 36 (11/25) | 8.33 × 10−4 | [171] | |
| No difference | 1.032 | 58 | Storz Lymphoma | 9 (3/6) | 0.447 | [172] | |
| STEAP4 | Overexpressed | 3.226 | 11 | Compagno Lymphoma | 64 (44/20) | 8.6 × 10−10 | [170] |
| Overexpressed | 1.129 | 30 | Brune Lymphoma | 36 (11/25) | 0.009 | [171] | |
| No difference | −1.236 | 38 | Storz Lymphoma | 12 (6/6) | 0.144 | [172] | |
| Burkitt’s Lymphoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 1.715 | 33 | Basso Lymphoma | 42 (17/25) | 0.045 | [141] |
| No difference | −1.049 | 40 | Brune Lymphoma | 30 (5/25) | 0.264 | [171] | |
| STEAP2 | No difference | −1.003 | 49 | Brune Lymphoma | 30 (5/25) | 0.47 | [171] |
| STEAP3 | Overexpressed | 1.219 | 25 | Brune Lymphoma | 30 (5/25) | 0.006 | [171] |
| STEAP4 | No difference | 1.078 | 50 | Brune Lymphoma | 30 (5/25) | 0.078 | [171] |
| Hodgkin’s Lymphoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 1.109 | 37 | Brune Lymphoma | 37 (12/25) | 0.038 | [171] |
| No difference | 1.524 | 35 | Eckerle Lymphoma | 45 (4/41) | 0.055 | [173] | |
| STEAP2 | Overexpressed | 1.103 | 24 | Brune Lymphoma | 37 (12/25) | 0.008 | [171] |
| No difference | 1.277 | 40 | Eckerle Lymphoma | 45 (4/41) | 0.070 | [173] | |
| STEAP3 | Overexpressed | 1.882 | 3 | Brune Lymphoma | 37 (12/25) | 4.93 × 10−6 | [171] |
| Overexpressed | 1.506 | 5 | Eckerle Lymphoma | 45 (4/41) | 9.87 × 10−4 | [173] | |
| STEAP4 | Overexpressed | 1.202 | 20 | Eckerle Lymphoma | 45 (4/41) | 0.018 | [173] |
| Overexpressed | 1.066 | 39 | Brune Lymphoma | 37 (12/25) | 0.045 | [171] | |
3.14. Melanoma
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Melanoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 4.635 | 23 | Haqq Melanoma | 9 (6/3) | 0.015 | [177] |
| Overexpressed | 2.319 | 19 | Riker Melanoma | 18 (14/4) | 0.042 | [178] | |
| No difference | −1.317 | 37 | Talantov Melanoma | 52 (45/7) | 0.082 | [179] | |
| No difference | −1.1 | 14 | Critchley-Thorne Melanoma | 46 (23/23) | 0.174 | [176] | |
| STEAP2 | No difference | 1.038 | 59 | Haqq Melanoma | 9 (6/3) | 0.402 | [177] |
| No difference | 1.05 | 23 | Critchley-Thorne Melanoma | 46 (23/23) | 0.219 | [176] | |
| Underexpressed | −2.195 | 11 | Riker Melanoma | 18 (14/4) | 0.006 | [178] | |
| STEAP3 | No difference | 1.217 | 37 | Haqq Melanoma | 9 (6/3) | 0.080 | [177] |
| Underexpressed | −2.669 | 4 | Talantov Melanoma | 52 (45/7) | 1.88 × 10−7 | [179] | |
| No difference | 1.003 | 54 | Critchley-Thorne Melanoma | 46 (23/23) | 0.473 | [176] | |
| No difference | −1.185 | 37 | Riker Melanoma | 18 (14/4) | 0.152 | [178] | |
| STEAP4 | Overexpressed | 1.119 | 3 | Critchley-Thorne Melanoma | 46 (23/23) | 0.036 | [176] |
| Underexpressed | −2.802 | 18 | Haqq Melanoma | 9 (6/3) | 0.036 | [177] | |
| No difference | 1.131 | 58 | Talantov Melanoma | 52 (45/7) | 0.414 | [179] | |
| Underexpressed | −2.521 | 14 | Riker Melanoma | 18 (14/4) | 0.01 | [178] | |
3.15. Ovarian Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Ovarian Serous Adenocarcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 1.495 | 8 | Lu Ovarian | 25 (20/5) | 0.001 | [185] |
| No difference | 1.602 | 27 | Adib Ovarian | 10 (6/4) | 0.088 | [186] | |
| No difference | 1.03 | 49 | Hendrix Ovarian | 45 (41/4) | 0.185 | [187] | |
| No difference | −1.249 | 48 | Yoshihara Ovarian | 53 (43/10) | 0.206 | [188] | |
| STEAP2 | Overexpressed | 1.21 | 24 | Lu Ovarian | 25 (20/5) | 0.040 | [185] |
| No difference | 1.238 | 36 | Yoshihara Ovarian | 50 (40/10) | 0.289 | [188] | |
| STEAP3 | Overexpressed | 2.876 | 4 | Yoshihara Ovarian | 53 (43/10) | 5.16 × 10−7 | [188] |
| Overexpressed | 1.307 | 8 | Hendrix Ovarian | 45 (41/4) | 1.27 × 10−5 | [187] | |
| Overexpressed | 1.559 | 4 | Lu Ovarian | 25 (20/5) | 1.18 × 10−4 | [185] | |
| STEAP4 | No difference | 1.083 | 44 | Lu Ovarian | 25 (20/5) | 0.184 | [185] |
| No difference | −1.009 | 53 | Hendrix Ovarian | 45 (41/4) | 0.432 | [187] | |
| Underexpressed | −25.706 | 4 | Yoshihara Ovarian | 33 (23/10) | 1.58 × 10−10 | [188] | |
| Ovarian Endometrioid Adenocarcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 1.542 | 3 | Lu Ovarian | 14 (9/5) | 7.91 × 10−4 | [185] |
| No difference | 1.031 | 50 | Hendrix Ovarian | 41 (37/4) | 0.207 | [187] | |
| STEAP2 | No difference | 1.033 | 59 | Lu Ovarian | 14 (9/5) | 0.354 | [185] |
| STEAP3 | Overexpressed | 1.368 | 6 | Hendrix Ovarian | 41 (37/4) | 1.96 × 10−6 | [187] |
| Overexpressed | 1.399 | 2 | Lu Ovarian | 14 (9/5) | 0.004 | [185] | |
| STEAP4 | No difference | 1.064 | 51 | Lu Ovarian | 14 (9/5) | 0.247 | [185] |
| No difference | −1024 | 50 | Hendrix Ovarian | 41 (37/4) | 0.326 | [187] | |
| Ovarian Clear Cell Adenocarcinoma vs. Normal | |||||||
| STEAP1 | No difference | 1.074 | 50 | Lu Ovarian | 12 (7/5) | 0.227 | [185] |
| Overexpressed | 1.124 | 20 | Hendrix Ovarian | 17 (13/4) | 0.004 | [187] | |
| STEAP2 | Underexpressed | −1.195 | 3 | Lu Ovarian | 12 (7/5) | 0.003 | [185] |
| STEAP3 | Overexpressed | 1.467 | 2 | Hendrix Ovarian | 12 (8/4) | 1.30 × 10−6 | [187] |
| No difference | 1.162 | 31 | Lu Ovarian | 12 (7/5) | 0.084 | [185] | |
| STEAP4 | No difference | 2.347 | 60 | Lu Ovarian | 14 (9/5) | 0.346 | [185] |
| No difference | −1.035 | 48 | Hendrix Ovarian | 12 (8/4) | 0.286 | [187] | |
| Ovarian Mucinous Adenocarcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 1.969 | 1 | Lu Ovarian | 14 (9/5) | 1.13 × 10−4 | [185] |
| Overexpressed | 1.124 | 20 | Hendrix Ovarian | 17 (13/4) | 0.004 | [187] | |
| STEAP2 | Overexpressed | 1.546 | 1 | Lu Ovarian | 14 (9/5) | 1.14 × 10−4 | [185] |
| STEAP3 | Overexpressed | 1.429 | 3 | Hendrix Ovarian | 17 (13/4) | 1.87 × 10−6 | [187] |
| Overexpressed | 1.272 | 2 | Lu Ovarian | 14 (9/5) | 0.001 | [185] | |
| STEAP4 | Overexpressed | 2.347 | 10 | Lu Ovarian | 14 (9/5) | 0.019 | [185] |
| No difference | −1.016 | 53 | Hendrix Ovarian | 17 (13/4) | 0.397 | [187] | |
| Ovarian Carcinoma vs. Normal | |||||||
| STEAP1 | No difference | −1.301 | 40 | Bonome Ovarian | 195 (185/10) | 0.136 | [189] |
| STEAP3 | No difference | 1.081 | 56 | Bonome Ovarian | 195 (185/10) | 0.094 | [189] |
| STEAP4 | Overexpressed | 1.086 | 41 | Bonome Ovarian | 195 (185/10) | 0.006 | [189] |
3.16. Pancreatic Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Pancreatic Ductal Adenocarcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 4.841 | 1 | Badea Pancreas | 78 (39/39) | 1.63 × 10−13 | [192] |
| Overexpressed | 1.77 | 7 | Grutzmann Pancreas | 22 (11/11) | 0.028 | [197] | |
| Overexpressed | 4.528 | 10 | Iacobuzio-Donahue Pancreas 2 | 17 (12/5) | 0.007 | [196] | |
| No difference | 1.278 | 22 | Ishikawa Pancreas | 49 (24/25) | 0.137 | [198] | |
| No difference | −1.036 | 54 | Buchholz Pancreas | 10 (5/5) | 0.467 | [193] | |
| STEAP2 | Overexpressed | 4.826 | 1 | Iacobuzio-Donahue Pancreas 2 | 17 (12/5) | 2.58 × 10−5 | [196] |
| Overexpressed | 2.45 | 3 | Badea Pancreas | 78 (39/39) | 1.72 × 10−11 | [192] | |
| No difference | 1.084 | 23 | Buchholz Pancreas | 14 (8/6) | 0.103 | [193] | |
| No difference | 1.186 | 40 | Ishikawa Pancreas | 49 (24/25) | 0.282 | [198] | |
| No difference | 1.271 | 55 | Grutzmann Pancreas | 22 (11/11) | 0.341 | [197] | |
| STEAP3 | Overexpressed | 1.726 | 5 | Grutzmann Pancreas | 22 (11/11) | 0.020 | [197] |
| Overexpressed | 1.832 | 6 | Ishikawa Pancreas | 49 (24/25) | 0.029 | [198] | |
| No difference | −1.128 | 33 | Buchholz Pancreas | 14 (8/6) | 0.168 | [193] | |
| No difference | 1.143 | 51 | Badea Pancreas | 78 (39/39) | 0.144 | [192] | |
| STEAP4 | Overexpressed | 1.72 | 38 | Badea Pancreas | 78 (39/39) | 0.004 | [192] |
| No difference | 1.147 | 47 | Grutzmann Pancreas | 22 (11/11) | 0.270 | [197] | |
| No difference | −1.246 | 39 | Iacobuzio-Donahue Pancreas 2 | 16 (11/5) | 0.269 | [196] | |
| Underexpressed | −1.528 | 13 | Buchholz Pancreas | 14 (8/6) | 0.017 | [193] | |
| No difference | −1.159 | 49 | Ishikawa Pancreas | 49 (24/25) | 0.302 | [198] | |
| Pancreatic Carcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.983 | 2 | Segara Pancreas | 17 (11/6) | 6.05 × 10−5 | [194] |
| Overexpressed | 2.673 | 14 | Pei Pancreas | 52 (36/16) | 7.00 × 10−4 | [195] | |
| Underexpressed | −1.476 | 15 | Buchholz Pancreas | 27 (23/5) | 0.05 | [193] | |
| STEAP2 | No difference | −1.045 | 38 | Buchholz Pancreas | 29 (23/6) | 0.251 | [193] |
| Overexpressed | 1.775 | 21 | Pei Pancreas | 52 (36/16) | 0.004 | [195] | |
| STEAP3 | Overexpressed | 1.35 | 30 | Pei Pancreas | 52 (36/16) | 0.025 | [195] |
| No difference | 1.048 | 41 | Buchholz Pancreas | 30 (24/6) | 0.362 | [193] | |
| No difference | −1.165 | 29 | Segara Pancreas | 17 (11/6) | 0.087 | [194] | |
| STEAP4 | No difference | 1.048 | 48 | Segara Pancreas | 17 (11/6) | 0.189 | [194] |
| No difference | −1.115 | 27 | Buchholz Pancreas | 30 (24/6) | 0.14 | [193] | |
| Underexpressed | −1.435 | 24 | Pei Pancreas | 52 (36/16) | 0.013 | [195] | |
3.17. Prostate Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Prostate Carcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.092 | 1 | Singh Prostate | 102 (52 / 50) | 1.88 × 10−6 | [206] |
| Overexpressed | 2.995 | 8 | Welsh Prostate | 34 (25/9) | 2.42 × 10−4 | [207] | |
| Overexpressed | 1.829 | 9 | Yu Prostate | 112 (65/23) | 8.08 × 10−4 | [208] | |
| No difference | 1.346 | 12 | Holzbeierlein Prostate | 54 (40/4) | 0.264 | [209] | |
| Ove-expressed | 1.551 | 9 | Liu Prostate | 57 (44/13) | 0.006 | [210] | |
| Overexpressed | 2.292 | 11 | Tomlins Prostate | 52 (30/22) | 0.002 | [211] | |
| Overexpressed | 1.391 | 7 | Taylor Prostate 3 | 185 (131/29) | 4.79 × 10−4 | [205] | |
| Overexpressed | 2.073 | 10 | Grasso Prostate | 122 (59/28) | 4.50 × 10−4 | [204] | |
| No difference | 1.419 | 15 | Luo Prostate 2 | 30 (15/15) | 0.061 | [212] | |
| No difference | 1.842 | 32 | LaTulippe Prostate | 35 (23/3) | 0.206 | [213] | |
| No difference | 1.057 | 36 | Lapointe Prostate | 112 (60/40) | 0.069 | [214] | |
| No difference | 1.212 | 49 | Arredouani Prostate | 21 (13/8) | 0.172 | [215] | |
| No difference | 1.089 | 51 | Varambally Prostate | 19 (7/6) | 0.376 | [203] | |
| STEAP2 | No difference | 1.471 | 32 | Tomlins Prostate | 53 (30/23) | 0.09 | [211] |
| Overexpressed | 1.099 | 7 | Taylor Prostate 3 | 160 (131/29) | 5.91 × 10−4 | [205] | |
| Overexpressed | 1.256 | 24 | Lapointe Prostate | 103 (62/41) | 0.009 | [214] | |
| No difference | 1.347 | 22 | Luo Prostate 2 | 30 (15/15) | 0.098 | [212] | |
| Overexpressed | 1.368 | 24 | Grasso Prostate | 122 (59/28) | 0.027 | [204] | |
| No difference | 1.116 | 57 | Arredouani Prostate | 21 (13/8) | 0.267 | [215] | |
| No difference | −1.036 | 61 | Varambally Prostate | 13 (7/6) | 0.403 | [203] | |
| STEAP3 | Overexpressed | 1.419 | 2 | Varambally Prostate | 13 (7/6) | 0.001 | [203] |
| No difference | −1.141 | 39 | Tomlins Prostate | 48 (28/20) | 0.198 | [211] | |
| No difference | −1.075 | 20 | Liu Prostate | 57 (44/13) | 0.087 | [210] | |
| No difference | −1.179 | 27 | Luo Prostate 2 | 30 (15/15) | 0.135 | [212] | |
| Underexpressed | −1.378 | 15 | Grasso Prostate | 121 (59/27) | 7.28 × 10−4 | [204] | |
| No difference | −1.316 | 20 | Arredouani Prostate | 21 (13/8) | 0.059 | [215] | |
| Underexpressed | −1.112 | 9 | Taylor Prostate 3 | 160 (131/29) | 2.24 × 10−4 | [205] | |
| STEAP4 | Overexpressed | 2.039 | 7 | Grasso Prostate | 122 (59/28) | 8.96 × 10−5 | [204] |
| Overexpressed | 1.504 | 2 | Taylor Prostate 3 | 160 (131/29) | 1.49 × 10−7 | [205] | |
| Overexpressed | 1.802 | 6 | Lapointe Prostate | 95 (58/37) | 1.59 × 10−6 | [214] | |
| Overexpressed | 1.24 | 7 | Liu Prostate | 57 (44/13) | 0.004 | [210] | |
| No difference | 1.426 | 36 | Tomlins Prostate | 52 (29/23) | 0.127 | [211] | |
| Overexpressed | 1.872 | 11 | Luo Prostate 2 | 30 (15/15) | 0.040 | [212] | |
| No difference | 1.663 | 19 | Varambally Prostate | 13 (7/6) | 0.069 | [203] | |
| Overexpressed | 1.522 | 19 | Arredouani Prostate | 21 (13/8) | 0.024 | [215] | |
| Prostate Adenocarcinoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.221 | 9 | Vanaja Prostate | 40 (27/8) | 9.61 × 10−4 | [216] |
| No difference | −1.128 | 63 | Wallace Prostate | 89 (69/20) | 0.261 | [217] | |
| STEAP2 | Overexpressed | 1.574 | 27 | Vanaja Prostate | 40 (27/8) | 0.032 | [216] |
| STEAP3 | No difference | −1.084 | 52 | Wallace Prostate | 89 (69/20) | 0.111 | [217] |
| Underexpressed | −1.247 | 9 | Vanaja Prostate | 40 (27/8) | 0.015 | [216] | |
| STEAP4 | Overexpressed | 1.717 | 8 | Vanaja Prostate | 40 (27/8) | 7.36 × 10−4 | [216] |
| Overexpressed | 1.564 | 15 | Wallace Prostate | 89 (69/20) | 0.016 | [217] | |
| Prostatic Intraepithelial Neoplasia vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.661 | 12 | Tomlins Prostate | 34 (13/22) | 0.005 | [211] |
| STEAP2 | Overexpressed | 2.275 | 8 | Tomlins Prostate | 36 (13/23) | 0.002 | [211] |
| STEAP3 | No difference | −1.3 | 28 | Tomlins Prostate | 33 (13/20) | 0.095 | [211] |
| STEAP4 | Overexpressed | 2.887 | 12 | Tomlins Prostate | 36 (13/23) | 0.005 | [211] |
| Benign Prostatic Hyperplasia Epithelial vs. Normal | |||||||
| STEAP1 | No difference | 2.020 | 30 | Tomlins Prostate | 26 (4/22) | 0.212 | [211] |
| STEAP2 | Overexpressed | 4.054 | 1 | Tomlins Prostate | 27 (4/23) | 6.02 × 10−6 | [211] |
| STEAP3 | No difference | −1.037 | 40 | Tomlins Prostate | 42 (2/20) | 0.388 | [211] |
| STEAP4 | No difference | 1.017 | 54 | Tomlins Prostate | 27 (4/23) | 0.488 | [211] |
3.18. Sarcoma
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Pleomorphic Liposarcoma vs. Normal | |||||||
| STEAP1 | No difference | 2.209 | 21 | Detwiller Sarcoma | 18 (3/15) | 0.067 | [221] |
| Underexpression | −2.015 | 12 | Barretina Sarcoma | 32 (23/9) | 8.20 × 10−4 | [222] | |
| STEAP3 | No difference | 1.019 | 56 | Barretina Sarcoma | 32 (23/9) | 0.421 | [222] |
| No difference | −1.069 | 52 | Detwiller Sarcoma | 18 (3/15) | 0.427 | [221] | |
| STEAP4 | No difference | 2.227 | 41 | Detwiller Sarcoma | 18 (3/15) | 0.275 | [221] |
| Underexpression | −1.561 | 5 | Barretina Sarcoma | 32 (23/9) | 2.58 × 10−5 | [222] | |
| Fibrosarcoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.108 | 22 | Detwiller Sarcoma | 22 (7/15) | 0.036 | [221] |
| STEAP3 | No difference | −1.488 | 31 | Detwiller Sarcoma | 22 (7/15) | 0.096 | [221] |
| STEAP4 | No difference | −1.73 | 39 | Detwiller Sarcoma | 22 (7/15) | 0.168 | [221] |
| Synovial Sarcoma vs. Normal | |||||||
| STEAP1 | Overexpressed | 2.377 | 24 | Detwiller Sarcoma | 19 (4/15) | 0.031 | [221] |
| STEAP3 | Underexpression | −2.04 | 7 | Detwiller Sarcoma | 19 (4/15) | 0.002 | [221] |
| STEAP4 | No difference | −1.44 | 51 | Detwiller Sarcoma | 19 (4/15) | 0.296 | [221] |
| Dedifferentiated Liposarcoma vs. Normal | |||||||
| STEAP1 | No difference | 1.281 | 34 | Detwiller Sarcoma | 19 (4/15) | 0.206 | [221] |
| Underexpression | −2.595 | 4 | Barretina Sarcoma | 55 (46/9) | 1.56 × 10−6 | [222] | |
| STEAP3 | No difference | 1.025 | 54 | Barretina Sarcoma | 55 (46/9) | 0.377 | [222] |
| No difference | −1.577 | 24 | Detwiller Sarcoma | 19 (4/15) | 0.098 | [221] | |
| STEAP4 | No difference | 1.426 | 45 | Detwiller Sarcoma | 19 (4/15) | 0.351 | [221] |
| Underexpression | −1.525 | 8 | Barretina Sarcoma | 55 (46/9) | 4.38 × 10−5 | [222] | |
| Malignant Fibrous Histiocytoma vs. Normal | |||||||
| STEAP1 | No difference | 1.179 | 49 | Detwiller Sarcoma | 24 (9/15) | 0.354 | [221] |
| STEAP3 | Underexpression | −1.559 | 18 | Detwiller Sarcoma | 24 (9/15) | 0.020 | [221] |
| STEAP4 | No difference | 1.812 | 34 | Detwiller Sarcoma | 24 (9/15) | 0.097 | [221] |
| Leiomyosarcoma vs. Normal | |||||||
| STEAP1 | No difference | 1.116 | 49 | Detwiller Sarcoma | 21 (6/15) | 0.376 | [221] |
| Underexpression | −3.613 | 5 | Barretina Sarcoma | 35 (26/9) | 1.52 × 10−6 | [222] | |
| STEAP3 | Underexpression | −1.911 | 7 | Detwiller Sarcoma | 21 (6/15) | 0.003 | [221] |
| STEAP4 | No difference | 1.164 | 51 | Detwiller Sarcoma | 21 (6/15) | 0.419 | [221] |
| Underexpression | −1.616 | 7 | Barretina Sarcoma | 35 (26/9) | 1.21 × 10−5 | [222] | |
| Myxofibrosarcoma vs. Normal | |||||||
| STEAP1 | Underexpression | −2.438 | 11 | Barretina Sarcoma | 40 (31/9) | 1.21 × 10−4 | [222] |
| STEAP3 | No difference | 1.118 | 48 | Barretina Sarcoma | 40 (31/9) | 0.148 | [222] |
| STEAP4 | Underexpression | −1.529 | 9 | Barretina Sarcoma | 40 (31/9) | 3.93 × 10−5 | [222] |
| Myxoid/Round Cell Liposarcoma vs. Normal | |||||||
| STEAP1 | No difference | −1.003 | 55 | Detwiller Sarcoma | 19 (4/15) | 0.495 | [221] |
| Underexpression | −2.811 | 4 | Barretina Sarcoma | 29 (20/9) | 2.86 × 10−7 | [222] | |
| STEAP3 | No difference | 1.055 | 50 | Barretina Sarcoma | 29 (20/9) | 0.216 | [222] |
| No difference | −1.033 | 51 | Detwiller Sarcoma | 19 (4/15) | 0.430 | [221] | |
| STEAP4 | Underexpression | −2.181 | 19 | Detwiller Sarcoma | 19 (4/15) | 0.05 | [221] |
| Underexpression | −1.579 | 10 | Barretina Sarcoma | 29 (20/9) | 4.37 × 10−5 | [222] | |
3.19. Testicular Cancer
| Gene | Expression Level | Fold-Change | Rank (Top %) | Dataset | #Samples | p-Value | Reference |
|---|---|---|---|---|---|---|---|
| Testicular Seminoma vs. Normal | |||||||
| STEAP1 | No difference | 1.260 | 23 | Skotheim Testis | 6 (3/3) | 0.078 | [225] |
| No difference | 1.067 | 43 | Sperger Others | 41 (22/19) | 0.199 | [227] | |
| STEAP2 | No difference | −1.125 | 53 | Skotheim Testis | 6 (3/3) | 0.684 | [225] |
| Underexpressed | −1.329 | 35 | Sperger Others | 31 (14/17) | 0.042 | [227] | |
| STEAP3 | Underexpressed | −1.612 | 26 | Skotheim Testis | 6 (3/3) | 0.034 | [225] |
| STEAP4 | No difference | −1.200 | 63 | Skotheim Testis | 6 (3/3) | 0.867 | [225] |
| Seminoma, Not Otherwise Specified vs. Normal | |||||||
| STEAP1 | Underexpressed | −1.222 | 37 | Korkola Seminoma | 18 (12/6) | 0.033 | [226] |
| STEAP2 | Overexpressed | 1.111 | 36 | Korkola Seminoma | 18 (12/6) | 0.006 | [226] |
| STEAP3 | Overexpressed | 1.532 | 34 | Korkola Seminoma | 18 (12/6) | 0.0073 | [226] |
| STEAP4 | No difference | 1.008 | 64 | Korkola Seminoma | 18 (12/6) | 0.374 | [226] |
| Testicular Teratoma vs. Normal | |||||||
| STEAP1 | No difference | 1.254 | 24 | Skotheim Testis | 7 (4/3) | 0.133 | [225] |
| STEAP2 | No difference | 1.200 | 26 | Skotheim Testis | 7 (4/3) | 0.157 | [225] |
| STEAP3 | Underexpressed | −2.067 | 10 | Skotheim Testis | 7 (4/3) | 0.012 | [225] |
| STEAP4 | No difference | 1.060 | 44 | Skotheim Testis | 7 (4/3) | 0.45 | [225] |
| Teratoma, Not Otherwise Specified vs. Normal | |||||||
| STEAP1 | No difference | 1.194 | 64 | Korkola Seminoma | 20 (14/6) | 0.851 | [226] |
| STEAP2 | Overexpressed | 2.443 | 4 | Korkola Seminoma | 20 (14/6) | 2.79 × 10−8 | [226] |
| STEAP3 | Overexpressed | 1.751 | 9 | Korkola Seminoma | 20 (14/6) | 2.48 × 10−6 | [226] |
| STEAP4 | Overexpressed | 2.770 | 28 | Korkola Seminoma | 20 (14/6) | 0.002 | [226] |
| Testicular Yolk Sac Tumor vs. Normal | |||||||
| STEAP1 | No difference | 1.302 | 22 | Skotheim Testis | 7 (4/3) | 0.101 | [225] |
| STEAP2 | No difference | −1.051 | 56 | Skotheim Testis | 7 (4/3) | 0.682 | [225] |
| STEAP3 | Underexpressed | −1.935 | 16 | Skotheim Testis | 7 (4/3) | 0.013 | [225] |
| STEAP4 | No difference | −1.043 | 52 | Skotheim Testis | 7 (4/3) | 0.630 | [225] |
| Yolk Sac Tumor, Not Otherwise Specified vs. Normal | |||||||
| STEAP1 | No difference | 1.225 | 61 | Korkola Seminoma | 15 (9/6) | 0.755 | [226] |
| STEAP2 | Overexpressed | 1.283 | 33 | Korkola Seminoma | 15 (9/6) | 0.019 | [226] |
| STEAP3 | Overexpressed | 1.461 | 15 | Korkola Seminoma | 15 (9/6) | 8.90 × 10−4 | [226] |
| STEAP4 | Underexpressed | −1.784 | 32 | Korkola Seminoma | 15 (9/6) | 0.023 | [226] |
| Testicular Embryonal Carcinoma vs. Normal | |||||||
| STEAP1 | No difference | 1.288 | 22 | Skotheim Testis | 8 (5/3) | 0.106 | [225] |
| STEAP2 | No difference | 1.037 | 41 | Skotheim Testis | 8 (5/3) | 0.380 | [225] |
| STEAP3 | Underexpressed | −1.516 | 25 | Skotheim Testis | 8 (5/3) | 0.048 | [225] |
| STEAP4 | No difference | −1.185 | 62 | Skotheim Testis | 8 (5/3) | 0.792 | [225] |
| Embryonal Carcinoma, Not Otherwise Specified vs. Normal | |||||||
| STEAP1 | Underexpressed | −1.282 | 35 | Korkola Seminoma | 21 (15/6) | 0.016 | [226] |
| STEAP2 | Overexpressed | 1.220 | 35 | Korkola Seminoma | 21 (15/6) | 0.005 | [226] |
| STEAP3 | Overexpressed | 1.539 | 16 | Korkola Seminoma | 21 (15/6) | 5.66 × 10−5 | [226] |
| STEAP4 | No difference | 1.062 | 52 | Korkola Seminoma | 21 (15/6) | 0.076 | [226] |
| Testicular Intratubular Germ Cell Neoplasia vs. Normal | |||||||
| STEAP1 | Underexpressed | −1.214 | 9 | Skotheim Testis | 6 (3/3) | 0.045 | [225] |
| STEAP2 | No difference | −1.053 | 53 | Skotheim Testis | 6 (3/3) | 0.636 | [225] |
| STEAP3 | Underexpressed | −1.669 | 7 | Skotheim Testis | 6 (3/3) | 0.032 | [225] |
| STEAP4 | No difference | 1.169 | 21 | Skotheim Testis | 6 (3/3) | 0.190 | [225] |
| Mixed Germ Cell Tumor, Not Otherwise Specified vs. Normal | |||||||
| STEAP1 | No difference | −1.020 | 48 | Korkola Seminoma | 47 (41/6) | 0.408 | [226] |
| STEAP2 | Overexpressed | 1.356 | 15 | Korkola Seminoma | 47 (41/6) | 1.87 × 10−6 | [226] |
| STEAP3 | Overexpressed | 1.484 | 20 | Korkola Seminoma | 47 (41/6) | 2.29 × 10−5 | [226] |
| STEAP4 | Overexpressed | 1.141 | 41 | Korkola Seminoma | 47 (41/6) | 0.003 | [226] |
4. Conclusions
| Cancer Type | STEAP1 | STEAP2 | STEAP3 | STEAP4 | |
|---|---|---|---|---|---|
| Bladder | Infiltrating Bladder Urothelial Carcinoma | ▼ | ▼ | ▲▲▲ | ▼ |
| Superficial Bladder Cancer | ▼ | ▼ | ▲▲▲ | ▼▼ | |
| Brain/CNS | Glioblastoma | ▲▲▲▼ | ▲▼▼ | ▲▲▲▲▲▲ | ▼ |
| Astrocytoma | ▼ | n.s. | ▲▲ | ▲ | |
| Oligodendroglioma | ▼▼ | ▼▼ | ▲ | ▼ | |
| Breast | Invasive Ductal Breast Carcinoma | ▲▼▼▼▼▼ | ▼▼▼▼ | ▲▲▼▼ | ▼▼▼ |
| Lobular Breast Carcinoma | ▼▼ | ▲▼ | ▲▲ | ▲▼▼ | |
| Fibroadenoma | ▼▼ | n.s. | n.s. | n.s. | |
| Cervical | Cervical Squamous Cell Carcinoma | ▲▲ | n.s. | ▲▲▲ | n.s. |
| Colorectal | Carcinoma | ▲ | ▲▼ | ▲▲ | ▼ |
| Rectal Adenocarcinoma | ▲▲ | ▲ | ▲▲ | ▲ | |
| Colon Adenocarcinoma | ▲ | ▲▲ | ▲▲ | ▼ | |
| Esophageal | Barrett’s Esophagus | ▲▲ | ▲▲ | n.s. | ▼ |
| Esophageal Squamous Cell Carcinoma | ▲▲ | ▲ | ▲▲ | ▼▼ | |
| Esophageal Adenocarcinoma | ▲▲ | ▲▲ | ▲ | ▼ | |
| Gastric | Gastric Cancer | ▲▲ | ▲ | n.s. | n.s. |
| Gastric Intestinal Type Adenocarcinoma | ▲▲▲ | ▲▲▲ | n.s. | n.s. | |
| Diffuse Gastric Adenocarcinoma | ▲▲▲ | ▲▲ | n.s. | ▲ | |
| Head and Neck | Oral Cavity Carcinoma | ▲▲▲ | n.s. | ▲▲ | ▼▼ |
| Tongue Carcinoma | ▲▲▲▲ | n.s. | ▲▲ | n.s. | |
| Thyroid Gland Papillary Carcinoma | ▼ | ▼▼ | ▲▲ | ▲ | |
| Kidney | Clear Cell Renal Cell Carcinoma | ▼▲ | ▼ | ▲▲ | ▼▲▲ |
| Papillary Renal Cell Carcinoma | ▲ | n.s. | ▲ | ▼ | |
| Chromophobe Renal Cell Carcinoma | ▼ | n.s. | n.s. | ▲ | |
| Renal Wilms Tumor | n.s. | ▼ | ▲ | n.s. | |
| Renal Oncocytoma | ▼ | n.s. | n.s. | ▲ | |
| Leukemia | T-Cell Acute Lymphoblastic Leukemia | ▲ | ▲▼ | ▼ | ▲▼▼ |
| B-Cell Acute Lymphoblastic Leukemia | ▲ | ▲▼ | ▼ | ▲▼▼ | |
| Acute Myeloid Leukemia | ▲▼▼ | ▲▼ | ▼ | ▼▼ | |
| Chronic Lymphocytic Leukemia | ▼▼ | ▲ | ▼ | ▼ | |
| Liver | Hepatocellular Carcinoma | ▲▲▼ | ▲ | ▼▼▼▼ | ▼▼▼▼▼ |
| Lung | Squamous Cell Lung Carcinoma | ▲▲▲▲▲ | ▲ | ▲▲ | ▼▼▼ |
| Lung Adenocarcinoma | ▲▲▲▲▲▲ | ▲ | ▲▲▲▲▲▲ | ▼▼▼▼ | |
| Lymphoma | Follicular Lymphoma | ▲ | ▲ | ▲▲ | ▲▲ |
| Diffuse Large B-Cell Lymphoma | ▲▲▲ | ▲▲▲ | ▲▲ | ▲▲ | |
| Burkitt’s Lymphoma | ▲ | n.s. | ▲ | n.s. | |
| Hodgkin’s Lymphoma | ▲ | ▲ | ▲▲ | ▲▲ | |
| Melanoma | Melanoma | ▲▲ | ▼ | ▼ | ▲▼▼ |
| Ovarian | Ovarian Serous Adenocarcinoma | ▲ | ▲ | ▲▲▲ | ▼ |
| Ovarian Endometrioid Adenocarcinoma | ▲ | n.s. | ▲▲ | n.s. | |
| Ovarian Clear Cell Adenocarcinoma | ▲ | ▼ | ▲ | n.s. | |
| Ovarian Mucinous Adenocarcinoma | ▲▲ | ▲ | ▲▲ | ▲ | |
| Ovarian Carcinoma | n.s. | - | n.s. | ▲ | |
| Pancreatic | Pancreatic Ductal Adenocarcinoma | ▲▲▲ | ▲▲ | ▲▲ | ▲▼ |
| Pancreatic Carcinoma | ▲▲▼ | ▲ | ▲ | ▼ | |
| Prostate | Prostate Carcinoma | ▲▲▲▲▲▲▲ | ▲▲▲ | ▲ | ▲▲▲▲▲▲ |
| Prostate Adenocarcinoma | ▲ | ▲ | ▼ | ▲▲ | |
| Prostatic Intraepithelial Neoplasia | ▲ | ▲ | n.s. | ▲ | |
| Benign Prostatic Hyperplasia Epithelial | n.s. | ▲ | n.s. | n.s. | |
| Sarcoma | Pleomorphic Liposarcoma | ▼ | - | n.s. | ▼ |
| Fibrosarcoma | n.s. | - | n.s. | n.s. | |
| Synovial Sarcoma | n.s. | - | ▼ | n.s. | |
| Dedifferentiated Liposarcoma | ▼ | - | n.s. | ▼ | |
| Malignant Fibrous Histiocytoma | n.s. | - | ▼ | n.s. | |
| Leiomyosarcoma | ▼ | - | ▼ | ▼ | |
| Myxofibrosarcoma | ▼ | - | n.s. | ▼ | |
| Myxoid/Round Cell Liposarcoma | ▼ | - | n.s. | ▼▼ | |
| Testicular | Testicular Seminoma | n.s. | ▼ | ▼ | n.s. |
| Seminoma, Not Otherwise Specified | ▼ | ▲ | ▲ | n.s. | |
| Testicular Teratoma | n.s. | n.s. | ▼ | n.s. | |
| Teratoma, Not Otherwise Specified | n.s. | ▲ | ▲ | ▲ | |
| Testicular Yolk Sac Tumor | n.s. | n.s. | ▼ | n.s. | |
| Yolk Sac Tumor, Not Otherwise Specified | n.s. | ▲ | ▲ | ▼ | |
| Testicular Embryonal Carcinoma | n.s. | n.s. | ▼ | n.s. | |
| Embryonal Carcinoma, Not Otherwise Specified | ▼ | ▲ | ▲ | n.s. | |
| Testicular Intratubular Germ Cell Neoplasia | ▼ | n.s. | ▼ | n.s. | |
| Mixed Germ Cell Tumor, Not Otherwise Specified | n.s. | ▲ | ▲ | ▲ | |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hubert, R.S.; Vivanco, I.; Chen, E.; Rastegar, S.; Leong, K.; Mitchell, S.C.; Madraswala, R.; Zhou, Y.; Kuo, J.; Raitano, A.B.; et al. STEAP: A prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc. Natl. Acad. Sci. USA 1999, 96, 14523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.-J.; Wu, H.-T.; Li, C.-L.; Lin, Y.-K.; Fang, Z.-X.; Lin, W.-T.; Liu, J. Regulatory Roles of Six-Transmembrane Epithelial Antigen of the Prostate Family Members in the Occurrence and Development of Malignant Tumors. Front. Cell Dev. Biol. 2021, 9, 2988. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.M.; Maia, C.J.; Santos, C.R. STEAP proteins: From structure to applications in cancer therapy. Mol. Cancer Res. 2012, 10, 573–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porkka, K.P.; Helenius, M.A.; Visakorpi, T. Cloning and characterization of a novel six-transmembrane protein STEAP2, expressed in normal and malignant prostate. Lab. Investig. 2002, 82, 1573–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korkmaz, K.S.; Elbi, C.; Korkmaz, C.G.; Loda, M.; Hager, G.L.; Saatcioglu, F. Molecular cloning and characterization of STAMP1, a highly prostate-specific six transmembrane protein that is overexpressed in prostate cancer. J. Biol. Chem. 2002, 277, 36689–36696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porkka, K.P.; Nupponen, N.N.; Tammela, T.L.J.; Vessella, R.L.; Visakorpi, T. Human pHyde is not a classical tumor suppressor gene in prostate cancer. Int. J. Cancer 2003, 106, 729–735. [Google Scholar] [CrossRef]
- Lambe, T.; Simpson, R.J.; Dawson, S.; Bouriez-Jones, T.; Crockford, T.L.; Lepherd, M.; Latunde-Dada, G.O.; Robinson, H.; Raja, K.B.; Campagna, D.R.; et al. Identification of a Steap3 endosomal targeting motif essential for normal iron metabolism. Blood 2009, 113, 1805–1808. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, C.G.; Korkmaz, K.S.; Kurys, P.; Elbi, C.; Wang, L.; Klokk, T.I.; Hammarstrom, C.; Troen, G.; Svindland, A.; Hager, G.L.; et al. Molecular cloning and characterization of STAMP2, an androgen-regulated six transmembrane protein that is overexpressed in prostate cancer. Oncogene 2005, 24, 4934–4945. [Google Scholar] [CrossRef] [Green Version]
- Ohgami, R.S.; Campagna, D.R.; McDonald, A.; Fleming, M.D. The Steap proteins are metalloreductases. Blood 2006, 108, 1388–1394. [Google Scholar] [CrossRef]
- Oosterheert, W.; Gros, P. Cryo-electron microscopy structure and potential enzymatic function of human six-transmembrane epithelial antigen of the prostate 1 (STEAP1). J. Biol. Chem. 2020, 295, 9502–9512. [Google Scholar] [CrossRef]
- Oosterheert, W.; Reis, J.; Gros, P.; Mattevi, A. An elegant four-helical fold in NOX and STEAP enzymes facilitates electron transport across biomembranes—Similar vehicle, different destination. Acc. Chem. Res. 2020, 53, 1969–1980. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Nakamura, H.; Takada, K.; Hayasaka, N.; Kubo, T.; Umeyama, Y.; Iyama, S.; Miyanishi, K.; Kobune, M.; Kato, J. Six-transmembrane epithelial antigen of the prostate 1 accelerates cell proliferation by targeting c-Myc in liver cancer cells. Oncol. Lett. 2021, 22, 546. [Google Scholar] [CrossRef] [PubMed]
- Whiteland, H.; Spencer-Harty, S.; Morgan, C.; Kynaston, H.; Thomas, D.H.; Bose, P.; Fenn, N.; Lewis, P.; Jenkins, S.; Doak, S.H. A role for STEAP2 in prostate cancer progression. Clin. Exp. Metastasis 2014, 31, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Luo, J.; He, Z.H.; Liu, Y.Q.; Li, H.G.; Xie, D.; Cai, M.Y. STEAP3 promotes cancer cell proliferation by facilitating nuclear trafficking of EGFR to enhance RAC1-ERK-STAT3 signaling in hepatocellular carcinoma. Cell Death Dis. 2021, 12, 1052. [Google Scholar] [CrossRef]
- Li, W.; Yin, X.; Yan, Y.; Liu, C.; Li, G. STEAP4 knockdown inhibits the proliferation of prostate cancer cells by activating the cGMP-PKG pathway under lipopolysaccharide-induced inflammatory microenvironment. Int. Immunopharmacol. 2021, 101, 108311. [Google Scholar] [CrossRef]
- Gomes, I.M.; Rocha, S.M.; Gaspar, C.; Alvelos, M.I.; Santos, C.R.; Socorro, S.; Maia, C.J. Knockdown of STEAP1 inhibits cell growth and induces apoptosis in LNCaP prostate cancer cells counteracting the effect of androgens. Med. Oncol. 2018, 35, 40. [Google Scholar] [CrossRef]
- Sanchez-Pulido, L.; Rojas, A.M.; Valencia, A.; Martinez-A, C.; Andrade, M.A. ACRATA: A novel electron transfer domain associated to apoptosis and cancer. BMC Cancer 2004, 4, 98. [Google Scholar] [CrossRef] [Green Version]
- Qin, D.N.; Kou, C.Z.; Ni, Y.H.; Zhang, C.M.; Zhu, J.G.; Zhu, C.; Wang, Y.P.; Zhu, G.Z.; Shi, C.; Ji, C.B.; et al. Monoclonal antibody to the six-transmembrane epithelial antigen of prostate 4 promotes apoptosis and inhibits proliferation and glucose uptake in human adipocytes. Int. J. Mol. Med. 2010, 26, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Ye, C.L.; Du, Y.; Yu, X.; Chen, Z.Y.; Wang, L.; Zheng, Y.F.; Liu, X.H. STEAP3 Affects Ferroptosis and Progression of Renal Cell Carcinoma Through the p53/xCT Pathway. Technol. Cancer Res. Treat. 2022, 21, 15330338221078728. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K.; Arihara, Y.; Hayasaka, N.; Murase, K.; Iyama, S.; Kobune, M.; Miyanishi, K.; Kato, J. Six-transmembrane epithelial antigen of the prostate 1 protects against increased oxidative stress via a nuclear erythroid 2-related factor pathway in colorectal cancer. Cancer Gene Ther. 2019, 26, 313–322. [Google Scholar] [CrossRef]
- Grunewald, T.G.P.; Diebold, I.; Esposito, I.; Plehm, S.; Hauer, K.; Thiel, U.; Da Silva-Buttkus, P.; Neff, F.; Unland, R.; Müller-Tidow, C.; et al. STEAP1 is associated with the invasive and oxidative stress phenotype of Ewing tumors. Mol. Cancer Res. 2012, 10, 52–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Ye, S.; Fujiwara, T.; Manolagas, S.C.; Zhao, H. Steap4 plays a critical role in osteoclastogenesis in vitro by regulating cellular iron/reactive oxygen species (ROS) levels and cAMP response element-binding protein (CREB) activation. J. Biol. Chem. 2013, 288, 30064–30074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Xing, X.; Beamer, M.A.; Swindell, W.R.; Sarkar, M.K.; Roberts, L.W.; Voorhees, J.J.; Kahlenberg, J.M.; Harms, P.W.; Johnston, A.; et al. Six-transmembrane epithelial antigens of the prostate comprise a novel inflammatory nexus in patients with pustular skin disorders. J. Allergy Clin. Immunol. 2017, 139, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tao, Y.; Zhang, Z.; Guo, X.; An, P.; Shen, Y.; Wu, Q.; Yu, Y.; Wang, F. Metalloreductase steap3 coordinates the regulation of iron homeostasis and inflammatory responses. Haematologica 2012, 97, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrøm, N.; Jin, Y.; Nenseth, Z.; Kuzu, O.F.; Saatcioglu, F. STAMP2 Expression Mediated by Cytokines Attenuates Their Growth-Limiting Effects in Prostate Cancer Cells. Cancers 2021, 13, 1579. [Google Scholar] [CrossRef]
- Moreaux, J.; Kassambara, A.; Hose, D.; Klein, B. STEAP1 is overexpressed in cancers: A promising therapeutic target. Biochem. Biophys. Res. Commun. 2012, 429, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Gomes, I.M.; Santos, C.R.; Socorro, S.; Maia, C.J. Six transmembrane epithelial antigen of the prostate 1 is down-regulated by sex hormones in prostate cells. Prostate 2013, 73, 605–613. [Google Scholar] [CrossRef]
- Ihlaseh-Catalano, S.M.; Drigo, S.A.; de Jesus, C.M.N.; Domingues, M.A.C.; Trindade Filho, J.C.S.; de Camargo, J.L.V.; Rogatto, S.R. STEAP1 protein overexpression is an independent marker for biochemical recurrence in prostate carcinoma. Histopathology 2013, 63, 678–685. [Google Scholar] [CrossRef]
- Lee, C.H.; Chen, S.L.; Sung, W.W.; Lai, H.W.; Hsieh, M.J.; Yen, H.H.; Su, T.C.; Chiou, Y.H.; Chen, C.Y.; Lin, C.Y.; et al. The Prognostic Role of STEAP1 Expression Determined via Immunohistochemistry Staining in Predicting Prognosis of Primary Colorectal Cancer: A Survival Analysis. Int. J. Mol. Sci. 2016, 17, 592. [Google Scholar] [CrossRef] [Green Version]
- Rocha, S.M.; Barroca-Ferreira, J.; Passarinha, L.A.; Socorro, S.; Maia, C.J. The Usefulness of STEAP Proteins in Prostate Cancer Clinical Practice. Prostate Cancer 2021, 10, 139–154. [Google Scholar] [CrossRef]
- Burnell, S.E.A.; Spencer-Harty, S.; Howarth, S.; Bodger, O.; Kynaston, H.; Morgan, C.; Doak, S.H. STEAP2 Knockdown Reduces the Invasive Potential of Prostate Cancer Cells. Sci. Rep. 2018, 8, 6252. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ji, G.; Li, J. STEAP2 is down-regulated in breast cancer tissue and suppresses PI3K/AKT signaling and breast cancer cell invasion in vitro and in vivo. Cancer Biol. Ther. 2020, 21, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Isobe, T.; Baba, E.; Arita, S.; Komoda, M.; Tamura, S.; Shirakawa, T.; Ariyama, H.; Takaishi, S.; Kusaba, H.; Ueki, T.; et al. Human STEAP3 maintains tumor growth under hypoferric condition. Exp. Cell Res. 2011, 317, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Xu, R.; Wang, S.; Yang, N.; Ni, S.; Zhang, Q.; Xu, Y.; Zhang, X.; Zhang, C.; Wei, Y.; et al. Six-Transmembrane Epithelial Antigen of Prostate 3 Predicts Poor Prognosis and Promotes Glioblastoma Growth and Invasion. Neoplasia 2018, 20, 543–554. [Google Scholar] [CrossRef]
- Passer, B.J.; Nancy-Portebois, V.; Amzallag, N.; Prieur, S.; Cans, C.; De Climens, A.R.; Fiucci, G.; Bouvard, V.; Tuynder, M.; Susini, L.; et al. The p53-inducible TSAP6 gene product regulates apoptosis and the cell cycle and interacts with Nix and the Myt1 kinase. Proc. Natl. Acad. Sci. USA 2003, 100, 2284–2289. [Google Scholar] [CrossRef] [Green Version]
- Amson, R.B.; Nemani, M.; Roperch, J.P.; Israeli, D.; Bougueleret, L.; Le Gall, I.; Medhioub, M.; Linares-Cruz, G.; Lethrosne, F.; Pasturaud, P.; et al. Isolation of 10 differentially expressed cDNAs in p53-induced apoptosis: Activation of the vertebrate homologue of the Drosophila seven in absentia gene. Proc. Natl. Acad. Sci. USA 1996, 93, 3953–3957. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Wang, L.; Qu, S.; Sheng, X.; Kristian, A.; Mælandsmo, G.M.; Pällmann, N.; Yuca, E.; Tekedereli, I.; Gorgulu, K.; et al. STAMP 2 increases oxidative stress and is critical for prostate cancer. EMBO Mol. Med. 2015, 7, 315–331. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Bredell, B.X.; Anderson, E.R.; Martin, A.; Mays, C.; Nagao-Kitamoto, H.; Huang, S.; Győrffy, B.; Greenson, J.K.; Hardiman, K.; et al. Quantitative proteomics identifies STEAP4 as a critical regulator of mitochondrial dysfunction linking inflammation and colon cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9608–E9617. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Zhao, J.; Bulek, K.; Tang, F.; Chen, X.; Cai, G.; Jia, S.; Fox, P.L.; Huang, E.; Pizarro, T.T.; et al. Inflammation mobilizes copper metabolism to promote colon tumorigenesis via an IL-17-STEAP4-XIAP axis. Nat. Commun. 2020, 11, 900. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpande, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pandey, A.; Chinnaiyan, A.M. ONCOMINE: A Cancer Microarray Database and Integrated Data-Mining Platform. Neoplasia 2004, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamie, K.; Litwin, M.S.; Bassett, J.C.; Daskivich, T.J.; Lai, J.; Hanley, J.M.; Konety, B.R.; Saigal, C.S.; Project, T.U.D.A. Recurrence of high-risk bladder cancer: A population-based analysis. Cancer 2013, 119, 3219–3227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaseb, H.; Aeddula, N.R. Bladder Cancer. Essence Anesth. Pract. 2021, 4, 62. [Google Scholar]
- Azumi, M.; Kobayashi, H.; Aoki, N.; Sato, K.; Kimura, S.; Kakizaki, H.; Tateno, M. Six-Transmembrane Epithelial Antigen of the Prostate as an Immunotherapeutic Target for Renal Cell and Bladder Cancer. J. Urol. 2010, 183, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Challita-Eid, P.M.; Morrison, K.; Etessami, S.; An, Z.; Morrison, K.J.; Perez-Villar, J.J.; Raitano, A.B.; Jia, X.C.; Gudas, J.M.; Kanner, S.B.; et al. Monoclonal Antibodies to Six-Transmembrane Epithelial Antigen of the Prostate-1 Inhibit Intercellular Communication In vitro and Growth of Human Tumor Xenografts In vivo. Cancer Res. 2007, 67, 5798–5805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Ho, J.N.; Jin, H.; Lee, S.C.; Lee, S.E.; Hong, S.K.; Lee, J.W.; Lee, E.S.; Byun, S.S. Upregulated expression of BCL2, MCM7, and ccne1 indicate cisplatin-resistance in the set of two human bladder cancer cell lines: T24 cisplatin sensitive and T24R2 cisplatin resistant bladder cancer cell lines. Korean J. Urol. 2016, 57, 63–72. [Google Scholar] [CrossRef]
- Yap, K.L.; Kiyotani, K.; Tamura, K.; Antic, T.; Jang, M.; Montoya, M.; Campanile, A.; Yew, P.Y.; Ganshert, C.; Fujioka, T.; et al. Whole-exome sequencing of muscle-invasive bladder cancer identifies recurrent mutations of UNC5C and prognostic importance of DNA repair gene mutations on survival. Clin. Cancer Res. 2014, 20, 6605–6617. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Carbayo, M.; Socci, N.D.; Lozano, J.; Saint, F.; Cordon-Cardo, C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J. Clin. Oncol. 2006, 24, 778–789. [Google Scholar] [CrossRef]
- Dyrskjøt, L.; Kruhøffer, M.; Thykjaer, T.; Marcussen, N.; Jensen, J.L.; Møller, K.; Ørntoft, T.F. Gene expression in the urinary bladder: A common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004, 64, 4040–4048. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Leem, S.H.; Lee, S.Y.; Kim, S.C.; Park, E.S.; Kim, S.B.; Kim, S.K.; Kim, Y.J.; Kim, W.J.; Chu, I.S. Expression signature of E2F1 and its associated genes predict superficial to invasive progression of bladder tumors. J. Clin. Oncol. 2010, 28, 2660–2667. [Google Scholar] [CrossRef]
- Nabors, L.B.; Ammirati, M.; Bierman, P.J.; Brem, H.; Butowski, N.; Chamberlain, M.C.; DeAngelis, L.M.; Fenstermaker, R.A.; Friedman, A.; Gilbert, M.R.; et al. Central Nervous System Cancers: Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2013, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Adamson, D.C.; Rasheed, B.A.K.; McLendon, R.E.; Bigner, D.D. Central nervous system. Cancer Biomark. 2011, 9, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, C.; Yu, Q.; Zhong, C.; Peng, Y.; Chen, J.; Chen, G. Comprehensive landscape of STEAP family functions and prognostic prediction value in glioblastoma. J. Cell. Physiol. 2021, 236, 2988–3000. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Z.; Song, Z.; Wu, Y.; Jin, Q.; Zhao, Z. Predictive potential of STEAP family for survival, immune microenvironment and therapy response in glioma. Int. Immunopharmacol. 2021, 101, 108183. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Tian, Y.; Li, X. Large-Scale Analysis Reveals Gene Signature for Survival Prediction in Primary Glioblastoma. Mol. Neurobiol. 2020, 57, 5235–5246. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Nobusawa, S.; Watanabe, T.; Kleihues, P.; Ohgaki, H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin. Cancer Res. 2009, 15, 6002–6007. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, C.; Hentschel, B.; Wick, W.; Capper, D.; Felsberg, J.; Simon, M.; Westphal, M.; Schackert, G.; Meyermann, R.; Pietsch, T.; et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: Implications for classification of gliomas. Acta Neuropathol. 2010, 120, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Pappula, A.L.; Rasheed, S.; Mirzaei, G.; Petreaca, R.C.; Bouley, R.A. A Genome-Wide Profiling of Glioma Patients with an IDH1 Mutation Using the Catalogue of Somatic Mutations in Cancer Database. Cancers 2021, 13, 4299. [Google Scholar] [CrossRef]
- Lee, J.; Kotliarova, S.; Kotliarov, Y.; Li, A.; Su, Q.; Donin, N.M.; Pastorino, S.; Purow, B.W.; Christopher, N.; Zhang, W.; et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006, 9, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Diehn, M.; Watson, N.; Bollen, A.W.; Aldape, K.D.; Nicholas, M.K.; Lamborn, K.R.; Berger, M.S.; Botstein, D.; Brown, P.O.; et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc. Natl. Acad. Sci. USA 2005, 102, 5814–5819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murat, A.; Migliavacca, E.; Gorlia, T.; Lambiv, W.L.; Shay, T.; Hamou, M.F.; De Tribolet, N.; Regli, L.; Wick, W.; Kouwenhoven, M.C.M.; et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J. Clin. Oncol. 2008, 26, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Program—National Cancer Institute. Available online: https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga (accessed on 3 March 2020).
- Shai, R.; Shi, T.; Kremen, T.J.; Horvath, S.; Liau, L.M.; Cloughesy, T.F.; Mischel, P.S.; Nelson, S.F. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene 2003, 22, 4918–4923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Hui, A.M.; Su, Q.; Vortmeyer, A.; Kotliarov, Y.; Pastorino, S.; Passaniti, A.; Menon, J.; Walling, J.; Bailey, R.; et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 2006, 9, 287–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bredel, M.; Bredel, C.; Juric, D.; Harsh, G.R.; Vogel, H.; Recht, L.D.; Sikic, B.I. Functional network analysis reveals extended gliomagenesis pathway maps and three novel MYC-interacting genes in human gliomas. Cancer Res. 2005, 65, 8679–8689. [Google Scholar] [CrossRef] [Green Version]
- French, P.J.; Swagemakers, S.M.A.; Nagel, J.H.A.; Kouwenhoven, M.C.M.; Brouwer, E.; Van Der Spek, P.; Luider, T.M.; Kros, J.M.; Van Den Bent, M.J.; Smitt, P.A.S. Gene expression profiles associated with treatment response in oligodendrogliomas. Cancer Res. 2005, 65, 11335–11344. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.N.; Dave, R.; Sanadya, J.; Sharma, P.; Sharma, K.K. Various types and management of breast cancer: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 109–126. [Google Scholar]
- Maia, C.J.B.; Socorro, S.; Schmitt, F.; Santos, C.R.A. STEAP1 is over-expressed in breast cancer and down-regulated by 17beta-estradiol in MCF-7 cells and in the rat mammary gland. Endocrine 2008, 34, 108–116. [Google Scholar] [CrossRef]
- Mudalagiriyappa, C.; Pillai, S.; Watson, M.; Aft, R. Abstract 5258: Expression analysis of STEAP1 in breast cancer patients as therapeutic target. Cancer Res. 2015, 75, 5258. [Google Scholar]
- Xie, J.; Yang, Y.; Sun, J.; Jiao, Z.; Zhang, H.; Chen, J. STEAP1 Inhibits Breast Cancer Metastasis and Is Associated with Epithelial-Mesenchymal Transition Procession. Clin. Breast Cancer 2019, 19, e195–e207. [Google Scholar] [CrossRef] [Green Version]
- Orfanou, I.M.; Argyros, O.; Papapetropoulos, A.; Tseleni-Balafouta, S.; Vougas, K.; Tamvakopoulos, C. Discovery and Pharmacological Evaluation of STEAP4 as a Novel Target for HER2 Overexpressing Breast Cancer. Front. Oncol. 2021, 11, 908. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, G.; Gatza, M.L.; Beck, A.H.; Wilkerson, M.D.; Rhie, S.K.; Pastore, A.; Zhang, H.; McLellan, M.; Yau, C.; Kandoth, C.; et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 2015, 163, 506–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.T.; Chen, W.J.; Xu, Y.; Shen, J.X.; Chen, W.T.; Liu, J. The Tumor Suppressive Roles and Prognostic Values of STEAP Family Members in Breast Cancer. Biomed Res. Int. 2020, 2020, 9578484. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.J.; Dahiya, S.; Richardson, E.; Erlander, M.; Sgroi, D.C. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009, 11, R7. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Langerød, A.; Ji, Y.; Nowels, K.W.; Nesland, J.M.; Tibshirani, R.; Bukholm, I.K.; Kåresen, R.; Botstein, D.; Børresen-Dale, A.L.; et al. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol. Biol. Cell 2004, 15, 2523–2536. [Google Scholar] [CrossRef] [Green Version]
- Sørlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef] [Green Version]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [Green Version]
- Perou, C.M.; Sørile, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Ress, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Radvanyi, L.; Singh-Sandhu, D.; Gallichan, S.; Lovitt, C.; Pedyczak, A.; Mallo, G.; Gish, K.; Kwok, K.; Hanna, W.; Zubovits, J.; et al. The gene associated with trichorhinophalangeal syndrome in humans is overexpressed in breast cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 11005–11010. [Google Scholar] [CrossRef] [Green Version]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Turashvili, G.; Bouchal, J.; Baumforth, K.; Wei, W.; Dziechciarkova, M.; Ehrmann, J.; Klein, J.; Fridman, E.; Skarda, J.; Srovnal, J.; et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer 2007, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, A.L.; Wang, Z.C.; De Nicolo, A.; Lu, X.; Brown, M.; Miron, A.; Liao, X.; Iglehart, J.D.; Livingston, D.M.; Ganesan, S. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell 2006, 9, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Xu, H.; Zhang, L.; Qiao, Y. Cervical cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2020, 32, 720. [Google Scholar] [CrossRef] [PubMed]
- Kessler, T.A. Cervical Cancer: Prevention and Early Detection. Semin. Oncol. Nurs. 2017, 33, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e6. [Google Scholar] [CrossRef] [Green Version]
- Biewenga, P.; Buist, M.R.; Moerland, P.D.; van Themaat, E.V.L.; van Kampen, A.H.C.; Kate, F.J.W.; Baas, F. Gene expression in early stage cervical cancer. Gynecol. Oncol. 2008, 108, 520–526. [Google Scholar] [CrossRef]
- Zhai, Y.; Kuick, R.; Nan, B.; Ota, I.; Weiss, S.J.; Trimble, C.L.; Fearon, E.R.; Cho, K.R. Gene expression analysis of preinvasive and invasive cervical squamous cell carcinomas identifies HOXC10 as a key mediator of invasion. Cancer Res. 2007, 67, 10163–10172. [Google Scholar] [CrossRef] [Green Version]
- Scotto, L.; Narayan, G.; Nandula, S.V.; Arias-Pulido, H.; Subramaniyam, S.; Schneider, A.; Kaufmann, A.M.; Wright, J.D.; Pothuri, B.; Mansukhani, M.; et al. Identification of copy number gain and overexpressed genes on chromosome arm 20q by an integrative genomic approach in cervical cancer: Potential role in progression. Genes Chromosom. Cancer 2008, 47, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Pyeon, D.; Newton, M.A.; Lambert, P.F.; Den Boon, J.A.; Sengupta, S.; Marsit, C.J.; Woodworth, C.D.; Connor, J.P.; Haugen, T.H.; Smith, E.M.; et al. Fundamental differences in cell cycle deregulation in human papillomavirus-positive and human papillomavirus-negative head/neck and cervical cancers. Cancer Res. 2007, 67, 4605–4619. [Google Scholar] [CrossRef] [Green Version]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Bhatlekar, S.; Addya, S.; Salunek, M.; Orr, C.R.; Surrey, S.; McKenzie, S.; Fields, J.Z.; Boman, B.M. Identification of a developmental gene expression signature, including hox genes, for the normal human colonic crypt stem cell niche: Overexpression of the signature parallels stem cell overpopulation during colon tumorigenesis. Stem Cells Dev. 2014, 23, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barresi, V.; Trovato-Salinaro, A.; Spampinato, G.; Musso, N.; Castorina, S.; Rizzarelli, E.; Condorelli, D.F. Transcriptome analysis of copper homeostasis genes reveals coordinated upregulation of SLC31A1, SCO1, and COX11 in colorectal cancer. FEBS Open Bio 2016, 6, 794–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, T.T.; Selaru, F.M.; Xu, Y.; Shustova, V.; Yin, J.; Mori, Y.; Shibata, D.; Sato, F.; Wang, S.; Olaru, A.; et al. Application of cDNA microarrays to generate a molecular taxonomy capable of distinguishing between colon cancer and normal colon. Oncogene 2002, 21, 4855–4862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrzypczak, M.; Goryca, K.; Rubel, T.; Paziewska, A.; Mikula, M.; Jarosz, D.; Pachlewski, J.; Oledzki, J.; Ostrowsk, J. Modeling oncogenic signaling in colon tumors by multidirectional analyses of microarray data directed for maximization of analytical reliability. PLoS ONE 2010, 5, e13091. [Google Scholar] [CrossRef]
- Hong, Y.; Downey, T.; Eu, K.W.; Koh, P.K.; Cheah, P.Y. A “metastasis-prone” signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin. Exp. Metastasis 2010, 27, 83–90. [Google Scholar] [CrossRef]
- Gaedcke, J.; Grade, M.; Jung, K.; Camps, J.; Jo, P.; Emons, G.; Gehoff, A.; Sax, U.; Schirmer, M.; Becker, H.; et al. Mutated KRAS results in overexpression of DUSP4, a MAP-kinase phosphatase, and SMYD3, a histone methyltransferase, in rectal carcinomas. Genes Chromosom. Cancer 2010, 49, 1024–1034. [Google Scholar] [CrossRef] [Green Version]
- Sabates-Bellver, J.; Van Der Flier, L.G.; De Palo, M.; Cattaneo, E.; Maake, C.; Rehrauer, H.; Laczko, E.; Kurowski, M.A.; Bujnicki, J.M.; Menigatti, M.; et al. Transcriptome profile of human colorectal adenomas. Mol. Cancer Res. 2007, 5, 1263–1275. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, S.; Park, Y.K.; Franklin, J.L.; Halberg, R.B.; Yu, M.; Jessen, W.J.; Freudenberg, J.; Chen, X.; Haigis, K.; Jegga, A.G.; et al. Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol. 2007, 8, R131. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.K.; Jeung, H.C.; Chan, H.P.; Seung, H.K.; Gui, Y.L.; Won, S.L.; Nam, K.K.; Hyun, C.C.; Sun, Y.R. Whole genome analysis for liver metastasis gene signatures in colorectal cancer. Int. J. Cancer 2007, 121, 2005–2012. [Google Scholar] [CrossRef]
- Arnal, M.J.D.; Arenas, Á.F.; Arbeloa, Á.L. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. 2015, 21, 7933. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Jin, G.F.; Shen, H.B. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin. J. Cancer 2012, 31, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Triadafilopoulos, G.; Sahbaie, P.; Young, H.S.; Omary, M.B.; Lowe, A.W. Gene Expression Profiling Reveals Stromal Genes Expressed in Common Between Barrett’s Esophagus and Adenocarcinoma. Gastroenterology 2006, 131, 925–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimchi, E.T.; Posner, M.C.; Park, J.O.; Darga, T.E.; Kocherginsky, M.; Karrison, T.; Hart, J.; Smith, K.D.; Mezhir, J.J.; Weichselbaum, R.R.; et al. Progression of Barrett’s metaplasia to adenocarcinoma is associated with the suppression of the transcriptional programs of epidermal differentiation. Cancer Res. 2005, 65, 3146–3154. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Park, Y.Y.; Park, E.S.; Cho, J.Y.; Izzo, J.G.; Zhang, D.; Kim, S.B.; Lee, J.H.; Bhutani, M.S.; Swisher, S.G.; et al. Prognostic biomarkers for esophageal adenocarcinoma identified by analysis of tumor transcriptome. PLoS ONE 2010, 5, e15074. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Hu, N.; Yang, H.H.; Wang, C.; Takikita, M.; Wang, Q.H.; Giffen, C.; Clifford, R.; Hewitt, S.M.; Shou, J.Z.; et al. Global gene expression profiling and validation in esophageal squamous cell carcinoma and its association with clinical phenotypes. Clin. Cancer Res. 2011, 17, 2955–2966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, N.; Clifford, R.J.; Yang, H.H.; Wang, C.; Goldstein, A.M.; Ding, T.; Taylor, P.R.; Lee, M.P. Genome wide analysis of DNA copy number neutral loss of heterozygosity (CNNLOH) and its relation to gene expression in esophageal squamous cell carcinoma. BMC Genom. 2010, 11, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrumurthy, S.G.; Chaudry, M.A.; Hochhauser, D.; Mughal, M. The diagnosis and management of gastric cancer. BMJ 2013, 347, f6367. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.Y.; Jiang, J.N.; Fang, X.D.; Ji, F.J. STEAP1 Regulates Tumorigenesis and Chemoresistance During Peritoneal Metastasis of Gastric Cancer. Front. Physiol. 2018, 9, 1132. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Hou, W.; Zhang, C.; Tan, Y.; Zhang, D.; An, W.; Pan, S.; Wu, W.; Chen, Q.; Xu, H. A research of STEAP1 regulated gastric cancer cell proliferation, migration and invasion in vitro and in vivos. J. Cell. Mol. Med. 2020, 24, 14217–14230. [Google Scholar] [CrossRef]
- Cui, J.; Chen, Y.; Chou, W.C.; Sun, L.; Chen, L.; Suo, J.; Ni, Z.; Zhang, M.; Kong, X.; Hoffman, L.L.; et al. An integrated transcriptomic and computational analysis for biomarker identification in gastric cancer. Nucleic Acids Res. 2011, 39, 1197–1207. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, Y.G.; Li, D.P.; Xia, J.; Zhou, C.Z.; Yan, D.W.; Tang, H.M.; Peng, Z.H. Upregulated INHBA expression is associated with poor survival in gastric cancer. Med. Oncol. 2012, 29, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Lim, J.Y.; Cheong, J.H.; Park, Y.Y.; Yoon, S.L.; Kim, S.M.; Kim, S.B.; Kim, H.; Hong, S.W.; Park, Y.N.; et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin. Cancer Res. 2011, 17, 1850–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Leung, S.Y.; Yuen, S.T.; Chu, K.M.; Ji, J.; Li, R.; Chan, A.S.Y.; Law, S.; Troyanskaya, O.G.; Wong, J.; et al. Variation in gene expression patterns in human gastric cancers. Mol. Biol. Cell 2003, 14, 3208–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Errico, M.; de Rinaldis, E.; Blasi, M.F.; Viti, V.; Falchetti, M.; Calcagnile, A.; Sera, F.; Saieva, C.; Ottini, L.; Palli, D.; et al. Genome-wide expression profile of sporadic gastric cancers with microsatellite instability. Eur. J. Cancer 2009, 45, 461–469. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Sougnez, C.; Lichtenstein, L.; Cibulskis, K.; Lander, E.; Gabriel, S.B.; Getz, G.; Ally, A.; Balasundaram, M.; Birol, I.; et al. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Toruner, G.A.; Ulger, C.; Alkan, M.; Galante, A.T.; Rinaggio, J.; Wilk, R.; Tian, B.; Soteropoulos, P.; Hameed, M.R.; Schwalb, M.N.; et al. Association between gene expression profile and tumor invasion in oral squamous cell carcinoma. Cancer Genet. Cytogenet. 2004, 154, 27–35. [Google Scholar] [CrossRef]
- Peng, C.H.; Liao, C.T.; Peng, S.C.; Chen, Y.J.; Cheng, A.J.; Juang, J.L.; Tsai, C.Y.; Chen, T.C.; Chuang, Y.J.; Tang, C.Y.; et al. A novel molecular signature identified by systems genetics approach predicts prognosis in oral squamous cell carcinoma. PLoS ONE 2011, 6, e23452. [Google Scholar] [CrossRef]
- Estilo, C.L.; O-Charoenrat, P.; Talbot, S.; Socci, N.D.; Carlson, D.L.; Ghossein, R.; Williams, T.; Yonekawa, Y.; Ramanathan, Y.; Boyle, J.O.; et al. Oral tongue cancer gene expression profiling: Identification of novel potential prognosticators by oligonucleotide microarray analysis. BMC Cancer 2009, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Talbot, S.G.; Estilo, C.; Maghami, E.; Sarkaria, I.S.; Pham, D.K.; O-charoenrat, P.; Socci, N.D.; Ngai, I.; Carlson, D.; Ghossein, R.; et al. Gene expression profiling allows distinction between primary and metastatic squamous cell carcinomas in the lung. Cancer Res. 2005, 65, 3063–3071. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Yu, T.; Temam, S.; Ziober, B.L.; Wang, J.; Schwartz, J.L.; Mao, L.; Wong, D.T.; Zhou, X. Transcriptomic dissection of tongue squamous cell carcinoma. BMC Genom. 2008, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuriakose, M.A.; Chen, W.T.; He, Z.M.; Sikora, A.G.; Zhang, P.; Zhang, Z.Y.; Qiu, W.L.; Hsu, D.F.; McMunn-Coffran, C.; Brown, S.M.; et al. Selection and validation of differentially expressed genes in head and neck cancer. Cell. Mol. Life Sci. 2004, 61, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Kallam, A. Clear Cell Renal Carcinoma; StatPearls: 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557644/ (accessed on 5 September 2021).
- Rossi, S.H.; Klatte, T.; Usher-Smith, J.; Stewart, G.D. Epidemiology and screening for renal cancer. World J. Urol. 2018, 36, 1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Shinghal, R.; Gill, H.; Reese, J.H.; Terris, M.; Cohen, R.J.; Fero, M.; Pollack, J.R.; Van de Rijn, M.; Brooks, J.D. Gene expression patterns in renal cell carcinoma assessed by complementary DNA microarray. Am. J. Pathol. 2003, 162, 925–932. [Google Scholar] [CrossRef] [Green Version]
- Yusenko, M.V.; Kuiper, R.P.; Boethe, T.; Ljungberg, B.; van Kessel, G.; Geurts, A.G.; Kovacs, G. High-resolution DNA copy number and gene expression analyses distinguish chromophobe renal cell carcinomas and renal oncocytomas. BMC Cancer 2009, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Borys, A.M.; Seweryn, M.; Gołąbek, T.; Bełch, Ł.; Klimkowska, A.; Totoń-Żurańska, J.; Machlowska, J.; Chłosta, P.; Okoń, K.; Wołkow, P.P. Patterns of gene expression characterize T1 and T3 clear cell renal cell carcinoma subtypes. PLoS ONE 2019, 14, e0216793. [Google Scholar] [CrossRef]
- Hong, Y.; Lin, M.; Ou, D.; Huang, Z.; Shen, P. A novel ferroptosis-related 12-gene signature predicts clinical prognosis and reveals immune relevancy in clear cell renal cell carcinoma. BMC Cancer 2021, 21, 831. [Google Scholar] [CrossRef]
- Kubota, S.; Yoshida, T.; Kageyama, S.; Isono, T.; Yuasa, T.; Yonese, J.; Kushima, R.; Kawauchi, A.; Chano, T. A risk stratification model based on four novel biomarkers predicts prognosis for patients with renal cell carcinoma. World J. Surg. Oncol. 2020, 18, 270. [Google Scholar] [CrossRef]
- Jones, J.; Otu, H.; Spentzos, D.; Kolia, S.; Inan, M.; Beecken, W.D.; Fellbaum, C.; Gu, X.; Joseph, M.; Pantuck, A.J.; et al. Gene signatures of progression and metastasis in renal cell cancer. Clin. Cancer Res. 2005, 11, 5730–5739. [Google Scholar] [CrossRef] [Green Version]
- Lenburg, M.E.; Liou, L.S.; Gerry, N.P.; Frampton, G.M.; Cohen, H.T.; Christman, M.F. Previously unidentified changes in renal cell carcinoma gene expression identified by parametric analysis of microarray data. BMC Cancer 2003, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Gumz, M.L.; Zou, H.; Kreinest, P.A.; Childs, A.C.; Belmonte, L.S.; LeGrand, S.N.; Wu, K.J.; Luxon, B.A.; Sinha, M.; Parker, A.S.; et al. Secreted frizzled-related protein 1 loss contributes to tumor phenotype of clear cell renal cell carcinoma. Clin. Cancer Res. 2007, 13, 4740–4749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutcliffe, C.; Kersey, D.; Huang, C.C.; Zeng, Y.; Walterhouse, D.; Perlman, E.J. Clear cell sarcoma of the kidney: Up-regulation of neural markers with activation of the sonic hedgehog and Akt pathways. Clin. Cancer Res. 2005, 11, 7986–7994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Types of Leukemia: Common, Rare and More Varieties|CTCA. Available online: https://www.cancercenter.com/cancer-types/leukemia/types (accessed on 6 August 2021).
- Andersson, A.; Ritz, C.; Lindgren, D.; Edén, P.; Lassen, C.; Heldrup, J.; Olofsson, T.; Råde, J.; Fontes, M.; Porwit-MacDonald, A.; et al. Microarray-based classification of a consecutive series of 121 childhood acute leukemias: Prediction of leukemic and genetic subtype as well as of minimal residual disease status. Leukemia 2007, 21, 1198–1203. [Google Scholar] [CrossRef] [Green Version]
- Stegmaier, K.; Ross, K.N.; Colavito, S.A.; O’Malley, S.; Stockwell, B.R.; Golub, T.R. Gene expression-based high-throughput screening (GE-HTS) and application to leukemia differentiation. Nat. Genet. 2004, 36, 257–263. [Google Scholar] [CrossRef]
- Valk, P.J.M.; Verhaak, R.G.W.; Beijen, M.A.; Erpelinck, C.A.J.; Doorn-Khosrovani, S.B.V.W.V.; Boer, J.M.; Beverloo, H.B.; Moorhouse, M.J.; Van Der Spek, P.J.; Löwenberg, B.; et al. Prognostically Useful Gene-Expression Profiles in Acute Myeloid Leukemia. N. Engl. J. Med. 2004, 350, 1617–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haferlach, T.; Kohlmann, A.; Wieczorek, L.; Basso, G.; Te Kronnie, G.; Béné, M.C.; De Vos, J.; Hernández, J.M.; Hofmann, W.K.; Mills, K.I.; et al. Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: Report from the international microarray innovations in leukemia study group. J. Clin. Oncol. 2010, 28, 2529–2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coustan-Smith, E.; Song, G.; Clark, C.; Key, L.; Liu, P.; Mehrpooya, M.; Stow, P.; Su, X.; Shurtleff, S.; Pui, C.H.; et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood 2011, 117, 6267–6276. [Google Scholar] [CrossRef]
- Basso, K.; Margolin, A.A.; Stolovitzky, G.; Klein, U.; Dalla-Favera, R.; Califano, A. Reverse engineering of regulatory networks in human B cells. Nat. Genet. 2005, 37, 382–390. [Google Scholar] [CrossRef]
- Haslinger, C.; Schweifer, N.; Stilgenbauer, S.; Döhner, H.; Lichter, P.; Kraut, N.; Stratowa, C.; Abseher, R. Microarray gene expression profiling of B-cell chronic lymphocytic leukemia subgroups defined by genomic aberrations and VH mutation status. J. Clin. Oncol. 2004, 22, 3937–3949. [Google Scholar] [CrossRef] [Green Version]
- Frager, S.Z.; Schwartz, J.M. Hepatocellular carcinoma: Epidemiology, screening, and assessment of hepatic reserve. Curr. Oncol. 2020, 27, S138. [Google Scholar] [CrossRef]
- Roessler, S.; Jia, H.L.; Budhu, A.; Forgues, M.; Ye, Q.H.; Lee, J.S.; Thorgeirsson, S.S.; Sun, Z.; Tang, Z.Y.; Qin, L.X.; et al. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. 2010, 70, 10202–10212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas, V.R.; Maluf, D.G.; Archer, K.J.; Yanek, K.; Kong, X.; Kulik, L.; Freise, C.E.; Olthoff, K.M.; Ghobrial, R.M.; McIver, P.; et al. Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma. Mol. Med. 2009, 15, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Zeballos, C.R.; Bouamar, H.; Gu, X.; Chen, Y.; Cigarroa, F.G.; Sun, L. The role of six transmembrane epithelial antigen of the prostate 2 in hepatocellular carcinoma. Cancer Res. 2017, 77, 5425. [Google Scholar]
- Coulouarn, C.; Derambure, C.; Lefebvre, G.; Daveau, R.; Hiron, M.; Scotte, M.; François, A.; Daveau, M.; Salier, J.P. Global gene repression in hepatocellular carcinoma and fetal liver, and suppression of dudulin-2 mRNA as a possible marker for the cirrhosis-to-tumor transition. J. Hepatol. 2005, 42, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Caillot, F.; Daveau, R.; Daveau, M.; Lubrano, J.; Saint-Auret, G.; Hiron, M.; Goria, O.; Scotte, M.; Francois, A.; Salier, J.-P. Down-regulated expression of the TSAP6 protein in liver is associated with a transition from cirrhosis to hepatocellular carcinoma. Histopathology 2009, 54, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Sonohara, F.; Hayashi, M.; Hishida, M.; Inokawa, Y.; Kanda, M.; Nishikawa, Y.; Takeda, S.; Sugimoto, H.; Fujii, T.; Kodera, Y.; et al. STEAP4 Inactivation Correlates Poor Prognosis and might be a Possible Cause of steatotic Change in Hepatocellular Carcinoma, Detected by Triple-Combination Array Analysis. J. Carcinog. Mutagen. 2014, 5, 201. [Google Scholar] [CrossRef]
- Yamada, N.; Yasui, K.; Dohi, O.; Gen, Y.; Tomie, A.; Kitaichi, T.; Iwai, N.; Mitsuyoshi, H.; Sumida, Y.; Moriguchi, M.; et al. Genome-wide DNA methylation analysis in hepatocellular carcinoma. Oncol. Rep. 2016, 35, 2228–2236. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Cheung, S.T.; So, S.; Fan, S.T.; Barry, C.; Higgins, J.; Lai, K.-M.; Ji, J.; Dudoit, S.; Ng, I.O.L.; et al. Gene Expression Patterns in Human Liver Cancers. Mol. Biol. Cell 2002, 13, 1929–1939. [Google Scholar] [CrossRef] [Green Version]
- Wurmbach, E.; Chen, Y.B.; Khitrov, G.; Zhang, W.; Roayaie, S.; Schwartz, M.; Fiel, I.; Thung, S.; Mazzaferro, V.; Bruix, J.; et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology 2007, 45, 938–947. [Google Scholar] [CrossRef]
- Lemjabbar-Alaoui, H.; Hassan, O.; Yang, Y.-W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta 2015, 1856, 189. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Ke, X.X.; Liu, Z.; Gao, W.L.; Fang, S.X.; Chen, C.; Song, Y.X.; Han, H.; Lu, H.L.; Xu, G. Evaluation of the Prognostic Value of STEAP1 in Lung Adenocarcinoma and Insights into Its Potential Molecular Pathways via Bioinformatic Analysis. Front. Genet. 2020, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.F.; Shang, W.L.; Yu, M.; Ren, X.P.; Wen, H.X.; Chai, C.Y.; Sun, L.; Hui, K.; Liu, L.H.; Wei, S.H.; et al. STEAP1 facilitates metastasis and epithelial-mesenchymal transition of lung adenocarcinoma via the JAK2/STAT3 signaling pathway. Biosci. Rep. 2020, 40, BSR20193169. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Niu, X.; Li, Y.; Xu, Z.; Chen, J.; Xu, G. Expression and prognostic analyses of the significance of STEAP1 and STEAP2 in lung cancer. World J. Surg. Oncol. 2022, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Guo, Q.Q.; Wei, Y.; Ren, K.M.; Zheng, F.S.; Tang, J.; Zhang, H.Y.; Zhao, J.G. Construction of a competing endogenous RNA network using differentially expressed lncRNAs, miRNAs and mRNAs in non-small cell lung cancer. Oncol. Rep. 2019, 42, 2402–2415. [Google Scholar] [CrossRef] [PubMed]
- Boelens, M.C.; van den Berg, A.; Fehrmann, R.S.; Geerlings, M.; de Jong, W.K.; Te Meerman, G.J.; Sietsma, H.; Timens, W.; Postma, D.S.; Groen, H.J. Current smoking-specific gene expression signature in normal bronchial epithelium is enhanced in squamous cell lung cancer. J. Pathol. 2009, 218, 182–191. [Google Scholar] [CrossRef]
- Never-Smoker, N.E. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Hou, J.; Aerts, J.; Den Hamer, B.; van IJcken, W.; Den Bakker, M.; Riegman, P.; van der Leest, C.; van der Spek, P.; Foekens, J.A.; Hoogsteden, H.C.; et al. Gene Expression-Based Classification of Non-Small Cell Lung Carcinomas and Survival Prediction. PLoS ONE 2010, 5, e10312. [Google Scholar] [CrossRef]
- Garber, M.E.; Troyanskaya, O.G.; Schluens, K.; Petersen, S.; Thaesler, Z.; Pacyna-Gengelbach, M.; Van De Rijn, M.; Rosen, G.D.; Perou, C.M.; Whyte, R.I.; et al. Diversity of gene expression in adenocarcinoma of the lung. Proc. Natl. Acad. Sci. USA 2001, 98, 13784–13789. [Google Scholar] [CrossRef] [Green Version]
- Wachi, S.; Yoneda, K.; Wu, R. Interactome-transcriptome analysis reveals the high centrality of genes differentially expressed in lung cancer tissues. Bioinformatics 2005, 21, 4205–4208. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, A.; Richards, W.G.; Staunton, J.; Li, C.; Monti, S.; Vasa, P.; Ladd, C.; Beheshti, J.; Bueno, R.; Gillette, M.; et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc. Natl. Acad. Sci. USA 2001, 98, 13790–13795. [Google Scholar] [CrossRef] [Green Version]
- Landi, M.T.; Dracheva, T.; Rotunno, M.; Figueroa, J.D.; Liu, H.; Dasgupta, A.; Mann, F.E.; Fukuoka, J.; Hames, M.; Bergen, A.W.; et al. Gene expression signature of cigarette smoking and its role in lung adenocarcinoma development and survival. PLoS ONE 2008, 3, e1651. [Google Scholar] [CrossRef] [PubMed]
- Stearman, R.S.; Dwyer-Nield, L.; Zerbe, L.; Blaine, S.A.; Chan, Z.; Bunn, P.A.; Johnson, G.L.; Hirsch, F.R.; Merrick, D.T.; Franklin, W.A.; et al. Analysis of orthologous gene expression between human pulmonary adenocarcinoma and a carcinogen-induced murine model. Am. J. Pathol. 2005, 167, 1763–1775. [Google Scholar] [CrossRef] [Green Version]
- Su, L.J.; Chang, C.W.; Wu, Y.C.; Chen, K.C.; Lin, C.J.; Liang, S.C.; Lin, C.H.; Whang-Peng, J.; Hsu, S.L.; Chen, C.H.; et al. Selection of DDX5 as a novel internal control for Q-RT-PCR from microarray data using a block bootstrap re-sampling scheme. BMC Genom. 2007, 8, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okayama, H.; Kohno, T.; Ishii, Y.; Shimada, Y.; Shiraishi, K.; Iwakawa, R.; Furuta, K.; Tsuta, K.; Shibata, T.; Yamamoto, S.; et al. Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 2012, 72, 100–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selamat, S.A.; Chung, B.S.; Girard, L.; Zhang, W.; Zhang, Y.; Campan, M.; Siegmund, K.D.; Koss, M.N.; Hagen, J.A.; Lam, W.L.; et al. Genome-scale analysis of DNA methylation in lung adenocarcinoma and integration with mRNA expression. Genome Res. 2012, 22, 1197–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Shaik, S.; Negi, B.S.; Rajguru, J.P.; Patil, P.B.; Parihar, A.S.; Sharma, U. Non-Hodgkin’s lymphoma: A review. J. Fam. Med. Prim. Care 2020, 9, 1834. [Google Scholar] [CrossRef]
- Compagno, M.; Lim, W.K.; Grunn, A.; Nandula, S.V.; Brahmachary, M.; Shen, Q.; Bertoni, F.; Ponzoni, M.; Scandurra, M.; Califano, A.; et al. Mutations of multiple genes cause deregulation of NF-B in diffuse large B-cell lymphoma. Nature 2009, 459, 717–721. [Google Scholar] [CrossRef] [Green Version]
- Brune, V.; Tiacci, E.; Pfeil, I.; Döring, C.; Eckerle, S.; Van Noesel, C.J.M.; Klapper, W.; Falini, B.; Von Heydebreck, A.; Metzler, D.; et al. Origin and pathogenesis of nodular lymphocyte-predominant Hodgkin lymphoma as revealed by global gene expression analysis. J. Exp. Med. 2008, 205, 2251–2268. [Google Scholar] [CrossRef]
- Storz, M.N.; Van De Rijn, M.; Kim, Y.H.; Mraz-Gernhard, S.; Hoppe, R.T.; Kohler, S. Gene expression profiles of cutaneous B cell lymphoma. J. Investig. Dermatol. 2003, 120, 865–870. [Google Scholar] [CrossRef] [Green Version]
- Eckerle, S.; Brune, V.; Döring, C.; Tiacci, E.; Bohle, V.; Sundström, C.; Kodet, R.; Paulli, M.; Falini, B.; Klapper, W.; et al. Gene expression profiling of isolated tumour cells from anaplastic large cell lymphomas: Insights into its cellular origin, pathogenesis and relation to Hodgkin lymphoma. Leukemia 2009, 23, 2129–2138. [Google Scholar] [CrossRef] [Green Version]
- Venanzi Rullo, E.; Maimone, M.G.; Fiorica, F.; Ceccarelli, M.; Guarneri, C.; Berretta, M.; Nunnari, G. Non-Melanoma Skin Cancer in People Living with HIV: From Epidemiology to Clinical Management. Front. Oncol. 2021, 11, 2896. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.T.; Ranmuthu, C.K.I.; Hall, P.N.; Funston, G.; Walter, F.M. Recognising Skin Cancer in Primary Care. Adv. Ther. 2020, 37, 603–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haqq, C.; Nosrati, M.; Sudilovsky, D.; Crothers, J.; Khodabakhsh, D.; Pulliam, B.L.; Federman, S.; Miller, J.R.; Allen, R.E.; Singer, M.I.; et al. The gene expression signatures of melanoma progression. Proc. Natl. Acad. Sci. USA 2005, 102, 6092–6097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riker, A.I.; Enkemann, S.A.; Fodstad, O.; Liu, S.; Ren, S.; Morris, C.; Xi, Y.; Howell, P.; Metge, B.; Samant, R.S.; et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med. Genom. 2008, 1, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Critchley-Thorne, R.J.; Yan, N.; Nacu, S.; Weber, J.; Holmes, S.P.; Lee, P.P. Down-regulation of the interferon signaling pathway in T lymphocytes from patients with metastatic melanoma. PLoS Med. 2007, 4, 897–911. [Google Scholar] [CrossRef] [Green Version]
- Talantov, D.; Mazumder, A.; Yu, J.X.; Briggs, T.; Jiang, Y.; Backus, J.; Atkins, D.; Wang, Y. Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin. Cancer Res. 2005, 11, 7234–7242. [Google Scholar] [CrossRef] [Green Version]
- Arora, T.; Mullangi, S.; Lekkala, M.R. Ovarian Cancer; StatPearls: 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK567760/ (accessed on 6 September 2021).
- Daniilidis, A.; Karagiannis, V. Epithelial ovarian cancer. Risk factors, screening and the role of prophylactic oophorectomy. Hippokratia 2007, 11, 63. [Google Scholar]
- Jiao, Z.; Huang, L.; Sun, J.; Xie, J.; Wang, T.; Yin, X.; Zhang, H.; Chen, J. Six-transmembrane epithelial antigen of the prostate 1 expression promotes ovarian cancer metastasis by aiding progression of epithelial-to-mesenchymal transition. Histochem. Cell Biol. 2020, 154, 215–230. [Google Scholar] [CrossRef]
- Broner, E.C.; Tropé, C.G.; Reich, R.; Davidson, B. TSAP6 is a novel candidate marker of poor survival in metastatic high-grade serous carcinoma. Hum. Pathol. 2017, 60, 180–187. [Google Scholar] [CrossRef]
- Yu, Z.; He, H.; Chen, Y.; Ji, Q.; Sun, M. A novel ferroptosis related gene signature is associated with prognosis in patients with ovarian serous cystadenocarcinoma. Sci. Rep. 2021, 11, 11486. [Google Scholar] [CrossRef]
- Lu, K.H.; Patterson, A.P.; Wang, L.; Marquez, R.T.; Atkinson, E.N.; Baggerly, K.A.; Ramoth, L.R.; Rosen, D.G.; Liu, J.; Hellstrom, I.; et al. Selection of potential markers for epithelial ovarian cancer with gene expression arrays and recursive descent partition analysis. Clin. Cancer Res. 2004, 10, 3291–3300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adib, T.R.; Henderson, S.; Perrett, C.; Hewitt, D.; Bourmpoulia, D.; Ledermann, J.; Boshoff, C. Predicting biomarkers for ovarian cancer using gene-expression microarrays. Br. J. Cancer 2004, 90, 686–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrix, N.D.; Wu, R.; Kuick, R.; Schwartz, D.R.; Fearon, E.R.; Cho, K.R. Fibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signaling in ovarian endometrioid adenocarcinomas. Cancer Res. 2006, 66, 1354–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshihara, K.; Tajima, A.; Komata, D.; Yamamoto, T.; Kodama, S.; Fujiwara, H.; Suzuki, M.; Onishi, Y.; Hatae, M.; Sueyoshi, K.; et al. Gene expression profiling of advanced-stage serous ovarian cancers distinguishes novel subclasses and implicates ZEB2 in tumor progression and prognosis. Cancer Sci. 2009, 100, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Bonome, T.; Levine, D.A.; Shih, J.; Randonovich, M.; Pise-Masison, C.A.; Bogomolniy, F.; Ozbun, L.; Brady, J.; Barrett, J.C.; Boyd, J.; et al. A gene signature predicting for survival in suboptimally debulked patients with ovarian cancer. Cancer Res. 2008, 68, 5478–5486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Liu, W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, Z.; Zhao, Y.; Zhou, Y.; Pei, H.; Bai, L. Bioinformatic mining and validation of the effects of ferroptosis regulators on the prognosis and progression of pancreatic adenocarcinoma. Gene 2021, 795, 145804. [Google Scholar] [CrossRef]
- Badea, L.; Herlea, V.; Dima, S.O.; Dumitrascu, T.; Popescu, I. Combined Gene Expression Analysis of Whole-Tissue and Microdissected Pancreatic Ductal Adenocarcinoma Identifies Genes Specifically Overexpressed in Tumor Epithelia. Hepatogastroenterology 2008, 55, 2016–2027. [Google Scholar]
- Buchholz, M.; Braun, M.; Heidenblut, A.; Kestler, H.A.; Klöppel, G.; Schmiegel, W.; Hahn, S.A.; Lüttges, J.; Gress, T.M. Transcriptome analysis of microdissected pancreatic intraepithelial neoplastic lesions. Oncogene 2005, 24, 6626–6636. [Google Scholar] [CrossRef] [Green Version]
- Segara, D.; Biankin, A.V.; Kench, J.G.; Langusch, C.C.; Dawson, A.C.; Skalicky, D.A.; Gotley, D.C.; Coleman, M.J.; Sutherland, R.L.; Henshall, S.M. Expression of HOXB2, a retinoic acid signaling target in pancreatic cancer and pancreatic intraepithelial neoplasia. Clin. Cancer Res. 2005, 11, 3587–3596. [Google Scholar] [CrossRef] [Green Version]
- Pei, H.; Li, L.; Fridley, B.L.; Jenkins, G.D.; Kalari, K.R.; Lingle, W.; Petersen, G.; Lou, Z.; Wang, L. FKBP51 Affects Cancer Cell Response to Chemotherapy by Negatively Regulating Akt. Cancer Cell 2009, 16, 259–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacobuzio-Donahue, C.A.; Maitra, A.; Olsen, M.; Lowe, A.W.; Van Heek, N.T.; Rosty, C.; Walter, K.; Sato, N.; Parker, A.; Ashfaq, R.; et al. Exploration of global gene expression patterns in pancreatic adenocarcinoma using cDNA microarrays. Am. J. Pathol. 2003, 162, 1151–1162. [Google Scholar] [CrossRef]
- Grützmann, R.; Pilarsky, C.; Ammerpohl, O.; Lüttges, J.; Böhme, A.; Sipos, B.; Foerder, M.; Alldinger, I.; Jahnke, B.; Schackert, H.K.; et al. Gene expression profiling of microdissected pancreatic ductal carcinomas using high-density DNA microarrays. Neoplasia 2004, 6, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, M.; Yoshida, K.; Yamashita, Y.; Ota, J.; Takada, S.; Kisanuki, H.; Koinuma, K.; Choi, Y.L.; Kaneda, R.; Iwao, T.; et al. Experimental trial for diagnosis of pancreatic ductal carcinoma based on gene expression profiles of pancreatic ductal cells. Cancer Sci. 2005, 96, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kaler, J.; Hussain, A.; Haque, A.; Naveed, H.; Patel, S. A Comprehensive Review of Pharmaceutical and Surgical Interventions of Prostate Cancer. Cureus 2020, 12, e11617. [Google Scholar] [CrossRef]
- Packer, J.R.; Maitland, N.J. The molecular and cellular origin of human prostate cancer. Biochim. Biophys. Acta 2016, 1863, 1238–1260. [Google Scholar] [CrossRef]
- Burnell, S.E.A.; Spencer-Harty, S.; Howarth, S.; Bodger, O.; Kynaston, H.; Morgan, C.; Doak, S.H. Utilisation of the STEAP protein family in a diagnostic setting may provide a more comprehensive prognosis of prostate cancer. PLoS ONE 2019, 14, e0220456. [Google Scholar] [CrossRef] [Green Version]
- Gomes, I.M.; Arinto, P.; Lopes, C.; Santos, C.R.; Maia, C.J. STEAP1 is overexpressed in prostate cancer and prostatic intraepithelial neoplasia lesions, and it is positively associated with Gleason score. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 53.e23–53.e29. [Google Scholar] [CrossRef]
- Varambally, S.; Yu, J.; Laxman, B.; Rhodes, D.R.; Mehra, R.; Tomlins, S.A.; Shah, R.B.; Chandran, U.; Monzon, F.A.; Becich, M.J.; et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell 2005, 8, 393–406. [Google Scholar] [CrossRef] [Green Version]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Febbo, P.G.; Ross, K.; Jackson, D.G.; Manola, J.; Ladd, C.; Tamayo, P.; Renshaw, A.A.; D’Amico, A.V.; Richie, J.P.; et al. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell 2002, 1, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Welsh, J.B.; Sapinoso, L.M.; Su, A.I.; Kern, S.G.; Wang-Rodriguez, J.; Moskaluk, C.A.; Frierson, H.F.; Hampton, G.M. Analysis of Gene Expression Identifies Candidate Markers and Pharmacological Targets in Prostate Cancer. Cancer Res. 2001, 61, 5974–5978. [Google Scholar] [PubMed]
- Yu, Y.P.; Landsittel, D.; Jing, L.; Nelson, J.; Ren, B.; Liu, L.; McDonald, C.; Thomas, R.; Dhir, R.; Finkelstein, S.; et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J. Clin. Oncol. 2004, 22, 2790–2799. [Google Scholar] [CrossRef]
- Holzbeierlein, J.; Lal, P.; LaTulippe, E.; Smith, A.; Satagopan, J.; Zhang, L.; Ryan, C.; Smith, S.; Scher, H.; Scardino, P.; et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am. J. Pathol. 2004, 164, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Ramachandran, S.; Seyed, M.A.; Scharer, C.D.; Laycock, N.; Dalton, W.B.; Williams, H.; Karanam, S.; Datta, M.W.; Jaye, D.L.; et al. Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res. 2006, 66, 4011–4019. [Google Scholar] [CrossRef] [Green Version]
- Tomlins, S.A.; Mehra, R.; Rhodes, D.R.; Cao, X.; Wang, L.; Dhanasekaran, S.M.; Kalyana-Sundaram, S.; Wei, J.T.; Rubin, M.A.; Pienta, K.J.; et al. Integrative molecular concept modeling of prostate cancer progression. Nat. Genet. 2007, 39, 41–51. [Google Scholar] [CrossRef]
- Luo, J.H.; Yu, Y.P.; Cieply, K.; Lin, F.; Deflavia, P.; Dhir, R.; Finkelstein, S.; Michalopoulos, G.; Becich, M. Gene expression analysis of prostate cancers. Mol. Carcinog. 2002, 33, 25–35. [Google Scholar] [CrossRef]
- LaTulippe, E.; Satagopan, J.; Smith, A.; Scher, H.; Scardino, P.; Reuter, V.; Gerald, W.L. Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic diseaseNo Title. Cancer Res. 2002, 62, 4499–4506. [Google Scholar]
- Lapointe, J.; Li, C.; Higgins, J.P.; Van De Rijn, M.; Bair, E.; Montgomery, K.; Ferrari, M.; Egevad, L.; Rayford, W.; Bergerheim, U.; et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc. Natl. Acad. Sci. USA 2004, 101, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Arredouani, M.S.; Lu, B.; Bhasin, M.; Eljanne, M.; Yue, W.; Mosquera, J.M.; Bubley, G.J.; Li, V.; Rubin, M.A.; Libermann, T.A.; et al. Identification of the transcription factor single-minded homologue 2 as a potential biomarker and immunotherapy target in prostate cancer. Clin. Cancer Res. 2009, 15, 5794–5802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanaja, D.K.; Cheville, J.C.; Iturria, S.J.; Young, C.Y. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 2003, 63, 3877–3882. [Google Scholar] [PubMed]
- Wallace, T.A.; Prueitt, R.L.; Yi, M.; Howe, T.M.; Gillespie, J.W.; Yfantis, H.G.; Stephens, R.M.; Caporaso, N.E.; Loffredo, C.A.; Ambs, S. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008, 68, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Hoang, N.T.; Acevedo, L.A.; Mann, M.J.; Tolani, B. A review of soft-tissue sarcomas: Translation of biological advances into treatment measures. Cancer Manag. Res. 2018, 10, 1089. [Google Scholar] [CrossRef] [Green Version]
- Skubitz, K.M.; D’Adamo, D.R. Sarcoma. Mayo Clin. Proc. 2007, 82, 1409–1432. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, D.; Grünewald, T.G.P.; Klar, R.; Schmidt, O.; Wohlleber, D.; Rubío, R.A.; Uckert, W.; Thiel, U.; Bohne, F.; Busch, D.H.; et al. Transgenic antigen-specific, HLA-A*02:01-allo-restricted cytotoxic T cells recognize tumor-associated target antigen STEAP1 with high specificity. Oncoimmunology 2016, 5, e1175795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detwiller, K.Y.; Fernando, N.T.; Segal, N.H.; Ryeom, S.W.; D’Amore, P.A.; Yoon, S.S. Analysis of hypoxia-related gene expression in sarcomas and effect of hypoxia on RNA interference of vascular endothelial cell growth factor A. Cancer Res. 2005, 65, 5881–5889. [Google Scholar] [CrossRef] [Green Version]
- Barretina, J.; Taylor, B.S.; Banerji, S.; Ramos, A.H.; Lagos-Quintana, M.; Decarolis, P.L.; Shah, K.; Socci, N.D.; Weir, B.A.; Ho, A.; et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat. Genet. 2010, 42, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Gaddam, S.J.; Chesnut, G.T. Testicle Cancer; StatPearls: 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563159/ (accessed on 6 August 2021).
- Batool, A.; Karimi, N.; Wu, X.-N.; Chen, S.-R.; Liu, Y.-X. Testicular germ cell tumor: A comprehensive review. Cell. Mol. Life Sci. 2019, 76, 1713–1727. [Google Scholar] [CrossRef]
- Skotheim, R.I.; Lind, G.E.; Monni, O.; Nesland, J.M.; Abeler, V.M.; Fosså, S.D.; Duale, N.; Brunborg, G.; Kallioniemi, O.; Andrews, P.W.; et al. Differentiation of human embryonal carcinomas in vitro and in vivo reveals expression profiles relevant to normal development. Cancer Res. 2005, 65, 5588–5598. [Google Scholar] [CrossRef] [Green Version]
- Korkola, J.E.; Houldsworth, J.; Chadalavada, R.S.; Olshen, A.B.; Dobrzynski, D.; Reuter, V.E.; Bosl, G.J.; Chaganti, R. Down-regulation of stem cell genes, including those in a 200-kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer Res. 2006, 66, 820–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperger, J.M.; Chen, X.; Draper, J.S.; Antosiewicz, J.E.; Chon, C.H.; Jones, S.B.; Brooks, J.D.; Andrews, P.W.; Brown, P.O.; Thomson, J.A. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 13350–13355. [Google Scholar] [CrossRef] [PubMed] [Green Version]




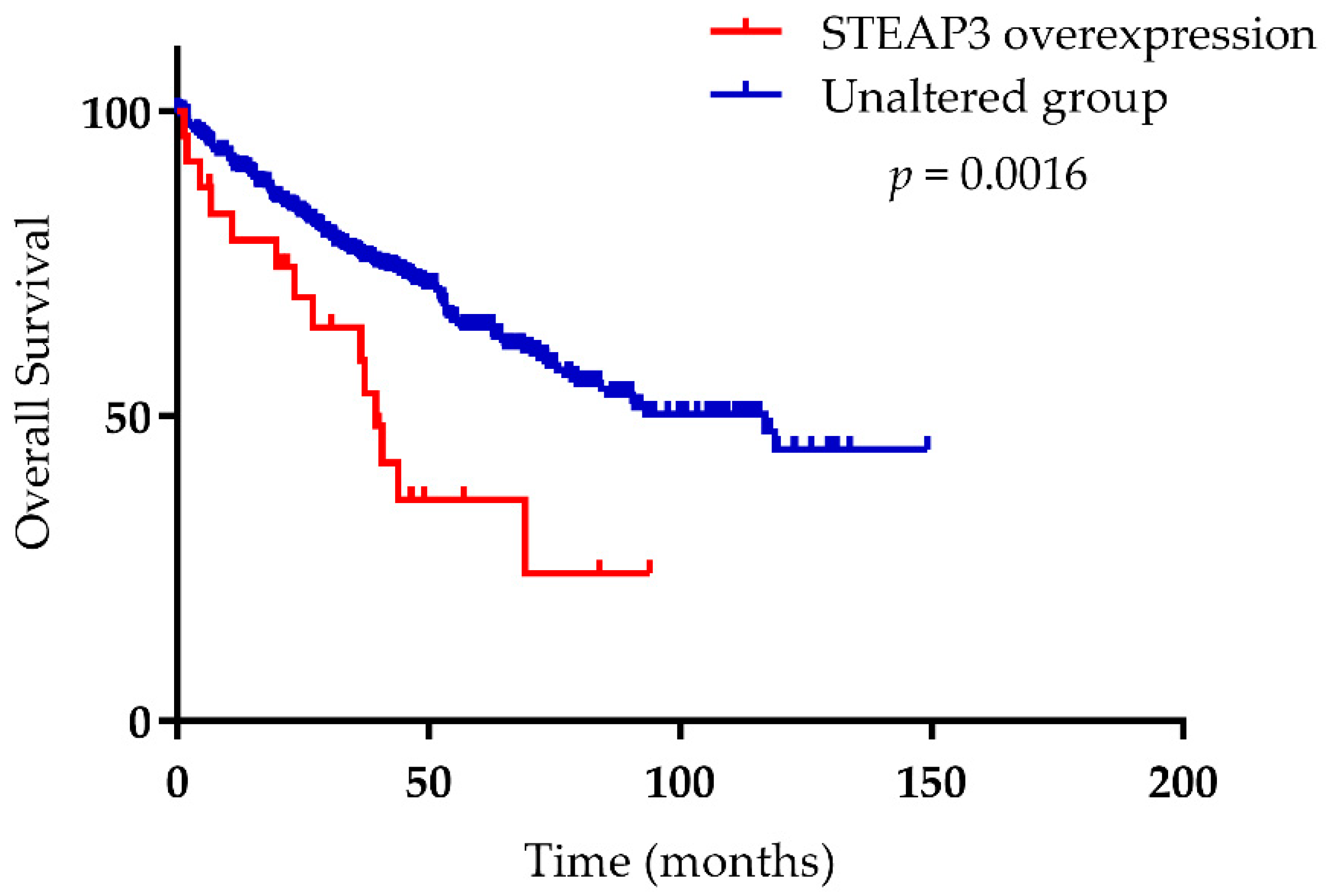
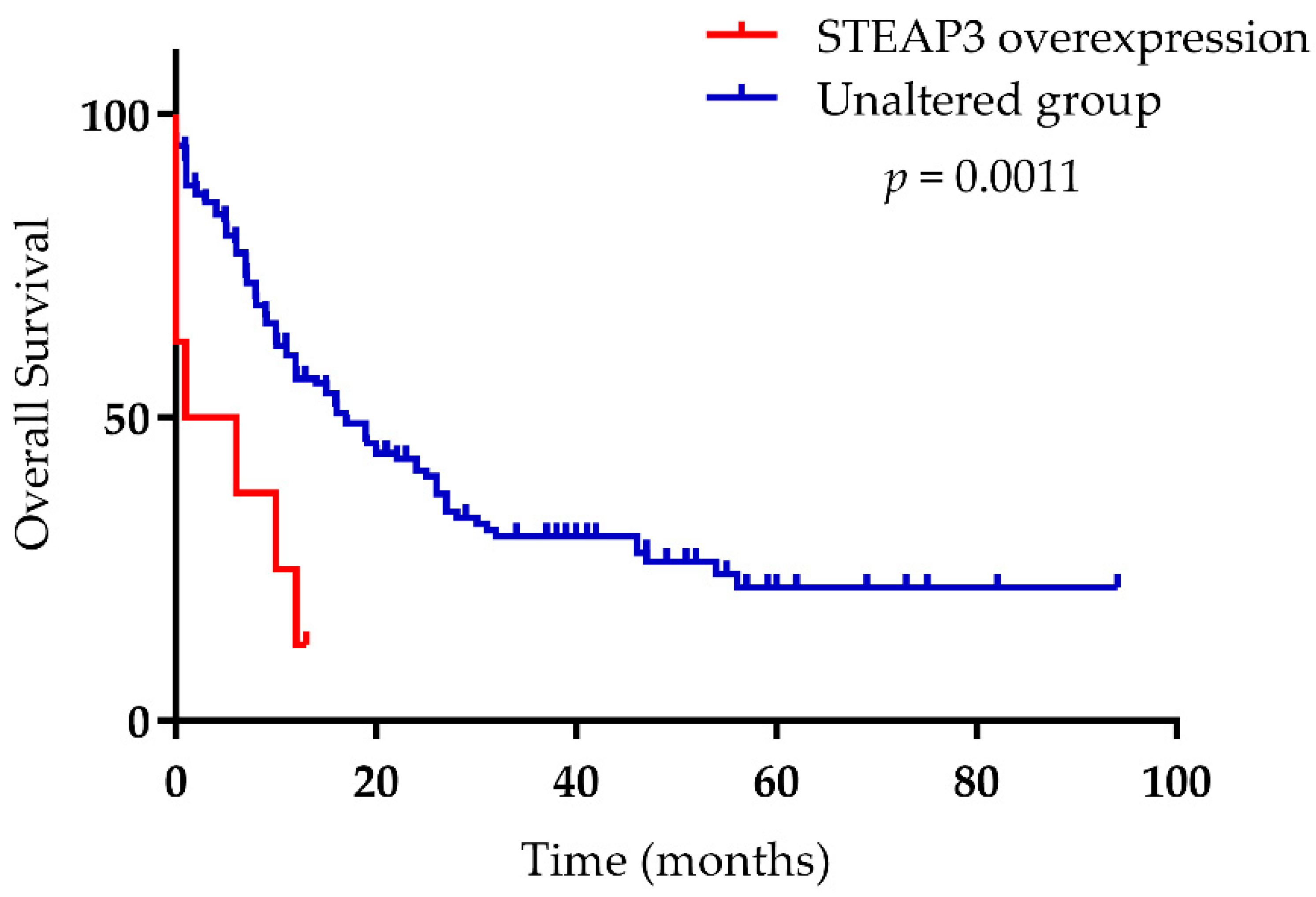



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, S.M.; Socorro, S.; Passarinha, L.A.; Maia, C.J. Comprehensive Landscape of STEAP Family Members Expression in Human Cancers: Unraveling the Potential Usefulness in Clinical Practice Using Integrated Bioinformatics Analysis. Data 2022, 7, 64. https://doi.org/10.3390/data7050064
Rocha SM, Socorro S, Passarinha LA, Maia CJ. Comprehensive Landscape of STEAP Family Members Expression in Human Cancers: Unraveling the Potential Usefulness in Clinical Practice Using Integrated Bioinformatics Analysis. Data. 2022; 7(5):64. https://doi.org/10.3390/data7050064
Chicago/Turabian StyleRocha, Sandra M., Sílvia Socorro, Luís A. Passarinha, and Cláudio J. Maia. 2022. "Comprehensive Landscape of STEAP Family Members Expression in Human Cancers: Unraveling the Potential Usefulness in Clinical Practice Using Integrated Bioinformatics Analysis" Data 7, no. 5: 64. https://doi.org/10.3390/data7050064
APA StyleRocha, S. M., Socorro, S., Passarinha, L. A., & Maia, C. J. (2022). Comprehensive Landscape of STEAP Family Members Expression in Human Cancers: Unraveling the Potential Usefulness in Clinical Practice Using Integrated Bioinformatics Analysis. Data, 7(5), 64. https://doi.org/10.3390/data7050064









