A Case of Craniocervical Junction Arteriovenous Fistulas with a Brainstem Mass Lesion on Imaging: Case Report and Literature Review
Abstract
:1. Introduction
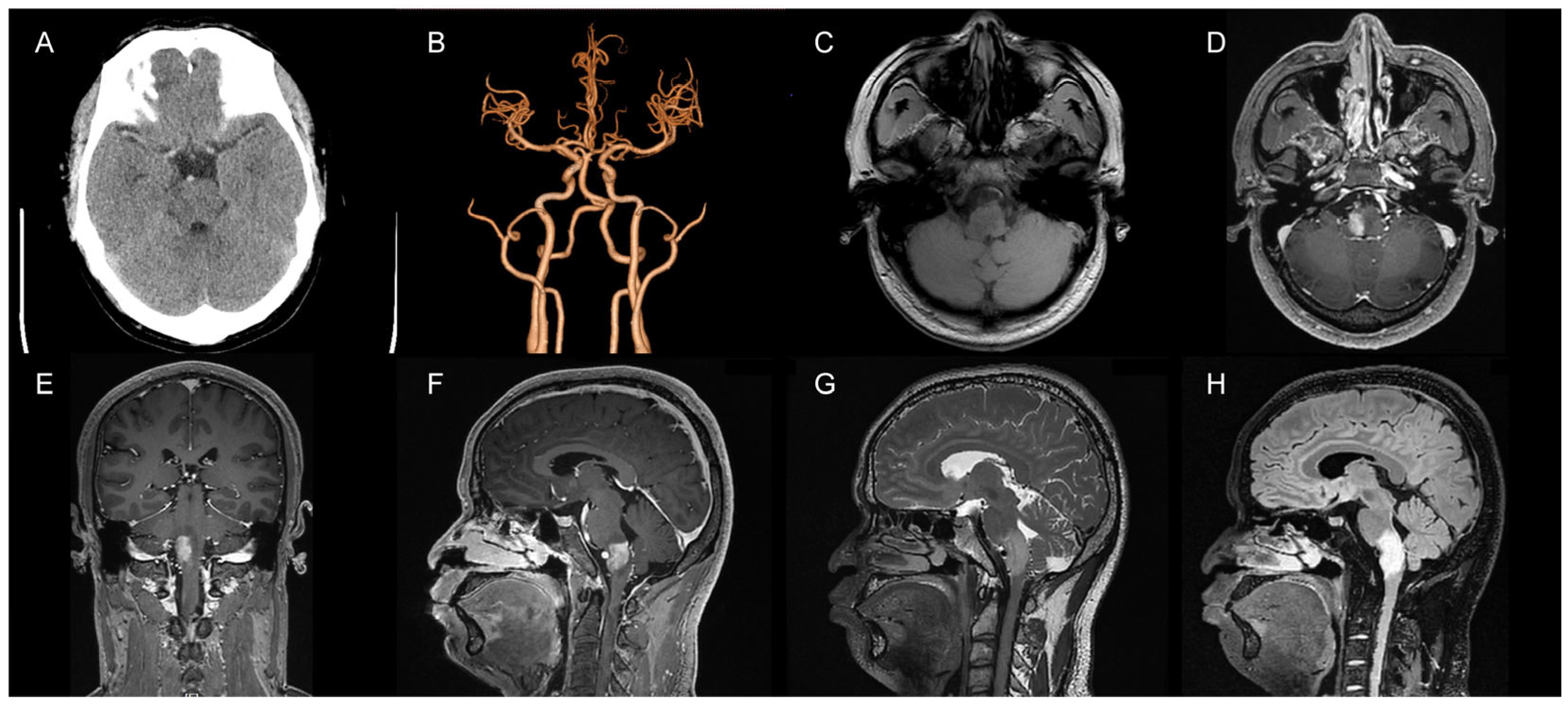
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freeman, W.D. Management of Intracranial Pressure. Continuum 2015, 21, 1299–1323. [Google Scholar] [CrossRef] [PubMed]
- Shimony, N.; Martinez-Sosa, M.; Osburn, B.; Jallo, G.I. Non-traumatic pediatric intracranial hypertension: Key points for different etiologies, diagnosis, and treatment. Acta Neurol. Belg. 2021, 121, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Timothy, J.; Cincu, R.; Agarwal, T.; Waghmare, L.B. Bradycardia in neurosurgery. Clin. Neurol. Neurosurg. 2008, 110, 321–327. [Google Scholar] [CrossRef] [PubMed]
- El Ahmadieh, T.Y.; Adel, J.G.; El Tecle, N.E.; Daou, M.R.; Aoun, S.G.; Nanney, A.D., 3rd; Bendok, B.R. Surgical treatment of elevated intracranial pressure: Decompressive craniectomy and intracranial pressure monitoring. Neurosurg. Clin. N. Am. 2013, 24, 375–391. [Google Scholar] [CrossRef]
- Neugebauer, H.; Witsch, J.; Zweckberger, K.; Juttler, E. Space-occupying cerebellar infarction: Complications, treatment, and outcome. Neurosurg. Focus 2013, 34, E8. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Schweizer, T.A. Spontaneous subarachnoid haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef]
- Varela, S.; Carrera, D.; Elazim, A.A.; Robinson, M.B.; Torbey, M.; Carlson, A.P. Continuous Tissue Plasminogen Activator Infusion Using a Minimally Invasive Irrigating Catheter for the Treatment of Intraparenchymal Hemorrhage Within the Basal Ganglia: Case Reports. Oper. Neurosurg. 2022, 23, e387–e391. [Google Scholar] [CrossRef]
- Goyal, M.; Ospel, J.M.; Demchuk, A.; McDonough, R.V. Intraparenchymal haemorrhages as a primary outcome measure. Lancet Neurol. 2021, 20, 595. [Google Scholar] [CrossRef]
- Nager, G.T. Acoustic neurinomas. Acta Otolaryngol. 1985, 99, 245–261. [Google Scholar] [CrossRef]
- Villena Martin, M.; Pena Pardo, F.J.; Jimenez Aragon, F.; Borras Moreno, J.M.; Garcia Vicente, A.M. Metabolic targeting can improve the efficiency of brain tumor biopsies. Semin. Oncol. 2020, 47, 148–154. [Google Scholar] [CrossRef]
- Stone, J.L.; Bailes, J.E.; Hassan, A.N.; Sindelar, B.; Patel, V.; Fino, J. Brainstem Monitoring in the Neurocritical Care Unit: A Rationale for Real-Time, Automated Neurophysiological Monitoring. Neurocrit. Care 2017, 26, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Elkady, A.; Rahametallah, M.; Bakheet, M.F. Dural Arteriovenous Fistula Presenting as a Rapidly Progressive Thalamic Dementia: A Case Report. Cureus 2022, 14, e29392. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, T.; Nishihori, M.; Izumi, T.; Motomura, K.; Ohka, F.; Goto, S.; Araki, Y.; Yokoyama, K.; Uda, K.; Saito, R. Dural Arteriovenous Fistula Mimicking a Brain Tumor on Methionine-positron Emission Tomography: A Case Report. NMC Case Rep. J. 2022, 9, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kida, S.; Neki, H.; Hiramatsu, H.; Kamio, Y.; Makita, I.; Shiraishi, Y.; Kurozumi, K. De novo dural arteriovenous fistula after mechanical thrombectomy for cerebral venous thrombosis: A case report. Surg. Neurol. Int. 2022, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Sasada, S.; Hiramatsu, M.; Kusumegi, A.; Fujimura, H.; Oshikata, S.; Takahashi, Y.; Nishida, K.; Yasuhara, T.; Date, I. Arteriovenous Fistula at the Craniocervical Junction Found after Cervical Laminoplasty for Ossification of the Posterior Longitudinal Ligament. Neurospine 2020, 17, 947–953. [Google Scholar] [CrossRef]
- Khan, M.I.; Tariq, M.; Rashid, D. Transient ischaemic attacks due to a pulsating mass in the neck produced after incision and drainage of parapharyngeal abscess. J. Ayub. Med. Coll. Abbottabad. 2008, 20, 143–145. [Google Scholar]
- Claassen, J.; Park, S. Spontaneous subarachnoid haemorrhage. Lancet 2022, 400, 846–862. [Google Scholar] [CrossRef]
- Nakamura, M.; Miyazaki, T.; Shinozaki, N.; Izumi, M.; Itabashi, T. Clinical Characteristics of Craniocervical Junction Arteriovenous Fistulas. No Shinkei Geka 2017, 45, 879–888. [Google Scholar] [CrossRef]
- Matsubara, S.; Toi, H.; Takai, H.; Miyazaki, Y.; Kinoshita, K.; Sunada, Y.; Yamada, S.; Tao, Y.; Enomoto, N.; Minami, Y.O.; et al. Variations and management for patients with craniocervical junction arteriovenous fistulas: Comparison of dural, radicular, and epidural arteriovenous fistulas. Surg. Neurol. Int. 2021, 12, 411. [Google Scholar] [CrossRef]
- Quinn, C.; Masood, K.; Mehta, T.; Topiwala, K.; Grande, A.; Tummala, R.; Jagadeesan, B.D. Trans-radial spinal angiography: A single-center experience. Interv. Neuroradiol. 2022, 15910199221135052. [Google Scholar] [CrossRef]
- Murakami, K.; Endo, T.; Tominaga, T. An analysis of flow dynamics in cerebral cavernous malformation and orbital cavernous angioma using indocyanine green videoangiography. Acta Neurochir. 2012, 154, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Duan, M.; Feng, H.; Fan, X.; Liu, G.; Yan, J.; Wang, Y. Potential epidural anesthesia risk of spinal dural arteriovenous fistula diagnosed and evaluated by indocyanine green fluorescence. Photodiagnosis Photodyn. Ther. 2022, 40, 103162. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.; Shibahara, I.; Inukai, M.; Koizumi, H.; Hyakutake, Y.; Niki, J.; Ishima, D.; Usui, R.; Kimura, A.; Hide, T.; et al. Coexistence of anterior cranial fossa dural arteriovenous fistula and arteriovenous malformation with the same drainage system: Illustrative case. J. Neurosurg. Case Lessons. 2022, 3, CASE2222. [Google Scholar] [CrossRef]
- Chen, X.; Ge, L.; Wan, H.; Huang, L.; Jiang, Y.; Lu, G.; Wang, J.; Zhang, X. Differential subsampling with cartesian ordering: A high spatial-temporal resolution dixon imaging sequence for assessment of dural arteriovenous fistula. Front. Neurol. 2022, 13, 1020749. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, M.P.; Chow, M.M.; Krishnaney, A.A.; Andrews-Hinders, D.; Benzel, E.C.; Masaryk, T.J.; Mayberg, M.R.; Rasmussen, P.A. Outcome after the treatment of spinal dural arteriovenous fistulae: A contemporary single-institution series and meta-analysis. Neurosurgery 2004, 55, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Zogopoulos, P.; Nakamura, H.; Ozaki, T.; Asai, K.; Ima, H.; Kidani, T.; Kadono, Y.; Murakami, T.; Fujinaka, T.; Yoshimine, T. Endovascular and Surgical Treatment of Spinal Dural Arteriovenous Fistulas: Assessment of Post-treatment Clinical Outcome. Neurol. Med. Chir. 2016, 56, 27–32. [Google Scholar] [CrossRef]
- Andres, R.H.; Barth, A.; Guzman, R.; Remonda, L.; El-Koussy, M.; Seiler, R.W.; Widmer, H.R.; Schroth, G. Endovascular and surgical treatment of spinal dural arteriovenous fistulas. Neuroradiology 2008, 50, 869–876. [Google Scholar] [CrossRef]
- Narvid, J.; Hetts, S.W.; Larsen, D.; Neuhaus, J.; Singh, T.P.; McSwain, H.; Lawton, M.T.; Dowd, C.F.; Higashida, R.T.; Halbach, V.V. Spinal dural arteriovenous fistulae: Clinical features and long-term results. Neurosurgery 2008, 62, 159–166. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Wang, Y.; Pang, C.; Li, X.; Zhuang, Z.; Li, W.; Hang, C. A Case of Craniocervical Junction Arteriovenous Fistulas with a Brainstem Mass Lesion on Imaging: Case Report and Literature Review. Brain Sci. 2023, 13, 839. https://doi.org/10.3390/brainsci13050839
Peng Z, Wang Y, Pang C, Li X, Zhuang Z, Li W, Hang C. A Case of Craniocervical Junction Arteriovenous Fistulas with a Brainstem Mass Lesion on Imaging: Case Report and Literature Review. Brain Sciences. 2023; 13(5):839. https://doi.org/10.3390/brainsci13050839
Chicago/Turabian StylePeng, Zheng, Yunfeng Wang, Cong Pang, Xiaojian Li, Zong Zhuang, Wei Li, and Chunhua Hang. 2023. "A Case of Craniocervical Junction Arteriovenous Fistulas with a Brainstem Mass Lesion on Imaging: Case Report and Literature Review" Brain Sciences 13, no. 5: 839. https://doi.org/10.3390/brainsci13050839
APA StylePeng, Z., Wang, Y., Pang, C., Li, X., Zhuang, Z., Li, W., & Hang, C. (2023). A Case of Craniocervical Junction Arteriovenous Fistulas with a Brainstem Mass Lesion on Imaging: Case Report and Literature Review. Brain Sciences, 13(5), 839. https://doi.org/10.3390/brainsci13050839





