Systematic Review of Pharyngeal and Esophageal Manometry in Healthy or Dysphagic Older Persons (>60 years)
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Participants
2.3. Interventions
2.4. Comparators
2.5. Outcomes
- Upper esophageal sphincter basal pressure (UES-BP in mmHg).
- Upper esophageal sphincter relaxation
- Duration (UES-RT)
- Integrated relaxation pressure in 0.25 s (UES-IRP in mmHg)
- UES opening extent on radiology or impedance base (UES Max Adm in milliSievert—mS)
- Intrabolus pressure above sphincter (IBP in mmHg at 1 cm above UES).
- Pharyngeal contractility—(PeakP or PhCI) and duration (milliseconds—ms)
- Esophagogastric junction barrier function (LES resting pressure in mmHg, EGJ contractile integral in mmHg.cm).
- Lower esophageal sphincter relaxation pressure (integrated relaxation pressure in 4 s IRP4 in mmHg).
- Contractility of the proximal esophagus (limited data) (proximal contractile integral/PCI—pressure × length × duration in mmHg.cm.s).
- Contractility of the distal esophagus (as mean peak pressure in mmHg or distal contractile integral—pressure × length × duration in mmHg.cm.s).
- Esophageal peristaltic success (% successful peristalsis).
2.6. Settings
2.7. Language
2.8. Search Strategy & Data Management
2.8.1. Information Sources
2.8.2. Data Management and Selection Process
2.8.3 Data Collection Process
2.8.4. Data, Outcomes and Prioritization
2.8.5. Risk of Bias
2.8.6. Data Synthesis
3. Results
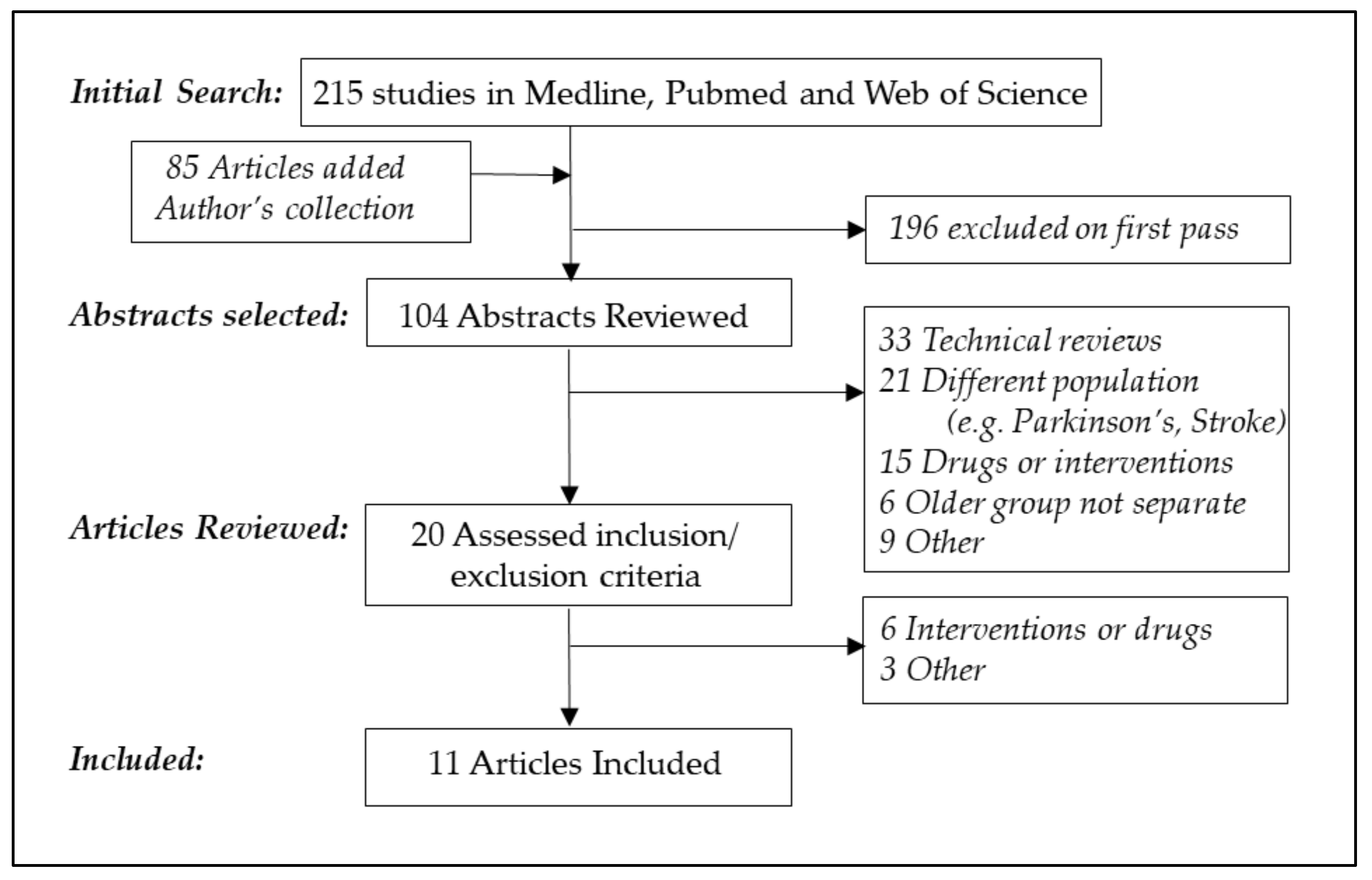
3.1. Literature Search and Study Characteristics
3.2. Results of Manometry Studies
3.3. Study Quality and Bias
4. Discussion
Limitations
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Smithard, D.G. Dysphagia: A Geriatric Giant? Med. Clin. Rev. 2016. [Google Scholar] [CrossRef]
- Altman, K.W.; Yu, G.-P.; Shaefer, S.D. Consequence of Dysphagia in the Hospitalized Patient. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Sura, L.; Madhavan, A.; Carnaby, G.; Crary, M. Dysphagia in the elderly: Management and nutritional considerations. Clinical Interventions in Aging 2010, 7, 287–298. [Google Scholar]
- Rommel, N.; Hamdy, S. Oropharyngeal dysphagia: Manifestation and diagnosis. Nat. Rev. Gastroenterol Hepatol 2016, 13, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Clavé, P.; Shaker, R. Dysphagia: Current reality and scope of the problem. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Eckberg, O.; Hamdy, S.; Woisard, V.; Wuttge-Hannig, A.; Ortega, P. Social and psychological burden of dysphagia: Its impact on diagnosis and treatment. Dysphagia 2002, 17, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Serra-Prat, M.; Palomera, M.; Gomez, C.; Sar-Shalom, D.; Saiz, A.; Montoya, J.G.; Navajas, M.; Palomera, E.; Clavé, P. Oropharyngeal dysphagia as a risk factor for malnutrition and lower respiratory tract infection in independently living older persons: A population-based prospective study. Age Ageing. 2012, 41, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H. Presbyphagia and Sarcopenic Dysphagia: Association between Aging, Sarcopenia and Deglutition Disorders. J. Frailty Aging 2014, 3, 97–103. [Google Scholar] [PubMed]
- Cesari, M.; Landi, F.; Vellas, B.; Bernabei, R.; Martezzi, E. Sarcopenia and Physical Frailty: Two Sides of the Same Coin. Front. Aging Neurosci. 2014, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Omari, T.I.; Kritas, S.; Cock, C.; Besanko, L.; Burgstad, C.; Thompson, A.; Rommel, N.; Heddle, R.; Fraser, R.J. Swallowing dysfunction in healthy older people using pharyngeal pressure-flow analysis. Neurogastroenterol. Motil. 2014, 26, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Cook, I. Oropharyngeal Dysphagia. Gastroenterol. Clin. North Am. 2009, 38, 411–431. [Google Scholar] [CrossRef] [PubMed]
- Shaker, R.; Lang, I.M. Effect of Aging on the Deglutitive Oral, Pharyngeal, and Esopahgeal Motor Function. Dysphagia 1994, 9, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Sears, V.W.; Castell, J.A.; Castell, D.O. Radial and longitudinal asymmetry of human pharyngeal pressures during swallowing. Gastroenterology 1991, 101, 1559–1563. [Google Scholar] [CrossRef]
- Meyer, J.P.; Jones, C.A.; Walzak, C.C.; McCulloch, T.M. Three-dimensional manometry of the upper esophageal sphincter in swallowing and nonswallowing tasks. Laryngoscope 2016, 126, 2539–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandolfino, J.E.; Kwaitek, M.A.; Nealis, T.; Bulsiewicz, W.; Post, J.; Kahrilas, P.J. Achalasia: A New Clinically Relevant Classification by High-Resolution Manometry. Gastroenterology 2008, 135, 1526–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahrilas, P.J.; Bredenoord, A.J.; Fox, M.; Gyawali, C.P.; Roman, S.; Smout, A.J.P.M.; Pandolfino, J.E. Advances in the management of oesophageal motility disorders in the era of high-resoltion manometry: A focus on achalasia syndromes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Pandolfino, J.E.; Fox, M.R.; Bredenoord, A.J.; Kahrilas, P.J. High-resolution manometry in clinical practice: Utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol. Motil. 2009, 21, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Bredenoord, A.J.; Fox, M.; Kahrilas, P.J.; Pandolfino, J.E.; Schwizer, W.; Smout, A.J.P.M. International High Resolution Manometry Working Group. Chicago Classification Criteria of Esophageal Motility Disorders Defined in High Resolution Esophageal Pressure Topography. Neurogastroenterol. Motil. 2012, 24 (Suppl. 1), 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, P.J.; Bredenoored, A.J.; Fox, M.; Gyawali, C.P.; Roman, S.; Smout, A.J.; Pandolfino, J.E. International High Resolution Manometry Working Group. The Chicago Classification of esophageal motility disorders, v 3.0. Neurogastroenterol. Motil. 2015, 27, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.R.; Bredenoord, A.J. Oeosphageal high-resolution manometry: Moving from research into clinical practice. Gut 2008, 57, 405–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, M.; Hebbard, G.; Janiak, P.; Brasseur, J.G.; Ghosh, S.; Thumshrin, M.; Fried, M.; Schwizer, W. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically inportnat abnormalities not detected by conventional manometry. Neurogastroenterol. Motil. 2004, 16, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P.; Bredenoord, A.J.; Conklin, J.L.; Fox, M.; Pandolfino, J.E.; Peters, J.H.; Roman, S.; Staiano, A.; Vaezi, M. Evaluation of esophageal motor function in clinical practice. Neurogastroenterol. Motil. 2013, 25, 99–133. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.C.; Klinger, P.J.; Hinder, R.A.; DeVault, K. Esophageal Manometry: A Comparison of Findings in Younger and Older Patients. Am. J. Gastroenterol. 1998, 93, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M.; Heddle, R.; Hebbard, G.S.; Checklin, H.; Besanko, L.; Fraser, R.J. Age and gender affect likely manometry diagnosis: Audit of a tertiary referral hospital clinical esophageal manometry service. J. Gastroenterol. Hepatol. 2009, 24, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Robson, K.M.; Glick, M.E. Dysphagia and Advancing Age. Are Manometric Abnormalities More Common in Older Patients? Dig. Dis. Sci. 2003, 48, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Alessandro, L.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamsheer, L.; Moher, D.; Clarke, M.; Ghershi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). World Report on Ageing and Health. Available online: http://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf?sequence=1 (accessed on 15 June 2018).
- Cock, C.; Omari, T. Diagnosis of Swallowing Disorders: How We Interpret Pharyngeal Manometry. Curr. Gastroenterol. Rep. 2017, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E. Cricopharyngeal function or dysfunction: What’s the deal? Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Cook, I. Clinical disorders of the upper esophageal sphincter. GI Motil. Online 2006. [Google Scholar] [CrossRef]
- Cook, I.J.; Dodds, W.J.; Dantas, R.O.; Massey, B.; Kern, M.K.; Lang, I.M.; Brasseur, J.G.; Hogan, W.J. Opening mechanisms of the human upper esophageal sphincter. Am. J. Physiol. 1989, 257, G748–G759. [Google Scholar] [CrossRef] [PubMed]
- Cock, C.; Jones, C.A.; Hammer, M.J.; Omari, T.I.; McCulloch, T.M. Modulation of Upper Esophageal Sphincter (UES) Relaxation and Opening During Volume Swallowing. Dysphagia 2017, 32, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Cock, C.; Besanko, L.; Kritas, S.; Burgstad, C.M.; Thompson, A.; Heddle, R.; Fraser, R.J.L.; Omari, T.I. Maximum upper esophageal sphincter (UES) admittance: A non-specific marker of UES dysfunction. Neurogastroenterol. Motil. 2016, 28, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Williams, R.B.; Cook, I.J.; Brasseur, J.G. Intrabolus pressure gradient identifies pathological constriction in the upper esophageal sphincter during flow. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G1037–G1048. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, A.; Humpries, K.; Lazar, A.; Martin-Harris, B. The pharyngeal contractile integral is a useful indicator of pharyngeal swallowing impairment. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J.; Castell, D.O. Classification of oesophageal motility abnormalities. Gut 2001, 49, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantas, R.O.; Alves, L.M.T.; Dalmazo, J.; Santos, C.M.; Cassiani, R.A.; Nascimento, W.V. Effect of Age on Proximal Esophageal Response to Swallowing. Arq. Gastroenterol. 2010, 47, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Nativ-Zetzer, N.; Logemann, J.A.; Zecker, S.G.; Kahrilas, P.J. Pressure topography metrics for high-resolution pharyngeal-esophageal manofluorography—A normative study of younger and older adults. Neurogastroenterol. Motil. 2016, 28, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.; Schar, M.; McCall, L.; Doeltgen, S.; Scholten, I.; Rommel, N.; Cock, C.; Omari, T. Characterization of swallow modulation in response to bolus volume in healthy subjects accounting for catheter diameter. Laryngoscope 2018, 128, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falk-Ytter, Y.; Alonso-Coelho, P.; Schünemann, H. GRADE: An emerging consensus on rating quality of evidence and strength of recommendation. BMJ 2008, 336, 924–926. [Google Scholar]
- Schünemann, H.; Oxman, A.D.; Brozek, J.; Glasziou, P.; Jaeschke, R.; Vist, G.E.; William, J.W.; Kunz, R.; Craig, J.; Montori, V.M.; et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008, 336, 1106–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaker, R.; Ren, J.; Podvrsan, B.; Dodds, W.J.; Hogan, W.J.; Kern, M.; Hoffmann, H.J. Effect of aging and bolus variables on pharyngeal and upper esophageal sphincter motor function. Am. J. Physiol. 1993, 264, G427–G432. [Google Scholar] [CrossRef] [PubMed]
- Dejaeger, E.; Pelemans, W.; Bibau, G.; Ponette, E. Manofluorographic Analysis of Swallwing in the Elderly. Dysphagia 1994, 9, 156–161. [Google Scholar] [CrossRef] [PubMed]
- McKee, G.J.; Johnston, B.T.; McBride, G.B.; Primrose, W.J. Does age and sex affect pharyngeal swallowing? Clin. Otolaryngol. 1998, 23, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Bardan, E.; Arndrofer, R.; Hofmann, C.; Ren, J.; Shaker, R. Comparison of Upper Esophegeal Sphincter Opening in Healthy Asymptomatic Young and Elderly Volunteers. Ann. Otol. Rhinol. Laryngol. 1999, 108, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Meier-Ewert, H.K.; van Herwaarden, M.A.; Gideon, R.M.; Castell, J.A.; Achem, S.; Castell, D.O. Effect of Age on Differences in Upper Esophageal Sphincter and Pharynx Pressures Between Patients With Dysphagia and Control Subjects. Am. J. Gastroenterol. 2002, 96, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Van Herwaarden, M.A.; Katz, P.O.; Gideon, R.M.; Barrett, J.; Castell, J.A.; Achenm, S.; Castell, D.O. Are Manometric Parameters of the Upper Esophageal Sphincter and Pharynx Affected by Age and Gender? Dysphagia 2003, 18, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Bardan, E.; Kern, M.; Arndorfer, R.C.; Hofmann, C.; Shaker, R. Effect of aging on bolus kinematics during the pharyngeal phase of swallowing. Am. J. Physiol. 2006, 290, G458–G465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, K.J.; Park, J.H.; Park, J.H.; Jung, I.S. Videofluoscopic and Manometric Evaluation of Pharyngeal and Upper esophageal Sphincter Function During Swallowing. JNM 2014, 20, 352–361. [Google Scholar] [PubMed]
- Cock, C.; Besanko, L.K.; Burgstad, C.M.; Thompson, A.; Kritas, S.; Heddle, R.; Fraser, R.J.L.; Omari, T.I. Age-related impairment of esophagogastric junction relaxation and bolus flow time. World J. Gastroenterol. 2017, 23, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Cock, C. Impaired bolus clearance in asymptomatic older adults during high-resolution impedance manometry. Neurogastroenterol. Motil. 2016, 28, 1890–1901. [Google Scholar] [Green Version]
- Besanko, L.K.; Burgstad, C.M.; Cock, C.; Heddle, R.; Fraser, A.; Fraser, R.J.L. Changes in Esophageal and Lower Esophageal Sphincter Motility with Healthy Aging. J. Gastrointest Liver Dis. 2014, 23, 243–248. [Google Scholar]
- Grande, L.; Lacima, G.; Ros, E.; Pera, M.; Ascaso, C.; Visa, J.; Pera, C. Deterioration of Esophageal Motility With Age: A Manometric Study of 79 Healthy Subjects. Am. J. Gastroenterol. 1999, 94, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Ferriolli, E.; Oliveira, R.B.; Matsuda, N.M.; Braga, F.J.H.N.; Dantas, R.O. Aging, Esophageal Motility, and Gastroesophageal Reflux. J. Am. Geriat. Soc. 1998, 46, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Hongo, M.; Yamada, M.; Kawakami, H.; Ueno, M.; Okuno, Y.; Toyota, T. Effect of Aging on the Esophageal Motor Functions. J. Smooth Muscle Res. 1996, 32, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.E.; Wu, W.C.; Johns, D.N.; Blackwell, J.N.; Nelson, J.L.; Castell, J.A.; Castell, D.O. Esophageal Manometry in 95 Healthy Adult Volunteers. Dig. Dis. Sci. 1987, 32, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Schragge, B.W.; Crispin, J.S.; Lind, J.L. Esophageal Motility in the Elderly. Dig. Dis. 1977, 22, 1049–1054. [Google Scholar] [CrossRef]
- Nakato, R.; Manabe, N.; Kamada, T.; Matsumoto, H.; Shiotani, A.; Hata, J.; Haruma, K. Age-Related Differences in Clinical Characteristics and Esophageal Motility in Patients with Dysphagia. Dysphagia 2017, 32, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.K.; Kim, N.; Park, Y.H.; Lee, J.C.; Sung, J.; Choi, Y.J.; Yoon, H.; Shin, C.M.; Park, Y.S.; Lee, D.H. Effects of Age on Esophageal Motility: Use of High-resolution Esophageal Impedance Manometry. J. Neurogastroenterol. Motil. 2017, 23, 229–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besanko, L.K.; Burgstad, C.M.; Mountifield, R.; Andrews, J.M.; Heddle, R.; Cheklin, H.; Fraser, R.J.L. Lower esophageal sphincter relaxation is impaired in older patients with dysphagia. World J. Gastroenterol. 2011, 17, 1326–1331. [Google Scholar]
- Andrews, J.M.; Fraser, R.J.; Heddle, R.; Hebbard, G.; Cheklin, H. Is esophageal dysphagia in the extreme elderly (≥ 80 years) different to dysphagia in younger adults? A clinical manometry service audit. Dis. Esophagus 2008, 21, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; Kieffer, E.J.; Powley, T.L. Aging of the myenteric plexus: Neuronal loss is specific to cholinergic neurons. Autonom. Neurosci. 2003, 106, 69–83. [Google Scholar] [CrossRef]
- Gregerson, H.; Pedersen, J.; Drewes, A.M. Deterioration of Muscle Function in the Human Esophagus with Age. Dig. Dis. Sci. 2008, 53, 3065–3070. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Pandolfino, J.E. High-Resolution Manometry in Clinical Practice. Gastroenterol. Hepatol. 2015, 11, 374–384. [Google Scholar]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Case control, Cohort, and Observational. | RCT (drug trails and therapeutic interventions), Review, Cases, and Case series. |
| At least one group ≥ 60 years of age. | Study focused on single disease process e.g., achalasia |
| Either healthy volunteers or a patient population with dysphagia included. | Surgery or radiotherapy involving the pharynx, UES or esophagus |
| Technical details of manometry procedure described. | Anorectal manometry |
| For pharyngeal studies the use of solid state sensors, 3 cm or less (“low-resolution”) or spaced at 1 cm or less “high-resolution” (HRPM). | For pharyngeal studies sensor spacing less than 3 cm |
| For esophageal studies both “low-resolution” (> 1 cm sensor spacing) and “high-resolution” (<1 cm or less) without or with impedance (HRM/HRIM). | Language other than English (LOTE) without available translation (simultaneous publication of English translation for LOTE articles). |
| Quality of Evidence | Strength of Recommendation |
|---|---|
| High quality (A) e.g., High Resolution Manometry | Strong recommendation for (1)/↑↑ |
| Moderate Quality (B) | Weak recommendation for (2)/↑ |
| Low Quality (C) e.g., Low Resolution Manometry | Weak recommendation against (2)/↓ |
| Very low quality (D) | Strong recommendation against (1)/↓↓ |
| Study | Population | Methods | Main Findings in Older |
|---|---|---|---|
| Pharyngeal Manometry | |||
| Shaker R et al. Effect of aging and bolus variables on pharyngeal and upper esophageal sphincter motor function. Am J Physiol 1993; 264:G427–G432 [43]. | Older (aged 76 ± 1.5 years) n = 12 Younger (aged 25 ± 1 years) n = 14 Healthy Volunteers | Videomanometry Gaeltec MMI spaced 1.5 cm | UES resting pressure lower Hypopharyngeal peak pressures increased Duration of hypopharyngeal pressure increased |
| Dejaeger E et al. Manofluorographic Analysis of Swallwing in the Elderly. Dysphagia 1994; 9:156–161 [44]. | Older (aged 80 ± 5 years) n = 16 Younger (aged 28 ± 8 years) n = 20 Healthy Volunteers | Video manometry Tranducers at 4 cm, 1.5 cm intervals | Incomplete UES relaxation in 18% Less negative pressure at UES in older |
| McKee GJ et al. Does age and sex affect pharyngeal swallowing? Clin Otolaryngol 1998; 23:100–106 [45]. | Older (60–85 years) n = 37 Younger (21–40 years) n = 36 Healthy Volunteers | Manometry 2 cm spacing Konigsberg | UES resting pressure lower UES opening earlier when referenced to UES closure (i.e., longer duration of UES relaxation) Less generation of negative pressure at the UES in older |
| Kern M et al. Comparison of Upper Esophegeal Sphincter Opening in Healthy Asymptomatic Young and Elderly Volunteers. Ann Otol Rhinol Laryngol 1999; 108:982–989 [46]. | Older (75 ± 2.8 years) n = 14 Younger (32 ± 2.7 years) n = 14 Healthy Volunteers | Videomanometry Gaeltec MMI spaced 1.5 cm 5 & 10 mL liquid barium boluses | Duration of UES opening longer Duration UES maximally relaxed longer Significantly higher IBP above UES (5 & 10 mL)UES opening decreased (in AP diameter for 5 mL) |
| *Meier-Ewert HK et al. Effect of Age on Differences in Upper Esophageal Sphincter and Pharynx Pressures Between Patients With Dysphagia and Control Subjects. Am J Gastroenterol 2002; 96:35–40 [47]. | Healthy Volunteers:Older (61–91 years) n = 15 Younger (32–59 years) n = 18 Patients:Older (60–88 years) n= 26 Younger (32–58 years) n = 15 | Manometry Konigsberg 1.5 cm/2 cm | UES resting pressure lower (significant in controls) Increased UES residual pressure during solid bolus swallows only in healthy volunteers Decreased pharyngeal peak pressure during solid bolus swallows only in patients |
| Van Herwaarden MA, et al. Are Manometric Parameters of the Upper Esophageal Sphincter and Pharynx Affected by Age and Gender? Dysphagia 2003; 18:211–217 [48]. | Older (>60 years) n = 23 Younger (<60 years) n = 61 Healthy Volunteers | Manometry Konigsberg 1.5 cm/2 cm | Decreasing UES resting pressure correlated with age (r = −0.41; p < 0.001) and lower UES residual pressure higher (liquids & solids) UES-RT shorter (liquids and solids); UES relaxation rate lower for all consistencies Pharyngeal amplitude increased Duration of contraction longer |
| Bardan E et al. Effect of aging on bolus kinematics during the pharyngeal phase of swallowing. Am J Physiol 2006; 290: G458-G465 [49]. | Older (70–85 years) n = 8 Younger (18–40 years) n = 8 | Videomanometry Study focused on bolus kinematics. | Bolus head (but not the bolus tail) slows significantly in the region between the epiglottis and UES only in older Negative pressure at the UES occurred less often: 41 vs. 53% for liquids (n.s.) and 55 vs. 83% of solids (p = 0.02) |
| Nativ-Zetzer et al. Pressure topography metrics for high-resolution pharyngeal-esophageal manofluorography—a normative study of younger and older adults. Neurogastroenterol Motil 2016; 28(5):721–731 [39]. | Older (aged 60–80 years) n = 22 Younger (aged 21–40 years) n = 22 | High-resolution manometry Manoscan 4.2 & 2.75 mm diameter catheters | Contractile integrals: PhCI, VPCI, TBI, and HPCI significantly greater (p < 0.05) Integrated UES relaxation pressure (UES-IRP) greater (p < 0.05) for all bolus trials. Proximal esophageal contraction (PCI) reduced |
| Cock et al. Maximum upper esophageal sphincter (UES) admittance: a non-specific marker of UES dysfunction. Neurogastroenterol Motil. 2016; 28:225–233 [34]. | Older (≥80 years) n=16 Younger (<60 years) n = 50 Also CPB (n = 11) & MND (n = 16) groups included | High-resolution manometry MMS Unisensor | UES admittance (opening extent) reduced UES IRP higher with age (liquid only) Duration of pharyngeal bolus presence during and following swallow (residue) increased Swallow risk index (aspiration risk) increased |
| Yoon et al. Videofluoroscopic and Manometric Evaluationof Pharyngeal and Upper Esophageal Sphincter Function During Swallowing J Neurogastroenterol Motil, Vol. 20 No. 3 July, 2014 [50]. | 26 asymptomatic volunteers (12 men and 14 women; age, 19–81 years). Correlation with age reported. | High-resolution manometry Given Imaging | A significant correlation was shown between decreasing hypopharyngeal CI vs. age Decreasing median intrabolus pressure at UES vs. age Decreasing nadir pressure at UES vs. Age |
| Omari et al. Swallowing dysfunction in healthy older people using pharyngeal pressure-flow analysis. Neurogastroenterol Motil 2014, 26:59–68 [10]. | Two older groups included 60–79 years (n = 18) & 80 + y (n = 20) | High-resolution manometry MMS Unisensor | Documented decrease in swallow function with pressure-flow parameters Increased SRI and increased PSIR Increased Flow Interval (Bolus Presence Time), Increased Nadir Impedance Correlations also of age vs. IBP (liquid) |
| Esophageal Manometry | |||
| Healthy Volunteers | |||
| Cock et al. Age-related impairment of esophagogastric junction relaxation and bolus flow time. World J Gastroenterol 2017; 23(15):2785–2794 [51] | Older (≥80 years) n = 15 Young (<60 years) n = 30 Asymptomatic volunteersGERD excluded by questionnaire | High-resolution impedance manometry (HRIM) MMS + Unisensor 5 and 10 mL liquid and viscous boluses in upright posture | Lower esophageal sphincter (LES) relaxation impaired (IRP4 11. 9 ± 2.3 vs. 5.9 ± 1.0 mmHg; p = 0.02). Bolus flow time through LES reduced (1.7 ± 0.3 vs. 3.8 ± 0.2 s; p < 0.001). Gastric resting pressure higher (9.4 ± 1.6 vs. 2.2 ± 1.5 mmHg). A novel index of LES contractility EGJ-contractile integral (contractility over three respiratory cycles at rest) similar in older |
| Cock et al. Impaired bolus clearance in asymptomatic older adults during high-resolution impedance manometry, Neurogastroenterol Motil 2016; 28(12):1890-1901 [52]. | Older (≥80 years) n = 15 Young (<60 years) n = 30 Asymptomatic volunteersGERD excluded by questionnaire | High-resolution impedance manometry (HRIM) MMS + Unisensor 5 and 10 mL liquid and viscous boluses in sitting posture | Overall average Chicago classification metrics were similar Higher proportion unsuccessful bolus transit for both liquids (60 vs. 80%) and viscous (40 vs. 80%) Failed bolus transit associated with reduced contractility and longer peristaltic breaks |
| Besanko et al. Changes in Esophageal and Lower Esophageal Sphincter Motility with Healthy Aging [53]. | Older ( ≥65 years) n = 10 Younger (<40 years) n = 10 | Low-resolution Water perfused Dentsleeve; Trace! | Reduced lower esophageal relaxation in older group in supine, as well as upright posture and with increased bolus consistencies. Trend towards lower LES resting pressure |
| Dantas et al. Effect of Age on Proximal Esophageal Response to Swallowing. Arq Gastroenterol 2010 Oct-Dec; 47(4)339–343 [38]. | Group I (18–30 years) n = 20 Group II (31–50 years) n = 27 Group III (51–74years) n = 22 Group C (III aged 51–59 years) n = 14 Group D (III aged ≥ 60 years) n = 8 | Low-resolution Medizintechnik Polygram Upper GI | No difference in amplitude. Duration longer in youngest group Trend towards lower amplitude in group aged over 60 years of age (not statistically significant) |
| Grande et al. Deterioration of Esophageal Motility With Age: A Manometric Study of 79 Healthy Subjects. Am J Gastroenterol 1999; 94(7): 1795–1801 [54]. | Six age cohorts (total n = 79) Sixth age cohort aged ≥ 65 years n = 13 | Low-resolution Arndorfer, Beckman instruments | LES resting pressure reduced LES overall length increased UES pressure and length reduced Maximum peristaltic wave amplitude reduced in the distal (but not significantly proximal) esophagus Simultaneous contractions occurred more commonly in older subjects |
| Ferriolli et al. Aging, Esophageal Motility, and Gastroesophageal Reflux. J Am Geriatric Soc 1998; 46:1534–1537 [55]. | Group I (20–30 years) n = 20 Group II (50–60 years) n = 10 Group III (70–80 years) n = 10 Healthy volunteers | Low-resolution Narco Bio 5 mL liquid and viscous boluses supine | LES resting pressure similar Contractile metrics similar Increased frequency of impaired peristalsis Clearance of scintigraphic reflux decreased |
| Nishimura et al. Effect of Aging on the Esophageal Motor Functions. J Smooth Muscle Res 1996; 32:43–50 [56]. | Group 1 (<50 years) n = 11 Group 2 (50–59 years) n = 15 Group 3 (60–69 years) n = 11 Group 4 (≥70 years) n = 10 | Low-resolution Arndorfer 3–5 mL liquids, supine | Trend towards lower LES resting pressure No difference in nadir LES pressure (relaxation) Lower proportion successful peristalsis ≥ 70 years Contractile amplitude reduced in ≥ 70 years |
| Richter et al. Esophageal Manometry in 95 Healthy Adult Volunteers. Dig Dis Sci 1987; 32:583–592 [57]. | 95 Healthy volunteers Older group (≥ 60 years) n = 13 | Low-resolution Arndorfer Beckman instruments 5 mL liquids, supine | No difference in LES resting pressure Contractile amplitudes similar Duration contraction longer |
| Khan et al. Esophageal Motility in the Elderly. Dig Dis 1977; 22(12):1049–1054 [58]. | Older group (≥60 years) n = 49 Young group (<40 years) n = 43 Asymptomatic per questionnaires | Low-resolution Water perfused 5 mL liquids | No difference in LES resting pressure LES relaxation reduced (82.2% vs. 94.1%; p < 0.003) Reduced amplitude distal and upper esophagus Increased “disordered” contractions (25.3 vs. 8.2%; p < 0.001) |
| Dysphagia Patients | |||
| Nakato et al. Age-Related Differences in Clinical Characteristics and Esophageal Motility in Patients with Dysphagia. Dysphagia 2017; 32:374–382 [59]. | Group A (≥ 65 years) n = 47 Group B (45–65 years) n = 42 Group C (<45 years) n = 27 Dysphagia symptoms | High-resolution impedance manometry (HRIM) Sandhill | Overall average Chicago classification metrics were similar Major motility disorders occurred in 28% of older and 39% of younger dysphagia cases. No difference in diagnoses between groups. |
| Shim et al. Effects of Age on Esophageal Motility: Use of High-resolution Esophageal Impedance Manometry. J Neurogastroenterol Motil 2017; 23:229–236 [60]. | Group A (≥65 years) n = 62 Group B (40–65 years) n = 185 Group C (<40 years) n = 32 All symptoms | High-resolution impedance manometry (HRIM) Sandhill | Overall average Chicago classification metrics were similar Upper esophageal sphincter resting pressures measured and reported to be lower in older (Group A 63.8 mmHg ± 32.2 vs. Group B 92.5 ± 49 mmHg and Group C 92.7 ± 46.0 mmHg; p < 0.001) |
| Besanko et al. Lower esophageal sphincter relaxation is impaired in older patients with dysphagia World J Gastroenterol 2011; 17(10):1326–1331 [61]. | Older group (≥80 years) n = 19 Young group (<50 years) n = 19 Dysphagia symptoms Achalasia excluded | Low-resolution Water perfused Dentsleeve; Trace! 5 mL liquids, solids Left lateral, upright | Resting LES pressure higher (23.4 ± 3.8 vs 14.9 ± 1.2 mmHg; p < 0.05) Nadir LES pressure higher 2.3 ± 0.6 vs. 0.7 ± 0.6 mmHg; p < 0.05) Restitution of LES earlier Amplitude and duration of contractions similar |
| Andrews et al. Age and gender affect likely manometric diagnosis: Audit of a tertiary referral hospital clinical esophageal manometry service. J Gastroenterol Hepatol 2009; 24:125–128 [24]. | Older group (≥65 years) n = 135 Young (n = 317): Group 1 (17–24 years) n = 14 Group 2 (25–44 years) n = 87 Group 3 (45–59 years) n = 216 All symptoms | Low-resolution Water perfused Dentsleeve; Trace! 5 mL liquids, solidsLeft lateral, upright | Increased abnormal studies (79% vs. 57%; p = 0.013) Trend towards increased spastic type motility (p = 0.06) |
| Andrews et al. Is esophageal dysphagia in the extreme elderly (≥ 80 years) different to dysphagia in younger adults? A clinical manometry service audit. Dis Esophagus 2008; 21:656–659 [62]. | Older group (≥80 years) n = 23 Young group (<50 years) n = 23 Dysphagia symptoms | Low-resolution Water perfused Dentsleeve; Trace! 5 mL liquids, solids Left lateral, upright | Resting LES pressure higher (26.1 ± 3.7 vs 16.8 ± 1.9 mmHg; p = 0.03) Increased failed peristalsis (63 vs. 32%; p = 0.006) Manometric diagnoses similar Fewer with heartburn symptom in addition |
| Robson & Glick. Dysphagia and Advancing Age. Are Manometric Abnormalities More Common in Older Patients? Dig Dis Sci 2003; 48(9): 1709–1712 [25]. | Older group (≥65 years) n = 53 Young group (18–45 years) n = 53 Dysphagia symptoms | Low-resolutionWater perfused Medtronic5 mL liquids, supine | Equal number of abnormal studies (82% vs. 77%; p = NS) and achalasia diagnoses (32% vs. 34%; p = NS) LES resting pressure, relaxation and esophageal contractility similar. Peristaltic failure in 53% older and 40% young (p = NS) |
| Ribeiro et al. Esophageal Manometry: A Comparison of Findings in Younger and Older Patients. Am J Gastroenterol 1998; 93:706–710 [23]. | Older Group (≥75 years) n = 66 Young (≤50 years) n = 122 All symptoms | Low-resolution Solid state Konigsberg 5 mL liquids | Dysphagia more common reason for referral LES resting pressure similar (28.6 mmHg vs. 27.2 mmHg). LES length similar. Peristaltic failure in 37% vs. 22% (p < 0.005) Amplitude of contractions similar More simultaneous contractions (15 vs. 4%; p < 0.02)Lower UES resting pressure (49.6 vs. 77.4 mmHg; p < 0.002) and less negative residual pressure Older patients more likely to have achalasia (15.2 vs. 4.1%; p < 0.05) or spastic disorders (16.6 vs. 5%; p < 0.05) Incomplete LES relaxation less |
| Study | Metric | Older | Younger | p-Value | Interpretation (Older Group Relative to Younger Group) |
|---|---|---|---|---|---|
| Upper Esophageal Sphincter Function | |||||
| UES Resting Pressure | |||||
| Shaker et al. 1993 [43] | UES-RP (mmHg) | 43 ± 5 | 71 ± 8 | <0.01 | Lower UES resting pressure |
| Mc Kee et al. 1997 [45] | UES-RP (mmHg) | 44 | 70 | <0.001 | Lower UES resting pressure |
| Meier-Ewert et al. 2001 (healthy volunteers) [47] | UES-RP (mmHg) | 52 ± 6 | 86 ± 9 | <0.05 | Lower UES resting pressure |
| Van Herwaarden et al. 2003 [48] | UES-RP (mmHg) | 46(20;116) | 78(34;164) | <0.001 | Lower UES resting pressure |
| Meier-Ewert et al. 2001 (patients) [47] | UES-RP (mmHg) | 65 ± 9 | 96 ± 15 | NS | Similar UES resting pressure |
| Intrabolus Pressure above UES (5 mL Liquids) | |||||
| Kern et al. 1999 [46] | Hypopharyngeal IBP | 14 ± 2 | 7 ± 1 | <0.05 | Higher |
| Omari et al. 2014 [10] | Mean Pharyngeal IBP | 10(4;30) | 7(2;13) | NS | Similar |
| Cock et al. 2016 [34] | Mean Pharyngeal IBP | 17(9;33) | 10(5;16) | <0.05 | Higher |
| UES Relaxation pressures (5 mL Liquids) | |||||
| Meier-Ewert et al. 2001 (healthy volunteers) [47] | UES residual pressure (mmHg) | 5.1 ± 1.2 | 7.4 ± 2.7 | NS | Similar residual pressure |
| Meier-Ewert et al. 2001 (patients) [47] | UES residual pressure (mmHg) | 3.5 ± 1.5 | −0.4 ± 3.5 | NS | Similar residual pressure |
| Van Herwaarden et al. 2003 [48] | UES residual pressure (mmHg) | 2.5(−8.4 to 14.5) | −3(−9.6 to 12) | <0.01 | Higher residual pressure Decreased extent UES relaxation |
| Cock et al. 2016 [34] | UES-IRP (mmHg) | 6(-1;23) | 3(1;9) | NS | Similar extent relaxation |
| Nativ-Zeltzer et al. 2016 [39] | UES-IRP (mmHg) | 4 ± 6 | -3 ± 4 | <0.05 | Decreased extent UES relaxation |
| Duration of UES relaxation/opening (5 mL Liquids) | |||||
| Kern et al. 1999 [46] | Total duration UES opening Maximum opening | 612 ± 9 ms 166 ± 14 ms (27%) | 571 ± 8 ms 128 ± 12 ms (22%) | <0.05 <0.05 | Increased duration UES relaxation |
| Meier-Ewert et al. 2001 (healthy volunteers) [47] | UES-RT (ms) | 554 ± 47 | 605 ± 38 | NS | Similar relaxation time |
| Meier-Ewert et al. 2001 (patients) [47] | UES-RT (ms) | 525 ± 35 | 470 ± 39 | <0.05 | Increased duration UES relaxation |
| Van Herwaarden et al. 2003 [48] | UES relaxation time (50% drop and return to 50% baseline) | 221 (75 to 379) | 260 (133 to 535) | < 0.05 | Decreased duration below 50% of baseline |
| UES Opening Extent (5 mL Liquids) | |||||
| Kern et al. 1999 [46] | Lateral projection/AP diameter (mm) AP projection/Lateral diameter (mm) | 11 ± 0.4 21 ± 4 | 12.6 ± 0.6 20 ± 5 | <0.05 NS | Decreased AP opening extent |
| Cock et al. 2016 [34] | UES Max Adm (mS) | 3.8(2.9;4.2) | 4.1(3.8;4.3) | <0.05 | Decreased opening extent |
| UES postswallow Contractility (5 mL Liquids) | |||||
| Nativ-Zeltzer et al. 2016 [39] | UES-CI (mmHg.cm.s) UES-PeakP (mmHg) | 405 ± 170 214 ± 72 | 408 ± 170 205 ± 46 | NS NS | Similar postswallow UES contractility |
| Pharyngeal Contractility (5 mL Liquids) | |||||
| Shaker et al. 1993 [43] | Hypopharyngeal PeakP (mmHg) Duration hypopharynx (ms) | 196 ± 12 437 ± 69 | 137 ± 9 204 ± 21 | <0.01 <0.01 | Increased hypopharyngeal contractile vigor and duration |
| Meier-Ewert et al. 2001 (healthy volunteers) [47]. | Pharyngeal PeakP (mmHg) Duration pharyngeal contraction (ms) | 182 ± 20 763 ± 64 | 139 ± 13 593 ± 55 | NS NS | Similar pharyngeal contractility |
| Meier-Ewert et al. 2001 (patients) [47]. | Pharyngeal PeakP (mmHg) Duration pharyngeal contraction (ms) | 96 ± 15 712 ± 64 | 144 ± 21 712 ± 58 | <0.05 NS | Decreased contractile vigor in patients |
| Van Herwaarden et al. 2003 [48]. | Pharyngeal PeakP (mmHg) Duration pharyngeal contraction (ms) | 152(44 to 379) 448(324 to 835) | 133(53 to 220) 396(187 to 628) | <0.05 <0.05 | Increased pharyngeal contractile vigor and duration |
| Omari et al 2014 [10]. | Mean Pharyngeal PeakP (mmHg) | 145(108;194) | 132(103;213) | NS | Similar pharyngeal contractility |
| Cock et al. 2016 [34]. | Mean Pharyngeal PeakP (mmHg) | 161(117;221) | 136(104;208) | NS | Similar pharyngeal contractility |
| Nativ-Zeltzer et al. 2016 [39] | P-max (PeakP) (mmHg) PhCI (mmHg.cm.s) | 249 ± 54 363 ± 110 | 211 ± 64 256 ± 84 | <0.05 <0.05 | Increased pharyngeal contractility |
| Esophageal Studies: | |||||
| Esophagogastric junction (EGJ) barrier function | |||||
| Healthy Volunteers | |||||
| Cock et al. 2017 [51] | EGJ contractile integral for 3 respiratory cycles (mmHg.cm) | 34 ± 5 | 25 ± 5 | NS | Similar EGJ-CI |
| Besanko et al. 2014 [53] | Lower esophageal sphincter resting pressure (LES-RP) (mmHg) | 16 ± 3 | 21 ± 1 | 0.08 | Lower (trend) LES-RP |
| Grande et al. 1999 [54] | LES-RP (mmHg) | 11–25 | 16–38 | <0.001 | Lower LES-RP |
| Ferrioli et al. 1998 [55] | LES-RP (mmHg) | 35 ± 9 | 31 ± 14 | NS | Similar LES-RP |
| Nishimura et al. 1996 [56] | LES-RP (mmHg) | 15(8;27) | 11(4;16) | NS | Similar LES-RP |
| Dysphagia Patients | |||||
| Besanko et al. 2011 [61] | LES-RP (mmHg) | 23 ± 4 | 15 ± 1 | <0.05 | Higher LES-RP |
| Andrews et al. 2008 [62] | LES-RP (mmHg) | 26 ± 4 | 17 ± 2 | 0.03 | Higher LES-RP |
| Robson et al. 2003 [25] | LES-RP (mmHg) | 33.3 | 32.5 | NS | Similar LES-RP |
| Lower esophageal sphincter (LES) relaxation | |||||
| Healthy Volunteers | |||||
| Cock et al. 2017 [51] | 4-second Integrated Relaxation Pressure (IRP4) (mmHg) | 12 ± 2 (Liquid) 14 ± 2 (Viscous) | 6 ± 1 (L) 7 ± 1 (V) | 0.02 0.02 | Decreased LES relaxation(Upright) |
| Cock et al. 2016 [52] | IRP4 (mmHg) | 9 ± 2 (L) 15 ± 2 (V) | 8 ± 1 (L) 8 ± 1 (V) | NS 0.002 | LES relaxation similar for liquids, but decreased for increased consistency (upright) |
| Besanko et al. 2014 [53] | IRP4 (mmHg) | 4 ± 1 (Right Lateral) 7 ± 1 (Upright Liquid) 8 ± 1 (Upright Solids) | 3 ± 1 (RL) 3 ± 1 (UL) 4 ± 1 (US) | NS <0.01 <0.001 | Decreased LES relaxation(upright) |
| Dysphagia Patients | |||||
| Nakato et al. 2017 [59] | IRP4 (mmHg) | 14 (8–27) | 17 (9–30) | NS | Similar LES relaxation |
| Besanko et al. 2011 [61] | Nadir LES pressure (mmHg) | 2.3 ± 0.6 | 0.7 ± 0.6 | <0.05 | Decreased LES relaxation |
| Robson et al. 2003 [25] | Proportion complete relaxation (%) | 24/53 (45) | 23/53 (43) | NS | Similar LES relaxation |
| Esophageal Contractility | |||||
| Healthy Volunteers | |||||
| Cock et al. 2016 [52] | Distal esophageal peak pressure (PeakP) (mmHg) Distal Contractile Integral (DCI) (mmHg.cm.s) | 56 ± 9 729 ± 224 | 66 ± 9 766 ± 123 | NS NS | Similar peak pressure and DCI |
| Besanko et al. 2014 [53] | Peak P (mmHg) DCI (mmHg.cm.s) | 38 ± 9 835 ± 260 | 41 ± 8 947 ± 201 | NS NS | Similar peak pressure and DCI |
| Grande et al. 1999 [54] | Distal amplitude (mmHg) | 40–77 | 56–158 | <0.001 | Lower mean distal amplitude |
| Ferrioli et al. 1998 [55] | Contractile amplitude (mmHg) | 97 ± 41 | 107 ± 35 | NS | Similar mean distal amplitude |
| Nishimura et al. 1996 [56] | 5 cm above LES (mmHg) | 37 (20;54) | 114 (58;142) | <0.05 | Lower mean distal amplitude |
| Dysphagia Patients | |||||
| Nakato et al. 2017 [59] | DCI (mmHg.cm.s) | 1005 (350;2063) | 464 (218–1227) | NS | Similar DCI |
| Besanko et al. 2011 [61] | Peak P (mmHg) | 54 ± 8 | 62 ± 6 | NS | Similar peak pressure and DCI |
| Robson et al. 2003 [25] | Contractile amplitude (mmHg) | 71 | 74 | NS | Similar mean distal amplitude |
| Esophageal Peristalsis (Success) | |||||
| Healthy Volunteers | |||||
| Cock et al. 2016 [52] | Percent successful peristaltic contractions (%) | 60 (Liquids) 40 (Viscous) | 82 (L) 83 (V) | <0.05 | Decrease in successful peristalsis |
| Nishimura et al. 1996 [56] | Percent successful peristaltic contractions (%) | 80 (60;100) Liquids | 100 (90;100) (L) | <0.05 | Decrease in successful peristalsis |
| Dysphagia Patients—no data | |||||
| Study | Comparative Diagnostic | GRADE Recommendation |
|---|---|---|
| Pharyngeal Studies in Dysphagia Patients | ||
| Ribeiro et al. Esophageal Manometry: A Comparison of Findings in Younger and Older Patients. Am J Gastroenterol 1998; 93:706–710 [23]. | Increase in “abnormal” studies | 2B |
| Meier-Ewert HK et al. Effect of Age on Differences in Upper Esophageal Sphincter and Pharynx Pressures Between Patients With Dysphagia and Control Subjects. Am J Gastroenterol 2002; 96:35–40 [47]. | Different mechanism | 2B |
| Esophageal Studies in Dysphagia Patients | ||
| Nakato et al. Age-Related Differences in Clinical Characteristics and Esophageal Motility in Patients with Dysphagia. Dysphagia 2017; 32:374–382 [59]. | Major diagnosis in 39 vs. 28% | 2B |
| Shim et al. Effects of Age on Esophageal Motility: Use of High-resolution Esophageal Impedance Manometry. J Neurogastroenterol Motil 2017; 23:229–236 [60]. | Similar numbers | 2C |
| Andrews et al. Age and gender affect likely manometric diagnosis: Audit of a tertiary referral hospital clinical esophageal manometry service. J Gastroenterol Hepatol 2009; 24:125–128 [24]. | Increase in “abnormal” studies | 2C |
| Robson & Glick. Dysphagia and Advancing Age. Are Manometric Abnormalities More Common in Older Patients? Dig Dis Sci 2003; 48(9): 1709–1712 [25]. | High proportion achalasia | 2B |
| Ribeiro et al. Esophageal Manometry: A Comparison of Findings in Younger and Older Patients. Am J Gastroenterol 1998; 93:706–710 [23]. | Increase in “abnormal” studies | 2B |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cock, C.; Omari, T. Systematic Review of Pharyngeal and Esophageal Manometry in Healthy or Dysphagic Older Persons (>60 years). Geriatrics 2018, 3, 67. https://doi.org/10.3390/geriatrics3040067
Cock C, Omari T. Systematic Review of Pharyngeal and Esophageal Manometry in Healthy or Dysphagic Older Persons (>60 years). Geriatrics. 2018; 3(4):67. https://doi.org/10.3390/geriatrics3040067
Chicago/Turabian StyleCock, Charles, and Taher Omari. 2018. "Systematic Review of Pharyngeal and Esophageal Manometry in Healthy or Dysphagic Older Persons (>60 years)" Geriatrics 3, no. 4: 67. https://doi.org/10.3390/geriatrics3040067
APA StyleCock, C., & Omari, T. (2018). Systematic Review of Pharyngeal and Esophageal Manometry in Healthy or Dysphagic Older Persons (>60 years). Geriatrics, 3(4), 67. https://doi.org/10.3390/geriatrics3040067





