Factors Predicting the Utilization of Center-Based Cardiac Rehabilitation Program
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Study Setting
2.4. Study Variables and Measures
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Comparison between Baseline and 3 Months
3.3. Predictors of CR Completion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Veronese, N.; Cereda, E.; Stubbs, B.; Solmi, M.; Luchini, C.; Manzato, E.; Sergi, G.; Manu, P.; Harris, T.; Fontana, L.; et al. Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: Results from a meta-analysis and exploratory meta-regression analysis. Ageing Res. Rev. 2017, 35, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Perel, P.; Avezum, Á.; Huffman, M.D.; Pais, P.; Rodgers, A.; Vedanthan, R.; Wood, D.; Yusuf, S. Reducing Premature Cardiovascular Morbidity and Mortality in People with Atherosclerotic Vascular Disease: The World Heart Federation Roadmap for Secondary Prevention of Cardiovascular Disease. Glob. Heart 2015, 10, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, J.M.; Duncan, M.S.; Shah, A.S.; Greevy, R.A.; Lindenfeld, J.; Keteyian, S.J.; Thomas, R.J.; Whooley, M.A.; Wang, T.J.; Freiberg, M.S. Association of Cardiac Rehabilitation With Decreased Hospitalizations and Mortality After Ventricular Assist Device Implantation. JACC Heart Fail. 2018, 6, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Ghisi, G.L.D.M.; Pesah, E.; Turk-Adawi, K.; Supervia, M.; Lopez-Jimenez, F.; Grace, S.L. Cardiac Rehabilitation Models around the Globe. J. Clin. Med. 2018, 7, 260. [Google Scholar] [CrossRef] [Green Version]
- Choo, C.C.; Chew, P.K.H.; Lai, S.-M.; Soo, S.-C.; Ho, C.S.H.; Ho, R.C.; Wong, R.C. Effect of Cardiac Rehabilitation on Quality of Life, Depression and Anxiety in Asian Patients. Int. J. Environ. Res. Public Health 2018, 15, 1095. [Google Scholar] [CrossRef] [Green Version]
- Del Pozo-Cruz, B.; Carrick-Ranson, G.; Reading, S.; Nolan, P.; Dalleck, L.C. The relationship between exercise dose and health-related quality of life with a phase III cardiac rehabilitation program. Qual. Life Res. 2018, 27, 993–998. [Google Scholar] [CrossRef]
- Duscha, B.D.; Piner, L.W.; Patel, M.P.; Craig, K.P.; Brady, M.; McGarrah, R.W.; Chen, C.; Kraus, W.E. Effects of a 12-week mHealth program on peak VO2 and physical activity patterns after completing cardiac rehabilitation: A randomized controlled trial. Am. Heart J. 2018, 199, 105–114. [Google Scholar] [CrossRef]
- Lane, D.; Carroll, D.; Ring, C.; Beevers, D.G.; Lip, G.Y.H. Mortality and Quality of Life 12 Months After Myocardial Infarction: Effects of Depression and Anxiety. Psychosom. Med. 2001, 63, 221–230. [Google Scholar] [CrossRef]
- Beauchamp, A.; Worcester, M.; Ng, A.; Murphy, B.; Tatoulis, J.; Grigg, L.; Newman, R.; Goble, A. Attendance at cardiac rehabilitation is associated with lower all-cause mortality after 14 years of follow-up. Heart 2013, 99, 620–625. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.; Thompson, D.R.; Oldridge, N. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Arena, R.; Williams, M.; Forman, D.E.; Cahalin, L.P.; Coke, L.; Myers, J.; Hamm, L.; Kris-Etherton, P.; Humphrey, R.; Bittner, V.; et al. Increasing Referral and Participation Rates to Outpatient Cardiac Rehabilitation: The Valuable Role of Healthcare Professionals in the Inpatient and Home Health Settings: A science advisory from the American Heart Association. Circulation 2012, 125, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, M.J.; Stevens, G.R.; Whalen, D.; Ozonoff, A.; Balady, G.J. Effect of an American Heart Association Get With the Guidelines Program-Based Clinical Pathway on Referral and Enrollment Into Cardiac Rehabilitation After Acute Myocardial Infarction. Am. J. Cardiol. 2008, 101, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Suaya, J.A.; Shepard, D.S.; Normand, S.-L.T.; Ades, P.A.; Prottas, J.; Stason, W.B. Use of Cardiac Rehabilitation by Medicare Beneficiaries After Myocardial Infarction or Coronary Bypass Surgery. Circulation 2007, 116, 1653–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bethell, H.J.; Evans, J.A.; Turner, S.C.; Lewin, R.J.P. The rise and fall of cardiac rehabilitation in the United Kingdom since 1998. J. Public Health 2007, 29, 57–61. [Google Scholar] [CrossRef]
- Grace, S.L.; Chessex, C.; Arthur, H.; Chan, S.; Cyr, C.; Dafoe, W.; Juneau, M.; Oh, P.; Suskin, N. Systematizing Inpatient Referral to Cardiac Rehabilitation 2010: Canadian Association of Cardiac Rehabilitation and Canadian Cardiovascular Society Joint Position Paper. Can. J. Cardiol. 2011, 27, 192–199. [Google Scholar] [CrossRef]
- Oldridge, N.B. Cardiac rehabilitation exercise programme. Compliance and compliance-enhancing strategies. Sports Med. 1988, 6, 42–55. [Google Scholar] [CrossRef]
- Ades, P.A.; Waldmann, M.L.; McCann, W.J.; Weaver, S.O. Predictors of cardiac rehabilitation participation in older coronary patients. Arch. Intern. Med. 1992, 152, 1033–1035. [Google Scholar] [CrossRef]
- Gallagher, R.; Roach, K.; Belshaw, J.; Kirkness, A.; Sadler, L.; Warrington, D. A pre-test post-test study of a brief educational intervention demonstrates improved knowledge of potential acute myocardial infarction symptoms and appropriate responses in cardiac rehabilitation patients. Aust. Crit. Care 2013, 26, 49–54. [Google Scholar] [CrossRef]
- Ruta, D. Short form 36 questionnaire. J. Epidemiol. Community Health. 1995, 49, 555. [Google Scholar] [CrossRef] [Green Version]
- Tiringer, I.; Simon, A.; Herrfurth, D.; Suri, I.; Szalai, K.; Veress, A. Occurrence of anxiety and depression disorders after acute cardiac events during hospital rehabilitation. Application of the Hospital Anxiety and Depression Scale as a screening instrument. Psychiatr. Hung. 2008, 23, 430–443. [Google Scholar]
- Failde, I.; Ramos, I. Validity and reliability of the SF-36 Health Survey Questionnaire in patients with coronary artery disease. J. Clin. Epidemiol. 2000, 53, 359–365. [Google Scholar] [CrossRef]
- Hunt-Shanks, T.; Blanchard, C.; Reid, R.; Fortier, M.; Cappelli, M. A psychometric evaluation of the Hospital Anxiety and Depression Scale in cardiac patients: Addressing factor structure and gender invariance. Br. J. Health Psychol. 2010, 15, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Stafford, L.; Berk, M.; Jackson, H.J. Validity of the Hospital Anxiety and Depression Scale and Patient Health Questionnaire-9 to screen for depression in patients with coronary artery disease. Gen. Hosp. Psychiatry 2007, 29, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Daffertshofer, A.; Lamoth, C.J.C.; Meijer, O.G.; Beek, P.J. PCA in studying coordination and variability: A tutorial. Clin. Biomech. 2004, 19, 415–428. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning. Data Mining Inference and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Soleimani, A.; Abbasi, A.; Nejatian, M.; Salarifar, M.; Darabian, S.; Karimi, A.A.; Davoodi, S.; Kassaian, E.; Abbasi, S.H.; Sheikhfathollahi, M. Factors predicting discontinuation of a hospital-based cardiac rehabilitation programme. Kardiol. Pol. 2009, 67, 147–148. [Google Scholar]
- Hripcsak, G.; Bakken, S.; Stetson, P.D.; Patel, V.L. Mining complex clinical data for patient safety research: A framework for event discovery. J. Biomed. Inform. 2003, 36, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Bucinski, A.; Bączek, T.; Krysiński, J.; Szoszkiewicz, R.; Załuski, J. Clinical data analysis using artificial neural networks (ANN) and principal component analysis (PCA) of patients with breast cancer after mastectomy. Rep. Pr. Oncol. Radiother. 2007, 12, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Du, K.-L.; Wang, H.; Lu, J.; Zhang, B. Neural Network Implementations for PCA and Its Extensions. ISRN Artif. Intell. 2012, 2012, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Mostaar, A.; Sattari, M.R.; Hosseini, S.; Deevband, M.R. Use of Artificial Neural Networks and PCA to Predict Results of Infertility Treatment in the ICSI Method. J. Biomed. Phys. Eng. 2019, 9, 679–686. [Google Scholar] [CrossRef]
- Bouwmans, C.A.M.; Vemer, P.; Van Straten, A.; Tan, S.S.; Roijen, L.H.-V. Health-Related Quality of Life and Productivity Losses in Patients with Depression and Anxiety Disorders. J. Occup. Environ. Med. 2014, 56, 420–424. [Google Scholar] [CrossRef]
- Pardaens, S.; De Smedt, D.; De Bacquer, D.; Willems, A.-M.; Verstreken, S.; De Sutter, J. Comorbidities and Psychosocial Characteristics as Determinants of Dropout in Outpatient Cardiac Rehabilitation. J. Cardiovasc. Nurs. 2017, 32, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Petrie, K.J.; Weinman, J.; Sharpe, N.; Buckley, J. Role of patients’ view of their illness in predicting return to work and functioning after myocardial infarction: Longitudinal study. BMJ 1996, 312, 1191–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridderstråle, M.; Johansson, L.E.; Rastam, L.; Lindblad, U. Increased risk of obesity associated with the variant allele of the PPARGC1A Gly482Ser polymorphism in physically inactive elderly men. Diabetologia 2006, 49, 496–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resurrección, D.M.; Motrico, E.; Rubio-Valera, M.; Mora-Pardo, J.A.; Moreno-Peral, P. Reasons for dropout from cardiac rehabilitation programs in women: A qualitative study. PLoS ONE 2018, 13, e0200636. [Google Scholar] [CrossRef] [Green Version]
- Ritchey, M.D.; Maresh, S.; McNeely, J.; Shaffer, T.; Jackson, S.L.; Keteyian, S.J.; Brawner, C.A.; Whooley, M.A.; Chang, T.; Stolp, H.; et al. Tracking Cardiac Rehabilitation Participation and Completion Among Medicare Beneficiaries to Inform the Efforts of a National Initiative. Circ. Cardiovasc. Qual. Outcomes 2020, 13, 005902. [Google Scholar] [CrossRef]
- Beauchamp, A.; Sheppard, R.; Wise, F.; Jackson, A. Health Literacy of Patients Attending Cardiac Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2020, 40, 249–254. [Google Scholar] [CrossRef]
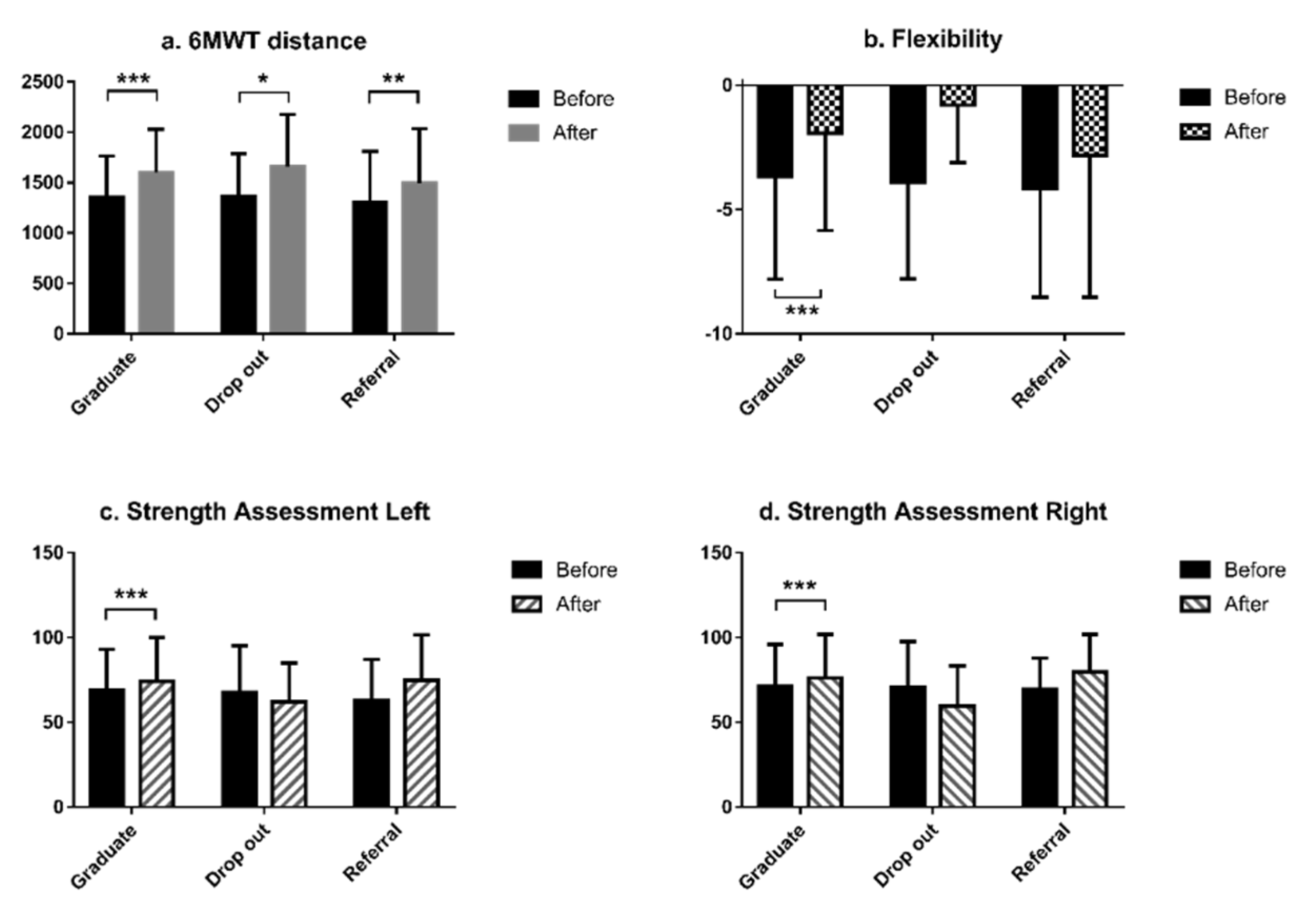
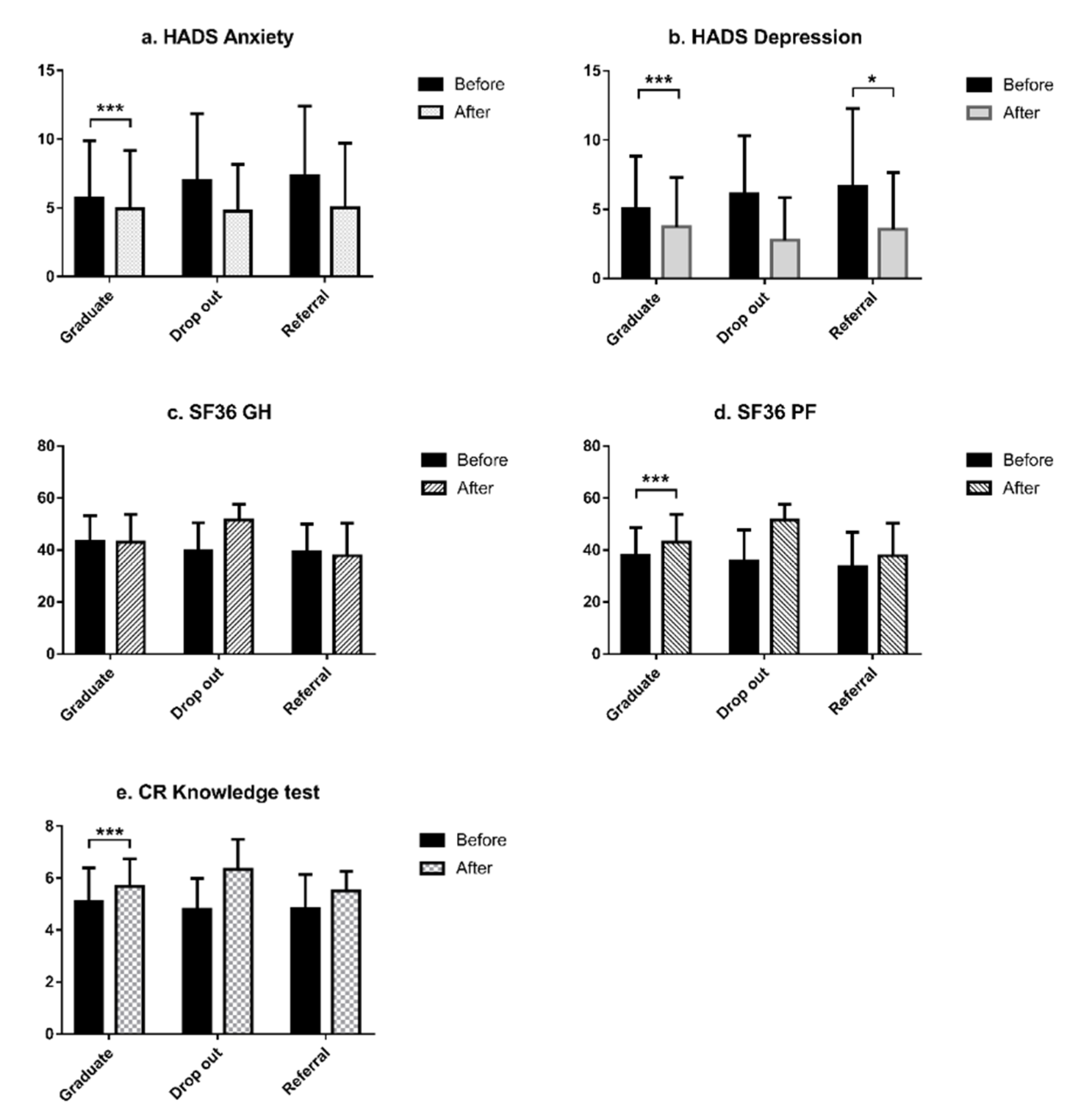

| Characteristic | Total | Graduate | Drop-Out | Referral | |
|---|---|---|---|---|---|
| Mean ± SD or N (%) | Mean ± SD or N (%) | Mean ± SD or N (%) | Mean ± SD or N (%) | ||
| Status (Graduate, Drop out, Referral) | 685 (100%) | 421 (61.4) | 217 (31.7) | 47 (6.9) | |
| Age | 64.0 ± 12.5 | 65.8 ± 11.7 | 60.9 ± 13.0 | 69.4 ± 14.3 | |
| Gender | male | 443 (64.7) | 274 (65.1) | 133 (61.3) | 36 (76.6) |
| female | 242 (35.3) | 147 (34.9) | 84 (38.7) | 11 (23.4) | |
| Primary diagnosis | Arrhythmia | 3 (0.4) | 2 (0.5) | 1 (0.5) | |
| Stable angina | 189 (27.6) | 115 (27.4) | 84 (38.7) | 17 (36.1) | |
| Acute coronary syndrome | 135 (19.7) | 76 (18.1) | 49 (22.6) | 10 (21.3) | |
| Stable heart failure | 54 (7.9) | 33 (7.8) | 18 (8.3) | 3 (6.4) | |
| CABG or PTCA | 288 (42.0) | 187 (44.4) | 57 (26.3) | 17 (36.2) | |
| Other | 16 (2.3) | 8 (1.9) | 8 (3.7) | ||
| Resting HR | 71.9 ± 12.1 | 71.4 ± 11.8 | 72.9 ± 12.8 | 73.6 ± 12.4 | |
| SBP | 127.1 ± 20.7 | 127.4 ± 19.8 | 126.8 ± 23.1 | 125.8 ± 16.9 | |
| DBP | 72.0 ± 12.3 | 72.7 ± 12.1 | 70.8 ± 13.1 | 71.1 ± 10.4 | |
| TC | 162.3 ± 49.4 | 159.9 ± 46.1 | 165.3 ± 54.2 | 162.0 ± 42.6 | |
| HDL | 42.5 ± 13.9 | 43.4 ± 13.3 | 41.2 ± 13.3 | 43.43 ± 20.8 | |
| Trig | 163.1 ± 272.0 | 139.2 ± 95.7 | 190.0 ± 401.1 | 177.8 ± 157.2 | |
| LDL | 91.5 ± 40.6 | 91.9 ± 40.5 | 91.7 ± 41.9 | 87.9 ± 33.0 | |
| FBG | 123.4 ± 93.2 | 123.8 ± 107.7 | 123.2 ± 63.3 | 121.4 ± 40.3 | |
| Hemoglobin A1c | 6.9 ± 6.0 | 7.0 ± 7.6 | 6.7 ± 1.9 | 6.8 ± 1.7 | |
| Weight | 201.7 ± 49.6 | 200.4 ± 49.8 | 203.2 ± 49.8 | 206.8 ± 47.5 | |
| Height | 67.6 ± 4.2 | 68.0 ± 3.8 | 67.1 ± 4.4 | 66.6 ± 5.7 | |
| BMI | 31.1 ± 8.0 | 30.4 ± 6.8 | 31.8 ± 8.0 | 34.0 ± 15.2 | |
| %BF | 34.1 ± 8.2 | 33.7 ± 8.4 | 34.7 ± 7.9 | 36.8 ± 6.1 | |
| Variables | F | p-Value |
|---|---|---|
| Age | 4.369 | 0.014 * |
| 6MWT distance | 0.216 | 0.806 |
| 6MWT Mets | 0.220 | 0.803 |
| Resting heart rate | 1.220 | 0.296 |
| Systolic BP | 0.149 | 0.861 |
| Diastolic BP | 1.821 | 0.163 |
| Limb flexibility | 0.276 | 0.759 |
| Left arm strength | 0.839 | 0.433 |
| Right arm strength | 0.047 | 0.954 |
| BMI | 5.117 | 0.006 ** |
| % of body fat | 2.564 | 0.078 |
| CR Knowledge test | 3.622 | 0.027 * |
| HADS Anxiety | 4.381 | 0.013 * |
| HADS Depression | 4.137 | 0.017 * |
| SF36GH | 6.717 | 0.001 *** |
| SF36PF | 2.966 | 0.052 |
| Variable | Component | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| SF36 GH | 0.872 | −0.094 | 0.066 |
| HADS Depression | −0.852 * | −0.030 | −0.057 |
| HADS Anxiety | −0.808 * | 0.187 | −0.037 |
| SF36 PF | 0.761 * | 0.342 | 0.145 |
| Age | 0.341 | −0.681 * | 0.325 |
| BMI | −0.213 | 0.096 | −0.835 * |
| CR Knowledge test | −0.362 | 0.469 | 0.516 |
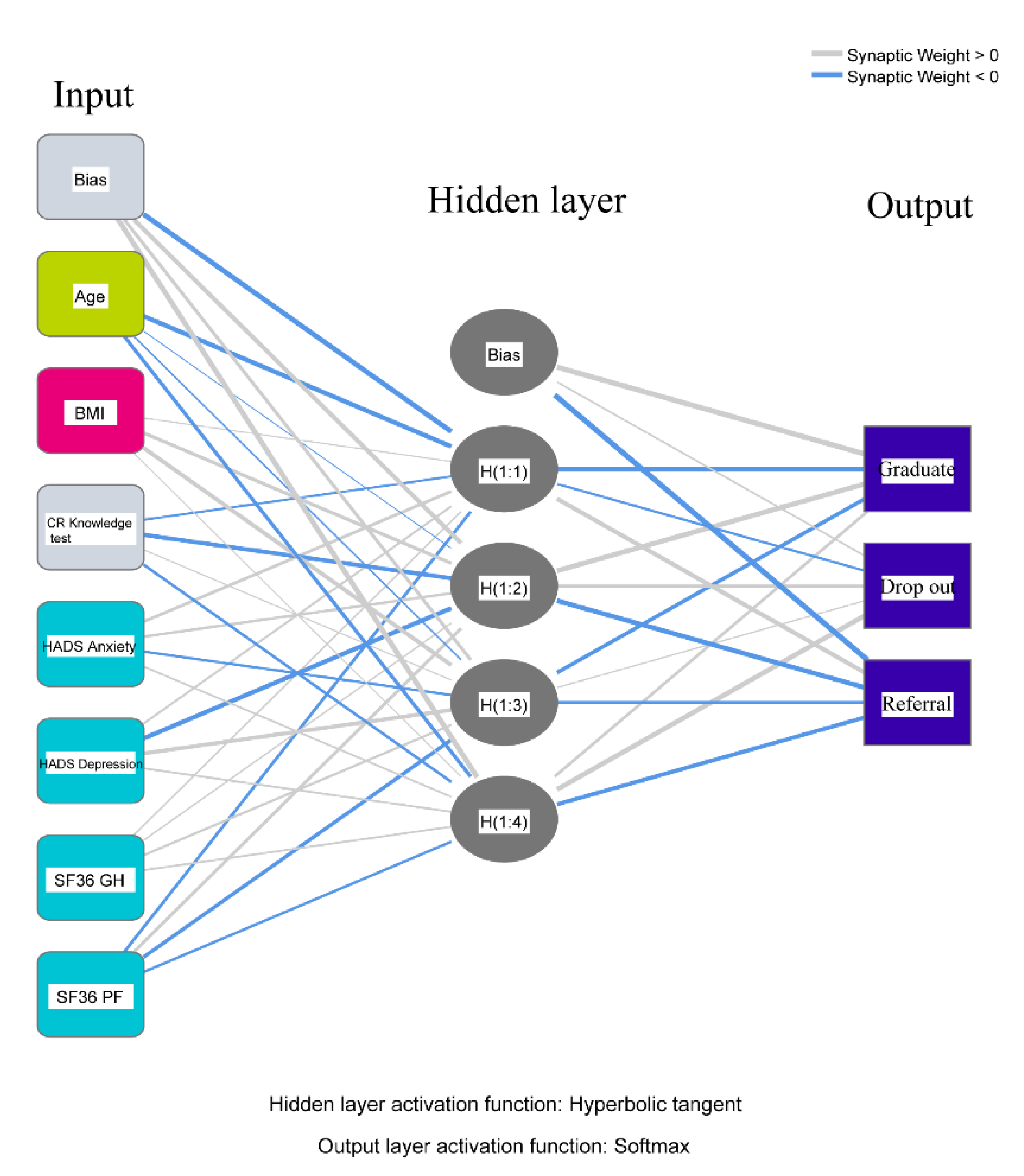
| Predictor | Predicted | |||||||
|---|---|---|---|---|---|---|---|---|
| Hidden Layer 1 | Output Layer | |||||||
| H(1:1) | H(1:2) | H(1:3) | H(1:4) | Graduate | Drop Out | Referral | ||
| Input Layer | (Bias) | −0.991 | 0.508 | 0.351 | 1.082 | |||
| Age | −0.669 | −0.014 | −0.094 | −0.393 | ||||
| BMI | 0.038 | 0.353 | 0.459 | 0.016 | ||||
| CR Knowledge test | −0.165 | −0.504 | 0.030 | −0.247 | ||||
| HADS Anxiety | 0.248 | 0.225 | −0.215 | 0.110 | ||||
| HADS Depression | 0.157 | −0.639 | 0.437 | 0.143 | ||||
| SF36GH | 0.096 | 0.081 | 0.184 | 0.134 | ||||
| SF36PF | −0.270 | 0.285 | −0.402 | −0.233 | ||||
| Hidden Layer 1 | (Bias) | 1.239 | 0.097 | −1.422 | ||||
| H(1:1) | −0.751 | −0.189 | 0.609 | |||||
| H(1:2) | 0.885 | 0.402 | −0.740 | |||||
| H(1:3) | −0.413 | 0.069 | −0.329 | |||||
| H(1:4) | 0.248 | 0.853 | −0.461 | |||||
| Training Model Summary | Value |
| Cross Entropy Error | 42.709 |
| Percent Incorrect Predictions | 19.7% |
| Stopping Rule Used | 1 consecutive step(s) with no decrease in error a |
| Training Time | 0:00:00.02 |
| Testing Model Summary | Value |
| Cross Entropy Error | 19.417 |
| Percent Incorrect Predictions | 22.9% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, L.; Zhang, Q.; Lian, E.; Roberts, K.; Weintraub, N.; Dong, Y.; Zhu, H.; Xu, H.; Schafer, P.; Dunlap, S. Factors Predicting the Utilization of Center-Based Cardiac Rehabilitation Program. Geriatrics 2020, 5, 66. https://doi.org/10.3390/geriatrics5040066
Young L, Zhang Q, Lian E, Roberts K, Weintraub N, Dong Y, Zhu H, Xu H, Schafer P, Dunlap S. Factors Predicting the Utilization of Center-Based Cardiac Rehabilitation Program. Geriatrics. 2020; 5(4):66. https://doi.org/10.3390/geriatrics5040066
Chicago/Turabian StyleYoung, Lufei, Qi Zhang, Eric Lian, Kimberly Roberts, Neal Weintraub, Yanbin Dong, Haidong Zhu, Hongyan Xu, Pascha Schafer, and Stephanie Dunlap. 2020. "Factors Predicting the Utilization of Center-Based Cardiac Rehabilitation Program" Geriatrics 5, no. 4: 66. https://doi.org/10.3390/geriatrics5040066





