Enhancement of Anticipatory Postural Adjustments by Virtual Reality in Older Adults with Cognitive and Motor Deficits: A Randomised Trial
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
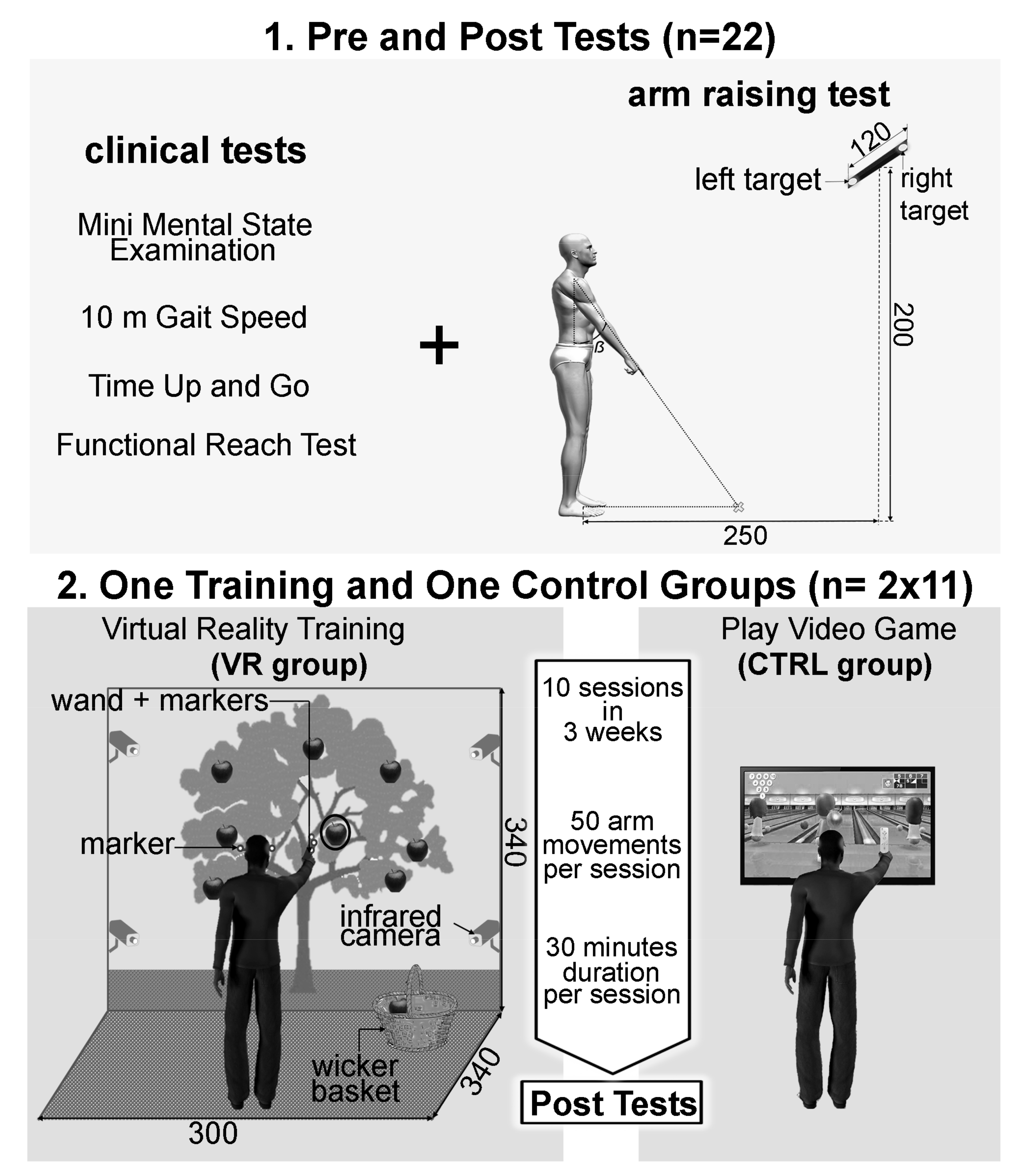
2.2. Experimental Design
2.3. Arm-Raising Procedure and Devices
2.4. Training Phase
- -
- The VR group: In order to propose rehabilitation exercises potentially increasing the management of balance abilities, a VR environment was designed using a visual immersion system, Cave Automatic Virtual Environment (CAVE). This environment is made up of two screens (resolution of 1024 by 768 pixels): (i) the first was a front wall measuring 2.70 m high and 3.40 m wide; and (ii) the second was a floor measuring 3 m depth and 3.40 m wide (Figure 2, panel 2). The interactivity between patients and the virtual environment was supported by two complementary technologies: (i) active stereoscopic vision (NVidia 3D Vision Pro) with special 3D glasses; and (ii) a tracking system composed of 4 infrared cameras (ART DTrack 2) operating at 60 frames per second with a precision of 1 mm. Two parts of the patients’ body are tracked with markers, which allowed an egocentric interaction in the virtual environment. Markers are positioned on the 3D glasses to capture the movements of the participant’s head. Other markers were placed on a wand, which is gripped by the participant to capture the movement of the dominant arm. Participants stood 3 m in front of the front wall screen. Patients received as instruction: “please pick the red apples”. Two different scenarios of the rehabilitation exercises were realized. (A) In the first scenario, called “picking ripe apples”, patients had to perform quite fast arm movements toward the virtual apples (apples played the role of targets). These apples had dynamic changing states: they appeared for the first time in the form of an apple blossom, became green, yellow, and then red, and finally were black apples. The changing state velocity was pseudo-randomized within a 500 to 5000 ms window depending on the patients’ abilities. These pseudo-randomized velocities were chosen by the medical staff during each training session. Once caught, the red apple had to be put in a real wicker basket which was integrated into the action space of the virtual environment in order to reinforce the ecological aspect of the motor tasks. Before each training exercise, the medical staff could adjust several other parameters accordingly to the patients’ state of health on the day: number of apples by exercise, each apples’ positions, the apples’ sizes, and the wicker basket position could be modulated. Overall, from these parameter modulations, the medical staff were able to induce an increase in the arm-raising velocities and thus biomechanically increase the difficulty level of the motor tasks with regard to equilibrium management for the patients. (B) The second scenario was called “the successful picking” and proposed in a virtual environment an apple tree with several red apples that had to be picked and put in the same wicker basket as used in the first scenario until a visual time gauge expired. Once again, medical staff could adjust several parameters in this motor exercise. Using both of these scenarios, the medical staff designed and regulated each training session in order to allow patients to perform 40 arm movements.
- -
- The CTRL group: From an upright standing position, patients played two parts of Wii Sport Bowling (Nintendo Wii), inducing a minimum of 50 arm movements. Such a protocol was systematically proposed for all patients in the Geriatric Medicine and rehabilitation service of Dijon University Hospital, France, in which this clinical research protocol was conducted. For ethical reasons, it was not possible to offer fewer motor stimulations to patients included in this study than the usual care protocols. This is why this group included the control group in the present clinical study.
2.5. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Mañas, L.; Feart, C.; Mann, G.; Vina, J.; Chatterji, S.; Chodzko-Zajko, W.; Harmand, M.G.-C.; Bergman, H.; Carcaillon, L.; Nicholson, C.; et al. Searching for an Operational Definition of Frailty: A Delphi Method Based Consensus Statement. The Frailty Operative Definition-Consensus Conference Project. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2013, 68, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Lobo, A.; Launer, L.J.; Fratiglioni, L.; Andersen, K.; Di Carlo, A.; Breteler, M.M.; Copeland, J.R.; Dartigues, J.F.; Jagger, C.; Martinez-Lage, J.; et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000, 54, 4–9. [Google Scholar]
- Ramaroson, H.; Helmer, C.; Barberger-Gateau, P.; Letenneur, L.; Dartigues, J.-F. Prevalence of dementia and Alzheimer’s disease among subjects aged 75 years or over: Updated results of the PAQUID cohort. Rev. Neurol. 2003, 159, 405–411. [Google Scholar]
- Horlings, C.; Carpenter, M.; Honegger, F.; Allum, J. Vestibular and Proprioceptive Contributions to Human Balance Corrections. Ann. N. Y. Acad. Sci. 2009, 1164, 1–12. [Google Scholar] [CrossRef]
- Buracchio, T.; Dodge, H.H.; Howieson, D.B.; Wasserman, D.; Kaye, J. The Trajectory of Gait Speed Preceding Mild Cognitive Impairment. Arch. Neurol. 2010, 67, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Albers, M.W.; Gilmore, G.; Kaye, J.; Murphy, C.; Wingfield, A.; Bennett, D.A.; Boxer, A.L.; Buchman, A.S.; Cruickshanks, K.J.; Devanand, D.P.; et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 70–98. [Google Scholar] [CrossRef] [Green Version]
- Cadore, E.; Rodríguez-Mañas, L.; Sinclair, A.; Izquierdo, M. Effects of Different Exercise Interventions on Risk of Falls, Gait Ability, and Balance in Physically Frail Older Adults: A Systematic Review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, S.; Gustafson, Y.; Lundin-Olsson, L. Risk factors for falls in people with and without a diagnose of dementia living in residential care facilities: A prospective study. Arch. Gerontol. Geriatr. 2008, 46, 293–306. [Google Scholar] [CrossRef]
- Tang, P.-F.; Woollacott, M.H. Balance Control in Older Adults: Training Effects on Balance Control and the Integration of Balance Control into Walking; Elsevier BV: Amsterdam, The Netherlands, 1996; pp. 339–367. [Google Scholar]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Association of Muscle Strength with the Risk of Alzheimer Disease and the Rate of Cognitive Decline in Community-Dwelling Older Persons. Arch. Neurol. 2009, 66, 1339–1344. [Google Scholar] [CrossRef] [Green Version]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35, ii7–ii11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapley, P.; Pozzo, T.; Grishin, A. The role of anticipatory postural adjustments during whole body forward reaching movements. NeuroReport 1998, 9, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Lepers, R.; Brenière, Y. The role of anticipatory postural adjustments and gravity in gait initiation. Exp. Brain Res. 1995, 107, 118–124. [Google Scholar] [CrossRef]
- Massion, J. Movement, posture and equilibrium: Interaction and coordination. Prog. Neurobiol. 1992, 38, 35–56. [Google Scholar] [CrossRef]
- Gillespie, L.D.; Robertson, M.C.; Gillespie, W.J.; Sherrington, C.; Gates, S.; Clemson, L.M.; E Lamb, S. Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2012, 2021, CD007146. [Google Scholar] [CrossRef]
- Kubicki, A.; Petrement, G.; Bonnetblanc, F.; Ballay, Y.; Mourey, F. Practice-Related Improvements in Postural Control During Rapid Arm Movement in Older Adults: A Preliminary Study. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2011, 67, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanekar, N.; Aruin, A.S. The Effect of a Four-Week Balance Training Program on Anticipatory Postural Adjustments in Older Adults: A Pilot Feasibility Study. Curr. Aging Sci. 2016, 9, 295–300. [Google Scholar] [CrossRef]
- Kubicki, A.; Bonnetblanc, F.; Petrement, G.; Mourey, F. Motor-prediction improvements after virtual rehabilitation in geriatrics: Frail patients reveal different learning curves for movement and postural control. Neurophysiol. Clin. Neurophysiol. 2014, 44, 109–118. [Google Scholar] [CrossRef]
- Rahmani, E.; Boren, S.A. Videogames and Health Improvement: A Literature Review of Randomized Controlled Trials. Games Health J. 2012, 1, 331–341. [Google Scholar] [CrossRef]
- Maillot, P.; Perrot, A.; Hartley, A. Effects of interactive physical-activity video-game training on physical and cognitive function in older adults. Psychol. Aging 2012, 27, 589–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krijn, M.; Emmelkamp, P.; Biemond, R.; Ligny, C.D.W.D.; Schuemie, M.J.; van der Mast, C.A. Treatment of acrophobia in virtual reality: The role of immersion and presence. Behav. Res. Ther. 2004, 42, 229–239. [Google Scholar] [CrossRef]
- Jorgensen, M.G.; Læssøe, U.; Hendriksen, C.; Nielsen, O.B.F.; Aagaard, P. Efficacy of Nintendo Wii Training on Mechanical Leg Muscle Function and Postural Balance in Community-Dwelling Older Adults: A Randomized Controlled Trial. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2012, 68, 845–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Purser, J.L.; Kuchibhatla, M.N.; Fillenbaum, G.G.; Harding, T.; Peterson, E.D.; Alexander, K.P. Identifying Frailty in Hospitalized Older Adults with Significant Coronary Artery Disease. J. Am. Geriatr. Soc. 2006, 54, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar]
- Duncan, P.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional Reach: A New Clinical Measure of Balance. J. Gerontol. 1990, 45, M192–M197. [Google Scholar] [CrossRef]
- Kubicki, A.; Fautrelle, L.; Bourrelier, J.; Rouaud, O.; Mourey, F. The Early Indicators of Functional Decrease in Mild Cognitive Impairment. Front. Aging Neurosci. 2016, 8, 193. [Google Scholar] [CrossRef]
- Fautrelle, L.; Prablanc, C.; Berret, B.; Ballay, Y.; Bonnetblanc, F. Pointing to double-step visual stimuli from a standing position: Very short latency (express) corrections are observed in upper and lower limbs and may not require cortical involvement. Neuroscience 2010, 169, 697–705. [Google Scholar] [CrossRef]
- Woltring, H.J. A Fortran package for generalized, cross-validatory spline smoothing and differentiation. Adv. Eng. Softw. 1986, 8, 104–113. [Google Scholar] [CrossRef]
- Crenna, P.; Frigo, C.A. A motor programme for the initiation of forward-oriented movements in humans. J. Physiol. 1991, 437, 635–653. [Google Scholar] [CrossRef] [PubMed]
- Hislop, H.J.; Montgomery, J. Évaluation des muscles innervés par les nerfs crâniens. Bilan Muscul. Daniels Worthingham 2009, 3, 293–351. [Google Scholar]
- Prablanc, C.; Martin, O. Automatic control during hand reaching at undetected two-dimensional target displacements. J. Neurophysiol. 1992, 67, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Desmurget, M.; Epstein, C.M.; Turner, R.S.; Prablanc, C.; Alexander, G.E.; Grafton, S.T. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat. Neurosci. 1999, 2, 563–567. [Google Scholar] [CrossRef]
- Bonnetblanc, F.; Martin, O.; Teasdale, N. Pointing to a target from an upright standing position: Anticipatory postural adjustments are modulated by the size of the target in humans. Neurosci. Lett. 2004, 358, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Saijo, N.; Murakami, I.; Nishida, S.; Gomi, H. Large-Field Visual Motion Directly Induces an Involuntary Rapid Manual Following Response. J. Neurosci. 2005, 25, 4941–4951. [Google Scholar] [CrossRef] [PubMed]
- Gomi, H. Implicit online corrections of reaching movements. Curr. Opin. Neurobiol. 2008, 18, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, V.; Yakovenko, S.; Kalaska, J.F. Integration of Predictive Feedforward and Sensory Feedback Signals for Online Control of Visually Guided Movement. J. Neurophysiol. 2009, 102, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Fautrelle, L.; Ballay, Y.; Bonnetblanc, F. Muscular synergies during motor corrections: Investigation of the latencies of muscle activities. Behav. Brain Res. 2010, 214, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Set Correlation and Contingency Tables. Appl. Psychol. Meas. 1988, 12, 425–434. [Google Scholar] [CrossRef]
- Ketcham, C.; Seidler, R.; Van Gemmert, A.; Stelmach, G.E. Age-Related Kinematic Differences as Influenced by Task Difficulty, Target Size, and Movement Amplitude. J. Gerontol. Ser. B 2002, 57, P54–P64. [Google Scholar] [CrossRef] [Green Version]
- Bouisset, S.; Do, M.-C. Posture, dynamic stability, and voluntary movement. Neurophysiol. Clin. Neurophysiol. 2008, 38, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.; Simmons, R.; Colberg, S.R.; Parson, H.K.; I Vinik, A. Supervised Balance Training and Wii Fit–Based Exercises Lower Falls Risk in Older Adults with Type 2 Diabetes. J. Am. Med. Dir. Assoc. 2018, 19, 185.e7–185.e13. [Google Scholar] [CrossRef] [PubMed]
- Aruin, A.S.; Kanekar, N.; Lee, Y.-J.; Ganesan, M. Enhancement of anticipatory postural adjustments in older adults as a result of a single session of ball throwing exercise. Exp. Brain Res. 2015, 233, 649–655. [Google Scholar] [CrossRef]
- Curuk, E.; Lee, Y.; Aruin, A.S. Individuals with stroke improve anticipatory postural adjustments after a single session of targeted exercises. Hum. Mov. Sci. 2020, 69, 102559. [Google Scholar] [CrossRef] [PubMed]
- Rossettini, G.; Carlino, E.; Testa, M. Clinical relevance of contextual factors as triggers of placebo and nocebo effects in musculoskeletal pain. BMC Musculoskelet. Disord. 2018, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.K.; Duncan, P.; Chandler, J.; Studenski, S.A. Functional Reach: A Marker of Physical Frailty. J. Am. Geriatr. Soc. 1992, 40, 203–207. [Google Scholar] [CrossRef]
- Newton, R.A. Validity of the Multi-Directional Reach Test: A Practical Measure for Limits of Stability in Older Adults. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2001, 56, M248–M252. [Google Scholar] [CrossRef] [Green Version]
- Ingemarsson, A.H.; Frändin, K.; Hellström, K.; Rundgren, Å. Balance function and fall-related efficacy in patients with newly operated hip fracture. Clin. Rehabil. 2000, 14, 497–505. [Google Scholar] [CrossRef]
- Sattin, R.W.; Easley, K.; Wolf, S.L.; Chen, Y.; Kutner, M.H. Reduction in Fear of Falling Through Intense Tai Chi Exercise Training in Older, Transitionally Frail Adults. J. Am. Geriatr. Soc. 2005, 53, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Levy, F.; Rautureau, G.; Komano, O.; Millet, B.; Jouvent, R.; Leboucher, P. Fear of falling: Efficacy of virtual reality associated with serious games in elderly people. Neuropsychiatr. Dis. Treat. 2016, 12, 877–881. [Google Scholar] [CrossRef] [Green Version]
- Tilborg, I.A.D.A.V.H.-V.; Scherder, E.J.A.; Hulstijn, W. Motor-Skill Learning in Alzheimer’s Disease: A Review with an Eye to the Clinical Practice. Neuropsychol. Rev. 2007, 17, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kizony, R.; Raz, L.; Katz, N.; Weingarden, H.; Weiss, P.L.T. Video-capture virtual reality system for patients with paraplegic spinal cord injury. J. Rehabil. Res. Dev. 2005, 42, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Van Diest, M.; Lamoth, C.J.C.; Stegenga, J.; Verkerke, G.J.; Postema, K. Exergaming for balance training of elderly: State of the art and future developments. J. Neuroeng. Rehabil. 2013, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Park, E.-C.; Kim, S.-G.; Lee, C.-W. The effects of virtual reality game exercise on balance and gait of the elderly. J. Phys. Ther. Sci. 2015, 27, 1157–1159. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourrelier, J.; Fautrelle, L.; Haratyk, E.; Manckoundia, P.; Mérienne, F.; Mourey, F.; Kubicki, A. Enhancement of Anticipatory Postural Adjustments by Virtual Reality in Older Adults with Cognitive and Motor Deficits: A Randomised Trial. Geriatrics 2021, 6, 72. https://doi.org/10.3390/geriatrics6030072
Bourrelier J, Fautrelle L, Haratyk E, Manckoundia P, Mérienne F, Mourey F, Kubicki A. Enhancement of Anticipatory Postural Adjustments by Virtual Reality in Older Adults with Cognitive and Motor Deficits: A Randomised Trial. Geriatrics. 2021; 6(3):72. https://doi.org/10.3390/geriatrics6030072
Chicago/Turabian StyleBourrelier, Julien, Lilian Fautrelle, Etienne Haratyk, Patrick Manckoundia, Frédéric Mérienne, France Mourey, and Alexandre Kubicki. 2021. "Enhancement of Anticipatory Postural Adjustments by Virtual Reality in Older Adults with Cognitive and Motor Deficits: A Randomised Trial" Geriatrics 6, no. 3: 72. https://doi.org/10.3390/geriatrics6030072
APA StyleBourrelier, J., Fautrelle, L., Haratyk, E., Manckoundia, P., Mérienne, F., Mourey, F., & Kubicki, A. (2021). Enhancement of Anticipatory Postural Adjustments by Virtual Reality in Older Adults with Cognitive and Motor Deficits: A Randomised Trial. Geriatrics, 6(3), 72. https://doi.org/10.3390/geriatrics6030072








