The Relationship between Metabolic Syndrome and Frailty in Older People: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Method of Review
2.4. Statistical Analysis
2.5. Sensitivity Analysis
- (1)
- Cross-sectional studies only;
- (2)
- Longitudinal studies only;
- (3)
- Caucasian studies only;
- (4)
- Asian studies only;
- (5)
- High-quality studies (those rated as ‘good’ on the NIH quality assessment tool) vs. lower quality studies (those rated ‘fair’ on the NIH quality assessment tool).
3. Results
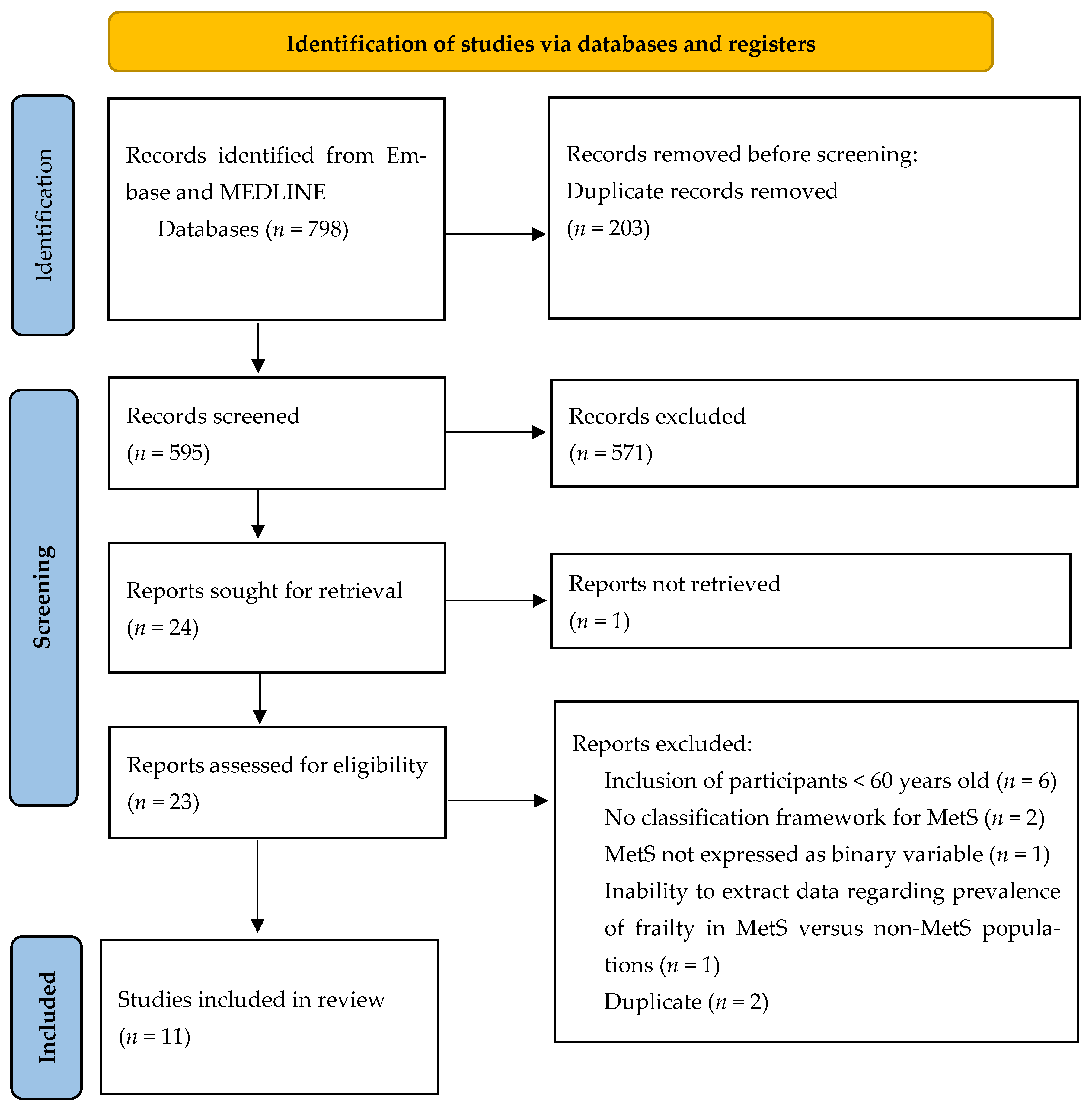
3.1. Study Selection and Characteristics
3.2. The Utilisation of Different Frameworks in Classifying Frailty
3.3. Meta-Analyses
3.4. Sensitivity Analysis
3.5. Risk of Bias Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Author | Criteria 1 | Criteria 2 | Criteria 3 | Criteria 4 | Criteria 5 | Criteria 6 | Criteria 7 | Criteria 8 | Criteria 9 | Criteria 10 | Criteria 11 | Criteria 12 | Criteria 13 | Criteria 14 | Quality Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barzilay 2007 [27] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Good 11 |
| Viscogliosi 2016 [17] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | No | Yes | No | Yes | Yes | Fair 9 |
| Hoogendijk 2017 [18] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good 12 |
| Perez-Tasigchana 2017 [26] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Good 11 |
| Veronese 2017 [19] | Yes | Yes | Yes | Yes | No | Yes | Yes | NA | Yes | Yes | Yes | No | NR | Yes | Good 10 |
| Chao 2019 [20] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | NR | Yes | Good 10 |
| Buchmann 2019 [21] | Yes | Yes | CD | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good 10 |
| Li 2019 [22] | Yes | Yes | Yes | Yes | No | No | CD | NA | Yes | No | Yes | No | NA | Yes | Fair 7 |
| Lee 2020 [24] | Yes | Yes | Yes | Yes | No | No | No | NA | Yes | No | Yes | No | NA | Yes | Fair 7 |
| Merchant 2020 [23] | Yes | Yes | Yes | No | No | No | No | Yes | Yes | No | Yes | No | NA | Yes | Fair 7 |
| Castellana 2021 [25] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | No | Yes | No | NA | Yes | Fair 8 |





References
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; Viveros-Ruiz, T. Recent advances in managing/understanding the metabolic syndrome. F1000Reserch 2019, 8, F1000 Faculty Rev-1370. [Google Scholar] [CrossRef] [Green Version]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef] [Green Version]
- van Vliet-Ostaptchouk, J.V.; Nuotio, M.L.; Slagter, S.N.; Doiron, D.; Fischer, K.; Foco, L.; Gaye, A.; Gögele, M.; Heier, M.; Hiekkalinna, T.; et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr. Disord. 2014, 14, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Márquez-Sandoval, F.; Macedo-Ojeda, G.; Viramontes-Hörner, D.; Fernández Ballart, J.D.; Salas Salvadó, J.; Vizmanos, B. The prevalence of metabolic syndrome in Latin America: A systematic review. Public Health Nutr. 2011, 14, 1702–1713. [Google Scholar] [CrossRef]
- Olijhoek, J.K.; van der Graaf, Y.; Banga, J.-D.; Algra, A.; Rabelink, T.J.; Visseren, F.L.J.; SMART Study Group. The Metabolic Syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. Eur. Heart J. 2004, 25, 342–348. [Google Scholar] [CrossRef]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef]
- Ju, S.-Y.; Lee, J.-Y.; Kim, D.-H. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly: A meta-analysis of prospective cohort studies. Medicine 2017, 96, e8491. [Google Scholar] [CrossRef]
- Dent, E.; Lien, C.; Lim, W.S.; Wong, W.C.; Wong, C.H.; Ng, T.P.; Woo, J.; Dong, B.; de la Vega, S.; Hua Poi, P.J.; et al. The Asia-Pacific Clinical Practice Guidelines for the Management of Frailty. J. Am. Med. Dir. Assoc. 2017, 18, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Mone, P.; Gambardella, J.; Lombardi, A.; Pansini, A.; De Gennaro, S.; Leo, A.L.; Famiglietti, M.; Marro, A.; Morgante, M.; Frullone, S.; et al. Correlation of physical and cognitive impairment in diabetic and hypertensive frail older adults. Cardiovasc. Diabetol. 2022, 21, 10. [Google Scholar] [CrossRef]
- Li, G.; Prior, J.C.; Leslie, W.D.; Thabane, L.; Papaioannou, A.; Josse, R.G.; Kaiser, S.M.; Kovacs, C.S.; Anastassiades, T.; Towheed, T.; et al. Frailty and Risk of Fractures in Patients With Type 2 Diabetes. Diabetes Care 2019, 42, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.S.; Solmi, M.; Schofield, P.; Veronese, N.; Stubbs, B. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Viscogliosi, G. The Metabolic Syndrome: A Risk Factor for the Frailty Syndrome? J. Am. Med. Dir. Assoc. 2016, 17, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Huisman, M.; van Ballegooijen, A.J. The role of frailty in explaining the association between the metabolic syndrome and mortality in older adults. Exp. Gerontol. 2017, 91, 5–8. [Google Scholar] [CrossRef]
- Veronese, N.; Sigeirsdottir, K.; Eiriksdottir, G.; Marques, E.A.; Chalhoub, D.; Phillips, C.L.; Launer, L.J.; Maggi, S.; Gudnason, V.; Harris, T.B. Frailty and Risk of Cardiovascular Diseases in Older Persons: The Age, Gene/Environment Susceptibility-Reykjavik Study. Rejuvenation Res. 2017, 20, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.T.; Lee, Y.H.; Li, C.M.; Han, D.S.; Huang, J.W.; Huang, K.C. Advanced Age and Chronic Kidney Disease Modify the Association Between Metabolic Syndrome and Frailty Among Community-Dwelling Elderly. Rejuvenation Res. 2020, 23, 333–340. [Google Scholar] [CrossRef]
- Buchmann, N.; Spira, D.; Konig, M.; Demuth, I.; Steinhagen-Thiessen, E. Frailty and the Metabolic Syndrome—Results of the Berlin Aging Study II (BASE-II). J. Frailty Aging 2019, 8, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Jiang, X.Y.; Stone, C.; Ma, Y.J.; Liu, Q.; Hu, Z.H.; Li, X.D.; Wang, X.F.; Li, S.J. A new physical-cognitive scale for assessment of frailty in Chinese Han elderly. Neurol. Res. 2019, 41, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.A.; Chan, Y.H.; Lim, J.Y.; Morley, J.E. Prevalence of Metabolic Syndrome and Association with Grip Strength in Older Adults: Findings from the HOPE Study. Diabetes Metab. Syndr. Obes. 2020, 13, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Peng, L.N.; Chen, L.K. Metabolic Syndrome and Its Components Are Associated with Frailty: A Nationwide Population-Based Study in Taiwan. Aging Med. Healthc 2020, 11, 47–52. [Google Scholar] [CrossRef]
- Castellana, F.; Lampignano, L.; Bortone, I.; Zupo, R.; Lozupone, M.; Griseta, C.; Daniele, A.; De Pergola, G.; Giannelli, G.; Sardone, R.; et al. Physical Frailty, Multimorbidity, and All-Cause Mortality in an Older Population From Southern Italy: Results from the Salus in Apulia Study. J. Am. Med. Dir. Assoc. 2021, 22, 598–605. [Google Scholar] [CrossRef]
- Pérez-Tasigchana, R.F.; León-Muñoz, L.M.; Lopez-Garcia, E.; Gutierrez-Fisac, J.L.; Laclaustra, M.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Metabolic syndrome and insulin resistance are associated with frailty in older adults: A prospective cohort study. Age Ageing 2017, 46, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Barzilay, J.I.; Blaum, C.; Moore, T.; Xue, Q.L.; Hirsch, C.H.; Walston, J.D.; Fried, L.P. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch. Intern. Med. 2007, 167, 635–641. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Li, D.; Yu, J.; Liu, Y.; Li, F.; Li, W.; Zhang, Q.; Gao, Y.; Zhang, W.; Zeng, Z.; et al. Subclinical cardiovascular disease and frailty risk: The atherosclerosis risk in communities study. BMC Geriatr. 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Clegg, A.; Hassan-Smith, Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. 2018, 6, 743–752. [Google Scholar] [CrossRef]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic Syndrome and Sarcopenia. Nutrients 2021, 13, 3519. [Google Scholar] [CrossRef]
- Kane, A.E.; Gregson, E.; Theou, O.; Rockwood, K.; Howlett, S.E. The association between frailty, the metabolic syndrome, and mortality over the lifespan. Geroscience 2017, 39, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.Z.; Wong, M.W.K.; Lim, J.Y.; Merchant, R.A. Frailty and Quality of Life in Older Adults with Metabolic Syndrome—Findings from the Healthy Older People Everyday (HOPE) Study. J. Nutr. Health Aging 2021, 25, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Author and Year | Country | Population | Size | Definition of Frailty | Prevalence of Frailty | Prevalence of MetS | Method of Classifying MetS | Association |
|---|---|---|---|---|---|---|---|---|
| Viscogliosi 2016 [17] | Italy | Mean age 76.1 years Outpatients of geriatric clinics (June–December 2015) | 118 | Fried’s criteria | Overall: 35.6% (42/118) In participants with MetS: 60.7% (34/56) In participants without MetS: 12.9% (8/62) | Overall: 47.5% (56/118) | National Cholesterol Education Program (NCEP) Adult Treatment Panel III | Cross-sectional |
| Hoogendijk 2017 [18] | The Netherlands | Mean age 75.4 years Participants of a population based study (The Longitudinal Aging Study Amsterdam) | 1247 | Fried’s criteria | Overall: 11.7% (146/1247) In participants with MetS: 16.7% (77/462) In participants without MetS: 8.8% (69/785) | Overall: 37.1% (462/1247) | National Cholesterol Education Program (NCEP) Adult Treatment Panel III | Cross-sectional |
| Perez-Tasigchana 2017 [26] | Spain | Aged ≥ 60 years Participants of a population based study (The Seniors-ENRICA cohort study) | 1499 | Fried’s criteria | At 3.5 year follow up: Overall: 5.6% (84/1499) In participants with MetS: 8.9% (41/462) In participants without MetS: 4.1% (43/1037) | Overall: 30.8% (462/1499) | Harmonised/joint statement International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity in 2009 | Longitudinal (prospective cohort study), 3.5-year follow up |
| Chao 2019 [20] | Taiwan | Mean age 73.4 years Community-dwelling underwent annual health examinations at National Taiwan University Hospital | 2862 | The Study of Osteoporotic Fractures criteria (SOF) | Overall: 2.6% (73/2862) In participants with MetS: 2.4% (12/502) In participants without MetS: 2.6% (61/2360) | Overall: 17.5% (502/2862) | American Association of Clinical Endocrinologists (AACE) | Cross-sectional |
| Buchmann 2019 [21] | Germany | Mean age 68.7 years Participants of a population based study (The Berlin Aging Study II) | 1486 | Fried’s criteria | Overall: 0.9% (13/1486) In participants with MetS: 1.4% (8/558) In participants without MetS: 0.5% (5/928) | 37.6% (558/1486) | Harmonised/joint statement International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity in 2009 | Cross-sectional |
| Lee 2020 [24] | Taiwan | Mean age 65.8 years Participants of a population based study | 1006 | Frailty Index (35 items, cut-point to define frailty: FI ≥0.25) | Overall: 12.9% 130/1006 In participants with MetS: 16.1% (59/366) In participants without MetS: 11.1% (71/640) | 36.4% (366/1006) | National Cholesterol Education Programme (NCEP) Adult Treatment Panel III (ATP III) guidelines | Cross-sectional |
| Barzilay 2007 [27] | United States | Aged 69–74 years Participants of the Cardiovascular Health Study | 2826 | Fried’s criteria | At 9 years: Overall: 8.3% (234/2826) In participants with MetS: 10.1% (60/596) In participants without MetS: 7.8% (174/2230) | 21.1% (596/2826) | National Cholesterol Education Programme (NCEP) Adult Treatment Panel III (ATP III) guidelines | Longitudinal/ Prospective cohort study |
| Veronese 2017 [19] | Iceland | Mean age 76.2 years Participants of a population based Study—The Age, Gene/Environment Susceptibility (AGES)— Reykjavik Study | 3818 | Fried’s criteria | Overall: 7.9% (300/3818) In participants with MetS: 10.3% (114/1111) In participants without MetS: 6.9% (186/2707) | 29.1% (1111/3818) | National Cholesterol Education Programme (NCEP) Adult Treatment Panel III (ATP III) guidelines | Cross-sectional |
| Li 2019 [22] | China | Mean age 75.3 years Participants of a population based Study (The RuLAS Rugao Longevity and Ageing Study) | 1757 | Fried’s criteria | Overall: 10.1% (178/1757) In participants with MetS: 13.2% (88/665) In participants without MetS: 8.2% (90/1092) | 37.8% (665/1757) | Joint statement between American Heart Association (AHA) and the National Heart, Lung, and Blood Institute (NHLBI) (2005) | Cross-sectional |
| Merchant 2020 [23] | Singapore | Mean age 71 +/− 5 years Participants of a population based study (The HOPE—Healthy Older People Everyday study) | 722 | 5-point FRAIL scale | Overall: 40/722 = 5.5% In participants with MetS: 7.1% (21/296) In participants without MetS: 4.5% (19/426) | 41.0% (296/722) | Modified National Cholesterol Education Programme (NCEP) Adult Treatment Panel III (ATP III) guidelines for Asians | Cross-sectional |
| Castellana 2021 [25] | Italy | Mean age 73.55 years Participants of a population based Study (the Salus in Apulia study) | 1929 | Fried’s criteria | Overall: 14.8% (286/1929) In participants with MetS: 19.1% (43/225) In participants without MetS: 14.3% (243/1704) | 11.7% (225/1929) | Harmonised/joint statement International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity in 2009 | Cross-sectional |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dao, H.H.H.; Burns, M.J.; Kha, R.; Chow, C.K.; Nguyen, T.N. The Relationship between Metabolic Syndrome and Frailty in Older People: A Systematic Review and Meta-Analysis. Geriatrics 2022, 7, 76. https://doi.org/10.3390/geriatrics7040076
Dao HHH, Burns MJ, Kha R, Chow CK, Nguyen TN. The Relationship between Metabolic Syndrome and Frailty in Older People: A Systematic Review and Meta-Analysis. Geriatrics. 2022; 7(4):76. https://doi.org/10.3390/geriatrics7040076
Chicago/Turabian StyleDao, Hiep Huu Hoang, Mason Jenner Burns, Richard Kha, Clara K. Chow, and Tu Ngoc Nguyen. 2022. "The Relationship between Metabolic Syndrome and Frailty in Older People: A Systematic Review and Meta-Analysis" Geriatrics 7, no. 4: 76. https://doi.org/10.3390/geriatrics7040076
APA StyleDao, H. H. H., Burns, M. J., Kha, R., Chow, C. K., & Nguyen, T. N. (2022). The Relationship between Metabolic Syndrome and Frailty in Older People: A Systematic Review and Meta-Analysis. Geriatrics, 7(4), 76. https://doi.org/10.3390/geriatrics7040076








