Early Feasibility of an Activity-Based Intervention for Improving Ingestive Functions in Older Adults with Oropharyngeal Dysphagia
Abstract
:1. Introduction
- (1)
- Does the ACT-ING program meet a set of a priori feasibility marks?
- (2)
- Is the ACT-ING program perceived as usable and acceptable by older adults with OD who are participating in the intervention?
- (3)
- Have the putative mediators of change of the ACT-ING program been accomplished?
2. Materials and Methods
2.1. Design
2.2. Setting
2.3. Participants
2.4. Intervention
2.5. Data Collection
2.5.1. Baseline Assessment
2.5.2. Feasibility Marks
- The demand for intervention by the target group was assessed as the proportion of eligible participants who were invited and agreed to participate. The success criterion was a proportion of ≥70%.
- Retention was assessed as the proportion of enrolled participants who completed the post-intervention assessments. The success criterion was a proportion of ≥85%.
- Intervention adherence was assessed as the proportion of enrolled participants who attended at least 75% of the planned therapy sessions. Adherence to self-training was assessed as the proportion of enrolled participants who completed at least 75% of their weekly food diary. The success criterion for both was a proportion of ≥70%.
- Safety was assessed during each therapy session using records on clinical signs of aspiration (e.g., wet voice, throat clearing, coughing, or gagging) which might increase briefly during exercise progression but are expected to decrease as the participants’ skills increase [41]. The success criterion was that clinical signs of aspiration occurred in less than 20% of the therapeutic swallowing attempts in 80% of the therapy sessions for 100% of the participants.
- Adverse events were assessed using records of any unexpected and unintended serious events related to the ingestion of training material during the therapy sessions (e.g., food allergy symptoms, severe pain, choking, and apnea). The success criterion was no record of adverse events.
- Tolerance was assessed at the end of each therapy session, when participants rated their experienced level of concern for aspiration on a 100 mm visual analog scale (VAS) with a horizontal line (left side = not concerned at all (0 mm); right side = extremely concerned (100 mm)). The distance from the left edge of the line to the mark placed by the participant was measured to the nearest millimeter and used in the analyses. The success criterion was that 80% of the aspiration concern VASs were ≤70 mm for at least 85% of participants.
2.5.3. Usability and Acceptability
- Intervention usability was assessed post intervention by the Intrinsic Motivation Inventory (IMI) "Value/usefulness” subscale with seven items addressing the content and level of motivation that a participant experiences during an intervention [42]. Items are scored on a seven-point Likert scale ranging from 1 (not at all true) to 7 (very true). A neutral score on the IMI is four (somewhat true), with a higher score indicative of a more positive result for motivation. The success criterion was that the “Value/usefulness” subscale score was >4 (average score across 7 items) for 100% of the participants.
- Acceptability was assessed during each intervention using fieldnote records of participants’ reactions and post intervention by a series of evaluation questions with a blend of closed- and open-ended questions based on the Theoretical Framework of Acceptability (TFA), which covers seven dimensions of intervention acceptability: (1) affective attitude (how the participant feels about it), (2) burden (perceived amount of effort required to participate), (3) ethicality (whether it fits with the participant’s value system), (4) intervention coherence (whether the participant understands it and how it works), (5) opportunity costs (whether benefits, profits, or values must be given up for participation), (6) perceived effectiveness (whether it is perceived as likely to achieve its purpose), and (7) self-efficacy (whether the participant has confidence in his/her own ability to perform the actions required to participate) [43]. The criterion was that the participants’ responses reflected that the intervention was acceptable.
2.5.4. Putative Mediators of Change
- The satisfaction of basic psychological needs was assessed post intervention by the Basic Psychological Needs in Exercise Scale (BPNES) [44], a participant-reported questionnaire concerning the extent to which the innate psychological need for autonomy (4 items), competence (4 items), and relatedness (4 items) are satisfied in the intervention. Items are rated on a five-point Likert scale ranging from 1 (do not agree at all) to 5 (completely agree), with higher scores indicating a high degree of satisfaction of basic needs. It was expected that the scores for the three dimensions were > 3 (the average score across four items for each dimension).
- In-therapy engagement was assessed during each therapy session using records on accompanying worksheets for the key features of the intervention in terms of external exercise loads reflecting practice complexity (task hierarchy levels exercised), practice variability (number of task hierarchy levels per session), practice distribution (number of sets per session), and practice amount (number of swallows across sets and sessions). The internal exercise load after each set was also obtained by the OMNI Perceived Exertion Scale for Resistance Exercise (OMNI-RES), ranging from 0 (extremely easy) to 10 (extremely hard) [45]. It was expected that all key features were implemented across the intervention.
- The perceived swallowing capacity when ingesting liquids and foods was assessed at baseline and post-intervention using a 100 mm VAS scale (left side (0) = unable to swallow; right side (100 mm) = no difficulties). The distance from the left edge of the line to the mark placed by the participant was measured to the nearest millimeter and used in the analyses. It was expected that the participants perceived their swallowing capacity to have improved.
2.5.5. Procedure
2.5.6. Data Analysis
3. Results
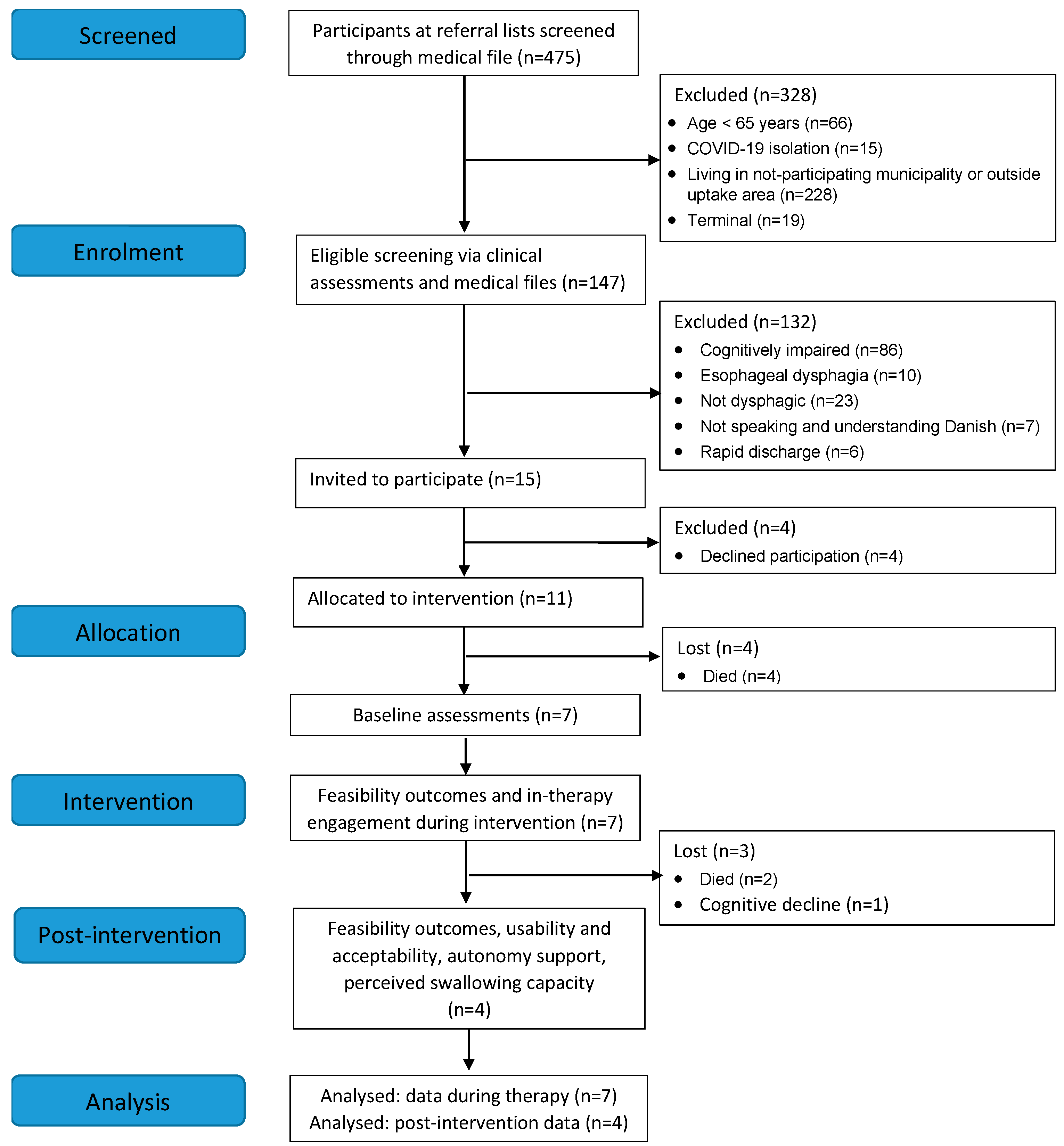
3.1. Demand for the Intervention and Retention
3.2. Baseline Characteristics and Assessments
3.3. Intervention Adherence
3.4. Safety, Adverse Events, and Tolerance
3.5. Intervention Usability and Acceptability
3.6. Putative Mediators of Change
4. Discussion
Methodological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajati, F.; Ahmadi, N.; Naghibzadeh, Z.A.; Kazeminia, M. The global prevalence of oropharyngeal dysphagia in different populations: A systematic review and meta-analysis. J. Transl. Med. 2022, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Ferrillo, M.; Lippi, L.; Agostini, F.; de Sire, R.; Ferrara, P.E.; Raguso, G.; Riso, S.; Roccuzzo, A.; Ronconi, G.; et al. Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review. Nutrients 2022, 14, 982. [Google Scholar] [CrossRef] [PubMed]
- Dibello, V.; Zupo, R.; Sardone, R.; Lozupone, M.; Castellana, F.; Dibello, A.; Daniele, A.; De Pergola, G.; Bortone, I.; Lampignano, L.; et al. Oral frailty and its determinants in older age: A systematic review. Lancet Healthy Longev. 2021, 2, e507–e520. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Kishima, M.; Itoda, M.; Fujishima, I.; Kunieda, K.; Ohno, T.; Shigematsu, T.; Oshima, F.; Mori, T.; Ogawa, N.; et al. Diagnosis and Treatment of Sarcopenic Dysphagia: A Scoping Review. Dysphagia 2021, 36, 523–531. [Google Scholar] [CrossRef]
- Nagano, A.; Ueshima, J.; Tsutsumichi, K.; Inoue, T.; Shimizu, A.; Mori, N.; Maeda, K. Effect of tongue strength on clinical outcomes of patients: A systematic review. Arch. Gerontol. Geriatr. 2022, 102, 104749. [Google Scholar] [CrossRef]
- Mizuno, S.; Wakabayashi, H.; Wada, F. Rehabilitation nutrition for individuals with frailty, disability, sarcopenic dysphagia, or sarcopenic respiratory disability. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 29–36. [Google Scholar] [CrossRef]
- Baumann, C.W.; Clark, B.C.; Phillips, B.E.; Szewczyk, N.J.; Consitt, L.A. Regenerative Rehabilitation in Sarcopenia, Dynapenia, and Frailty. In Regenerative Rehabilitation. From Basic Science to the Clinic, 1st ed.; Greising, S.M., Call, J.A., Eds.; Springer: Cham, Switzerland, 2022; pp. 121–176. [Google Scholar]
- Kagaya, H.; Inamoto, Y. Possible Rehabilitation Procedures to Treat Sarcopenic Dysphagia. Nutrients 2022, 14, 778. [Google Scholar] [CrossRef]
- Jannah, S.N.; Syahrul, S.; Kadar, K. The Effectiveness of Tongue Strengthening Exercise in Increasing Tongue Strength Among Older People with Dysphagia: A Systematic Review. Health Sci. Rev. 2022, 4, 100047. [Google Scholar] [CrossRef]
- Krekeler, B.N.; Rowe, L.M.; Connor, N.P. Dose in Exercise-Based Dysphagia Therapies: A Scoping Review. Dysphagia 2021, 36, 1–32. [Google Scholar] [CrossRef]
- Huckabee, M.L.; Flynn, R.; Mills, M. Expanding Rehabilitation Options for Dysphagia: Skill-Based Swallowing Training. Dysphagia 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Zimmerman, E.; Carnaby, G.; Lazarus, C.L.; Malandraki, G.A. Motor learning, neuroplasticity, and strength and skill training: Moving from compensation to retraining in behavioral management of dysphagia. Am. J. Speech. Lang. Pathol. 2020, 29, 1065–1077. [Google Scholar] [CrossRef]
- Carnaby, G.D.; LaGorio, L.; Silliman, S.; Crary, M. Exercise-based swallowing intervention (McNeill Dysphagia Therapy) with adjunctive NMES to treat dysphagia post-stroke: A double-blind placebo-controlled trial. J. Oral Rehabil. 2020, 47, 501–510. [Google Scholar] [CrossRef]
- Carnaby-Mann, G.D.; Crary, M.A. Adjunctive neuromuscular electrical stimulation for treatment-refractory dysphagia. Ann. Otol. Rhinol. Laryngol. 2008, 117, 279–287. [Google Scholar] [CrossRef]
- Hansen, T.; Thomassen, J.D.; Jensen, L.E.; Irgens, M.R.; Kjaersgaard, A. Development of an Intervention for Improving Ingestion in Elders with Oropharyngeal Dysphagia. Phys. Occup. Ther. Geriatr. 2021, 39, 70–95. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ (Clin. Res. Ed.) 2014, 348, g1687. [Google Scholar] [CrossRef]
- Mathiowetz, B. Task oriented approach to stroke rehabilitation. In Stroke Rehabilitation: A Function-Based Approach, 4th ed.; Gillen, G., Ed.; Elsevier Inc.: St. Louis, MD, USA, 2016; pp. 59–78. [Google Scholar]
- Hosseini, S.A.; Ghamari, N.; Hayatizadeh, Z. Review of task-oriented interventions in occupational therapy. J. Rehab. Med. 2017, 6, 239–248. [Google Scholar]
- Forsgren, E.; Åke, S.; Saldert, C. Person-centred care in speech-language therapy research and practice for adults: A scoping review. Int. J. Lang. Commun. Disord 2022, 57, 381–402. [Google Scholar] [CrossRef]
- Ryan, R.M.; Deci, E.L. Self-Determination Theory: Basic Psychological Needs in Motivation Development and Wellness, 1st ed.; Guilford Press: New York, NY, USA, 2017. [Google Scholar]
- Blamey, A.A.M.; MacMillan, F.; Fitzsimons, C.F.; Shaw, R.; Mutrie, N. Using programme theory to strengthen research protocol and intervention design within an RCT of a walking intervention. Evaluation 2013, 19, 5–23. [Google Scholar] [CrossRef]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.; et al. A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. BMJ 2021, 374, n2061. [Google Scholar] [CrossRef]
- Czajkowski, S.M.; Hunter, C.M. From ideas to interventions: A review and comparison of frameworks used in early phase behavioral translation research. Health Psychol. 2021, 40, 829–844. [Google Scholar] [CrossRef]
- Gonot-Schoupinsky, F.N.; Garip, G. A flexible framework for planning and evaluating early-stage health interventions: FRAME-IT. Eval. Program Plann. 2019, 77, 101685. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.K. Case Study Research and Applications: Design and Methods, 6th ed.; SAGE Publications, Inc.: Los Angeles, CA, USA, 2018. [Google Scholar]
- Creswell, J. Designing and Conducting Mixed Methods Research, 3rd ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2017. [Google Scholar]
- Schmidt, M.; Schmidt, S.A.J.; Adelborg, K.; Sundbøll, J.; Laugesen, K.; Ehrenstein, V.; Sørensen, H.T. The Danish health care system and epidemiological research: From health care contacts to database records. Clin. Epidemiol. 2019, 11, 563–591. [Google Scholar] [CrossRef] [PubMed]
- Umay, E.; Eyigor, S.; Karahan, A.Y.; Gezer, I.A.; Kurkcu, A.; Keskin, D.; Karaca, G.; Unlu, Z.; Tıkız, C.; Vural, M.; et al. The GUSS test as a good indicator to evaluate dysphagia in healthy older people: A multicenter reliability and validity study. Eur. Geriatr. Med. 2019, 10, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Crary, M.A.; Mann, G.D.; Groher, M.E. Initial Psychometric Assessment of a Functional Oral intake Scale for Dysphagia in Stroke Patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Bahia, M.M.; Lowell, S.Y. A Systematic Review of the Physiological Effects of the Effortful Swallow Maneuver in Adults with Normal and Disordered Swallowing. Am. J. Speech. Lang. Pathol. 2020, 29, 1655–1673. [Google Scholar] [CrossRef]
- The International Dysphagia Diet Standardisation Initiative (IDDSI). Complete IDDSI Framework: Detailed Definitions, Version 2.0. Published 2019. Available online: https://ftp.iddsi.org/Documents/Complete_IDDSI_Framework_Final_31July2019.pdf (accessed on 7 August 2019).
- Hall, W.H.; Ramachandran, R.; Narayan, S.; Jani, A.B.; Vijayakumar, S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer 2004, 4, 94. [Google Scholar] [CrossRef]
- Bahat, G.; Erdoğan, T.; İlhan, B. SARC-F and other screening tests for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 37–42. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Lee, S.H.; Gong, H.S. Measurement and Interpretation of Handgrip Strength for Research on Sarcopenia and Osteoporosis. J. Bone Metab. 2020, 27, 85–96. [Google Scholar] [CrossRef]
- Franciotti, R.; Di Maria, E.; D’Attilio, M.; Aprile, G.; Cosentino, F.G.; Perrotti, V. Quantitative Measurement of Swallowing Performance Using Iowa Oral Performance Instrument: A Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 2319. [Google Scholar] [CrossRef]
- IOPI Medical. Available online: https://iopimedical.com/normal-values/ (accessed on 12 January 2022).
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA®-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Hansen, T.; Melgaard Kristiansen, D. Revisiting the psychometric properties of a revised Danish version of the McGill ingestive skills assessment. Cogent Med. 2017, 4, 1–14. [Google Scholar] [CrossRef]
- Beets, M.W.; von Klinggraeff, L.; Weaver, R.G.; Armstrong, B.; Burkart, S. Small studies, big decisions: The role of pilot/feasibility studies in incremental science and premature scale-up of behavioral interventions. Pilot Feasibil. Stud. 2021, 7, 173. [Google Scholar] [CrossRef]
- Crary, M.A.; Carnaby, G.D. Adoption into clinical practice of two therapies to manage swallowing disorders: Exercise-based swallowing rehabilitation and electrical stimulation. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 172–180. [Google Scholar] [CrossRef]
- Center for Self-Determination Theory. Intrinsic Motivation Inventory (IMI). Available online: https://selfdeterminationtheory.org/intrinsic-motivation-inventory/ (accessed on 5 January 2020).
- Sekhon, M.; Cartwright, M.; Francis, J.J. Acceptability of healthcare interventions: An overview of reviews and development of a theoretical framework. BMC Health Serv. Res. 2017, 17, 88. [Google Scholar] [CrossRef]
- Vlachopoulos, S.P.; Michailidou, S. Development and initial validation of a measure of autonomy, competence, and relatedness in exercise: The Basic Psychological Needs in Exercise Scale. Meas. Phys. Educ. Exerc. Sci. 2006, 10, 179–201. [Google Scholar] [CrossRef]
- Lagally, K.M.; Robertson, R.J. Construct validity of the OMNI resistance exercise scale. J. Strength Cond. Res. 2006, 20, 252–256. [Google Scholar] [CrossRef]
- Ose, S.O. Using Excel and Word to structure qualitative data. J. Appl. Soc. Sci. 2016, 10, 147–162. [Google Scholar] [CrossRef]
- Forsat, N.D.; Palmowski, A.; Palmowski, Y.; Boers, M.; Buttgereit, F. Recruitment and Retention of Older People in Clinical Research: A Systematic Literature Review. J. Am. Geriatr. Soc. 2020, 68, 2955–2963. [Google Scholar] [CrossRef]
- Ganz, J.B.; Ayres, K.M. Methodological standards in single-case experimental design: Raising the bar. Res. Dev. Disabil. 2018, 79, 3–9. [Google Scholar] [CrossRef]
- Collado-Mateo, D.; Lavín-Pérez, A.M.; Peñacoba, C.; Del Coso, J.; Leyton-Román, M.; Luque-Casado, A.; Gasque, P.; Fernández-Del-Olmo, M.Á.; Amado-Alonso, D. Key Factors Associated with Adherence to Physical Exercise in Patients with Chronic Diseases and Older Adults: An Umbrella Review. Int. J. Environ. Res. Public Health 2021, 18, 2023. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.C.; Raatz, M.; Marshall, J.; Wishart, L.R.; Burns, C.L. Telepractice and Dysphagia Management: The Era of COVID-19 and Beyond. Dysphagia 2022, 37, 1386–1399. [Google Scholar] [CrossRef] [PubMed]
- Dalton, E.J.; Churilov, L.; Lannin, N.A.; Corbett, D.; Campbell, B.C.V.; Hayward, K.S. Multidimensional Phase I Dose Ranging Trials for Stroke Recovery Interventions: Key Challenges and How to Address Them. Neurorehabil. Neural Repair 2021, 35, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Bryant, L.; Hemsley, B. The true cost of dysphagia on quality of life: The views of adults with swallowing disability. Int. J. Lang. Commun. Disord. 2023, 58, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Bick, S.; Buxton, H.; Chase, R.P.; Ross, I.; Adriano, Z.; Capone, D.; Knee, J.; Brown, J.; Nalá, R.; Cumming, O.; et al. Using path analysis to test theory of change: A quantitative process evaluation of the MapSan trial. BMC Public Health 2021, 21, 1411. [Google Scholar] [CrossRef]
- Abu-Ghanem, S.; Graf, A.; Govind, J. Diagnosis of Sarcopenic Dysphagia in the Elderly: Critical Review and Future Perspectives. Dysphagia 2022, 37, 1093–1102. [Google Scholar] [CrossRef]
- Rossi, V.; Pourtois, G. Transient state-dependent fluctuations in anxiety measured using STAI, POMS, PANAS or VAS: A comparative review. Anxiety Stress. Coping 2012, 25, 603–645. [Google Scholar] [CrossRef]
- Baamer, R.M.; Iqbal, A.; Lobo, D.N.; Knaggs, R.D.; Levy, N.A.; Toh, L.S. Utility of unidimensional and functional pain assessment tools in adult postoperative patients: A systematic review. Br. J. Anaesth. 2022, 128, 874–888. [Google Scholar] [CrossRef]
- Facco, E.; Zanette, G.; Favero, L.; Bacci, C.; Sivolella, S.; Cavallin, F.; Manani, G. Toward the validation of visual analogue scale for anxiety. Anesth. Prog. 2011, 58, 8–13. [Google Scholar] [CrossRef]

| Case | Age | Gender | Admission Diagnosis | aCCI | GUSS | SARC-F | HGS | MTP | MNA-SF | MISA2 | FOIS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 86 | Male | Pneumonia | 7 | 18 | 7 | 20 | 20 | 4 | 82 | 5 |
| 2 | 87 | Female | Duodenal ulcer | 7 | 18 | 8 | 20 | 28 | 8 | 73 | 4 |
| 3 | 84 | Female | Aspiration pneumonia | 5 | 13 | 9 | 12.5 | 20 | 8 | 77 | 4 |
| 5 | 85 | Female | Diabetes mellitus | 7 | 3 | 8 | N/A | 16 | 6 | 42 | 1 |
| 7 | 86 | Male | Dehydration | 6 | 9 | 6 | 19.5 | 13 | 6 | 71 | 3 |
| 8 | 78 | Female | HNC sequelae # | 6 | 19 | 5 | 18.5 | 33 | 12 | 86 | 5 |
| 10 | 67 | Female | HNC sequelae # | 5 | 19 | 7 | 25 | 36 | 9 | 88 | 5 |
| Case | |||||||
|---|---|---|---|---|---|---|---|
| Ingestive Skills | 1 | 2 | 3 | 5 | 7 | 8 | 10 |
| Seals lips on cup/glass/utensil | x | x | |||||
| Controls liquid bolus in mouth before swallowing | x | x | |||||
| Uses functional chewing pattern | x | x | x | x | x | x | |
| Controls solid bolus in mouth before swallowing | x | ||||||
| Brings bolus into a cohesive unit | x | x | x | x | x | ||
| Transport bolus backwards in mouth | x | x | x | x | |||
| Swallows without extra effort | x | x | x | x | x | x | x |
| Swallows only once or twice | x | x | x | x | |||
| Maintains respiratory pattern | x | x | x | x | x | x | |
| Protects the airway from penetration/aspiration | x | x | x | x | x | x | x |
| Coughs or clears the airway efficiently if needed | x | x | x | ||||
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Attended/Planned (Adherence) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | IEE | EEE | EE | EE | CC | CC | EE | EC | EE | T | 15/20 (75%) | ||
| Case 2 | IEE | EE | CC | EC | EC | CC | CC | EE | EE | EF | 13/21 (62%) | ||
| Case 3 | IEE | EE | EE | EE | EC | EE | EE | EE | EE | EC | CC | F | 20/24 (83%) |
| Case 5 | I | IEE | EEE | EEE | EEE | EEE | EEE | ECC | EEE | EEE | T | 26/28 (93%) | |
| Case 7 | IEE | EE | EE | EE | EE | T | 11/11 (100%) | ||||||
| Case 8 | I | EE | EE | CC | EC | CC | EE | EC | EC | EC | EC | F | 13/22 (59%) |
| Case 10 | I | CC | IE | CC | EE | EC | EC | EC | CC | EC | F | 10/20 (50%) |
| Case ID | Safety Clinical Signs of Aspiration | Tolerance Aspiration Concern * |
|---|---|---|
| Therapy Sessions with <20% Aspiration N (%) | Therapy Sessions with VAS ≤ 70 mm N (%) | |
| Case 1 | 14/14 (100%) | 12/12 (100%) |
| Case 2 | 11/11 (100%) | 6/7 (85.7%) |
| Case 3 | 18/18 (100%) | 18/18 (100%) |
| Case 5 | 20/24 (83.3%) | 12/17 (70.6%) |
| Case 7 | 8/10 (80.0%) | 5/6 (83.3%) |
| Case 8 | 11/11 (100%) | 11/11 (100%) |
| Case 10 | 7/7 (100%) | 7/7 (100%) |
| Criterion | ≥80% of the therapy sessions for all participants | ≥80% of the therapy sessions for 85% of the participants |
| Case 2 | Case 3 | Case 8 | Case 10 | |
|---|---|---|---|---|
| Usefulness IMI: Mean (SD) | 7.00 (0.00) | 6.86 (0.38) | 6.00 (0.58) | 7.00 (0.00) |
| TFA domains [43]. | ||||
| Affective attitude Pleasurable Security | “It was cozy sitting with the foods and liquids”… “looked forward to the meetings”. “I felt secure that the therapist from the hospital was available during course of therapy”. | “It was exciting… inspired to buy some of the given food items”. | “Wonderful with the different taste samples”. “I think it has been good… There hasn’t been anything I didn’t like”. | “I liked getting the good advices”… “and the delicious food”… “I am so grateful for the help”. |
| Burden Appropriate Structure minimizes failure Diary burdensome | Duration, frequency, session length were rated appropriate. “The food diary was hard to remember”. | Duration, frequency, session length were rated appropriate. “The weekly visits helped correcting what I did wrong”. “The food diary was difficult”. | Duration, frequency, session length were rated appropriate. “The food diary was hard to remember”. | Duration, frequency, session length were rated appropriate. “I’ve worked with what I had been taught and then we have talked about it next time”. |
| Ethicality Support | “The therapy was quiet and not stressful… Not disturbing that therapist observed me during therapy- but if strangers … then I’m full”. | “No one has ever talked to me about my swallowing problem… I have been embarrassed by the way I eat… It helps me when there are someone helping me with it”. | “Being provided with good explanations on my problems and how to overcome them”. | “When I commit to something, I do it 100%.”… “How on earth would I have learned it on my own?”… “Being taken seriously is motivating”. |
| Intervention coherence Attentional focus Training materials assist in learning | “I constantly think about what I have learned and that I must bow my head and swallow hard… and then have breaks”. | “To try things out and talk about it”… “I had to be conscious in the beginning”. | “Using the various food and liquid samples were pleasant… help to experience that I could ingest more without pain”. | “Swallowing consciously and in small bites to begin with”. “The different foods and liquids have been very important—how would I have learned it without”. |
| Opportunity costs Flexible schedule | “Nothing has been given up”. | “It has not been a problem”… “We have solved it by looking in my calendar”. | “I have not given anything up to engage in the program… we have planned it”. | “We have solved it… It has been easy to fit the program into my daily life”. |
| Perceived effectiveness Improvements of ingestive skills | “Earlier, I coughed it up again… I have got my life back”. | “I am feeling better”… “It has helped me to eat properly- to drink something- to swallow the food”. | “Opening my mouth more widely when taking in foods and chewing has become better”. | “I can see that it helped, much more than I could imagine”… “It has really helped me. I do not choke that much anymore”. |
| Self-efficacy Capacity | Difficulty levels of training materials were rated appropriate. “I experienced it easy”. | Difficulty levels of training materials were rated appropriate. “In the beginning, I just had to be conscious…, but now it had become a habit to chew and swallow more normal”… “it has become a routine”. | Difficulty levels of training materials were rated appropriate. “There have been no obstacles”… “Chewing more texture without a feeling of danger”… “Feeling progression… then you want more”. | Difficulty levels of training materials were rated appropriate. “The effortful swallow became a routine quickly… although the therapist is not here, I still have to avoid my old way of eating”. |
| Case 1 | Case 2 | Case 3 | Case 5 | Case 7 | Case 8 | Case 10 | |
|---|---|---|---|---|---|---|---|
| BPNES (mean (SD)) | |||||||
| Autonomy | N/A | 4.50 (1.00) | 4.75 (0.50) | N/A | N/A | 4.50 (0.58) | 5.00 (0.00) |
| Competency | N/A | 4.75 (0.50) | 4.75 (0.50) | N/A | N/A | 4.50 (1.00) | 4.75 (0.50) |
| Relatedness | N/A | 5.00 (0.00) | 5.00 (0.00) | N/A | N/A | 5.00 (0.00) | 5.00 (0.00) |
| In-therapy engagement (median, min–max) | |||||||
| Task difficulty across sessions/practice complexity | 9 (1–16) | 10 (1–17) | 10 (1–17) | 2 (1–8) | 2 (1–4) | 13 (1–16) | 14 (1–17) |
| No. of task levels across sessions/practice variability | 5 (1–8) | 4 (1–6) | 5 (1–6) | 2 (1–3) | 5 (2–7) | 5 (2–7) | 4 (3–6) |
| No. of sets across sessions/practice distribution | 7 (2–11) | 5 (2–6) | 10 (1–15) | 5 (1–11) | 5 (1–8) | 6 (3–10) | 7 (5–8) |
| Swallow repetitions across sets/practice amount | 7 (3–9) | 7 (4–12) | 8 (5–12) | 5 (4–11) | 5 (4–9) | 8 (4–10) | 7 (5–11) |
| Swallow repetitions across sessions/practice amount | 46 (6–74) | 30 (10–53) | 87 (9–130) | 25 (5–61) | 25 (4–44) | 55 (12–84) | 44 (36–67) |
| Perceived swallowing capacity (pre/post-test) | |||||||
| Liquids: 100 mm VAS | N/A | 47/96 | 26/92 | N/A | N/A | 95/99 | 67/100 |
| Foods: 100 mm VAS | N/A | 19/66 | 26/83 | N/A | N/A | 71/99 | 19/100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, T.; Laursen, L.B.; Hansen, M.S. Early Feasibility of an Activity-Based Intervention for Improving Ingestive Functions in Older Adults with Oropharyngeal Dysphagia. Geriatrics 2023, 8, 44. https://doi.org/10.3390/geriatrics8020044
Hansen T, Laursen LB, Hansen MS. Early Feasibility of an Activity-Based Intervention for Improving Ingestive Functions in Older Adults with Oropharyngeal Dysphagia. Geriatrics. 2023; 8(2):44. https://doi.org/10.3390/geriatrics8020044
Chicago/Turabian StyleHansen, Tina, Louise Bolvig Laursen, and Maria Swennergren Hansen. 2023. "Early Feasibility of an Activity-Based Intervention for Improving Ingestive Functions in Older Adults with Oropharyngeal Dysphagia" Geriatrics 8, no. 2: 44. https://doi.org/10.3390/geriatrics8020044
APA StyleHansen, T., Laursen, L. B., & Hansen, M. S. (2023). Early Feasibility of an Activity-Based Intervention for Improving Ingestive Functions in Older Adults with Oropharyngeal Dysphagia. Geriatrics, 8(2), 44. https://doi.org/10.3390/geriatrics8020044





