Contribution of Different Brain Disorders and Multimorbidity to Delirium Superimposed Dementia (DSD)
Abstract
:1. Introduction
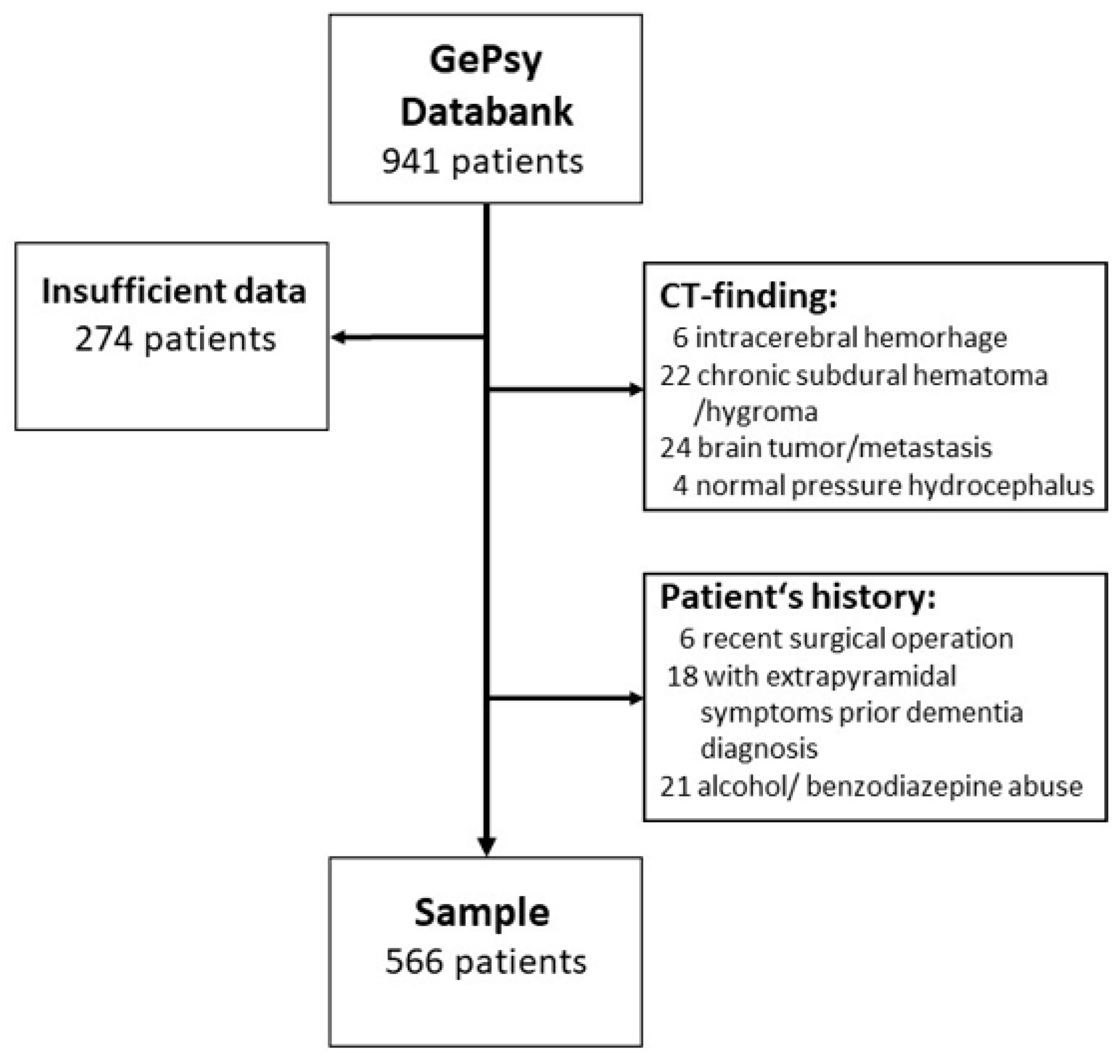
2. Materials and Methods
2.1. Subjects
2.2. Measures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morandi, A.; Lucchi, E.; Turco, R.; Morghen, S.; Guerini, F.; Santi, R.; Gentile, S.; Meagher, D.; Voyer, P.; Fick, D.M.; et al. Delirium superimposed on dementia: A quantitative and qualitative evaluation of informal caregivers and health care staff experience. J. Psychosom. Res. 2015, 79, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Fick, D.M.; Agostini, J.V.; Inouye, S.K. Delirium superimposed on dementia: A systematic review. J. Am. Geriatr. Soc. 2002, 50, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Y.C.; Rodrigues, N.G.; Klainin-Yobas, P.; Haugan, G.; Wu, X.V. Prevalence, risk factors, and Iipact of delirium on hospitalized older adults with dementia: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2022, 23, 23–32. [Google Scholar] [CrossRef]
- Fong, T.G.; Inouye, S.K. The inter-relationship between delirium and dementia: The importance of delirium prevention. Nat. Rev. Neurol. 2022, 18, 579–596. [Google Scholar] [CrossRef]
- Goldberg, T.E.; Chen, C.; Wang, Y.; Jung, E.; Swanson, A.; Ing, C.; Garcia, P.S.; Whittington, R.A.; Moitra, V. Association of delirium with long-term cognitive decline: A meta-analysis. JAMA Neurol. 2020, 77, 1373–1381. [Google Scholar] [CrossRef]
- Morandi, A.; Bellelli, G. Delirium superimposed on dementia. Eur. Geriatr. Med. 2020, 11, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.V.; Aung Thein, M.Z.; Nitchingham, A.; Caplan, G.A. Delirium in older adults is associated with development of new dementia: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2021, 36, 993–1003. [Google Scholar] [CrossRef]
- Tsui, A.; Searle, S.D.; Bowden, H.; Hoffmann, K.; Hornby, J.; Goslett, A.; Weston-Clarke, M.; Howes, L.H.; Street, R.; Perera, R.; et al. The effect of baseline cognition and delirium on long-term cognitive impairment and mortality: A prospective population-based study. Lancet Healthy Longev. 2022, 3, e232–e241. [Google Scholar] [CrossRef]
- Nitchingham, A.; Caplan, G.A. Current challenges in the recognition and management of delirium superimposed on dementia. Neuropsychiatr. Dis. Treat. 2021, 17, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Press: Washington, DC, USA, 2013. [Google Scholar]
- WHO. ICD-10. Geneve. Available online: https://apps.who.int/iris/handle/10665/37958 (accessed on 12 April 2023).
- WHO. ICD-11. Geneve. Available online: https://icd.who.int/browse11/l-m/en (accessed on 12 April 2023).
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef] [Green Version]
- Marcantonio, E.R. Delirium in hospitalized older adults. N. Engl. J. Med. 2017, 377, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular cognitive impairment and dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 3326–3344. [Google Scholar] [CrossRef] [PubMed]
- Kalvas, L.B.; Monroe, T.B. Structural brain changes in delirium: An integrative review. Biol. Res. Nurs. 2019, 21, 355–365. [Google Scholar] [CrossRef]
- Hasegawa, N.; Hashimoto, M.; Yuuki, S.; Honda, K.; Yatabe, Y.; Araki, K.; Ikeda, M. Prevalence of delirium among outpatients with dementia. Int. Psychogeriatr. 2013, 25, 1877–1883. [Google Scholar] [CrossRef] [Green Version]
- Bir, S.C.; Khan, M.W.; Javalkar, V.; Toledo, E.G.; Kelley, R.E. Emerging concepts in vascular dementia: A review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105864. [Google Scholar] [CrossRef] [PubMed]
- Cannistraro, R.J.; Badi, M.; Eidelman, B.H.; Dickson, D.W.; Middlebrooks, E.H.; Meschia, J.F. CNS small vessel disease: A clinical review. Neurology 2019, 92, 1146–1156. [Google Scholar] [CrossRef]
- Clancy, U.; Gilmartin, D.; Jochems, A.C.C.; Knox, L.; Doubal, F.N.; Wardlaw, J.M. Neuropsychiatric symptoms associated with cerebral small vessel disease: A systematic review and meta-analysis. Lancet Psychiatry 2021, 8, 225–236. [Google Scholar] [CrossRef]
- Nitchingham, A.; Kumar, V.; Shenkin, S.; Ferguson, K.J.; Caplan, G.A. A systematic review of neuroimaging in delirium: Predictors, correlates and consequences. Int. J. Geriatr. Psychiatry 2018, 33, 1458–1478. [Google Scholar] [CrossRef]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Violan, C.; Foguet-Boreu, Q.; Flores-Mateo, G.; Salisbury, C.; Blom, J.; Freitag, M.; Glynn, L.; Muth, C.; Valderas, J.-M. Prevalence, determinants and patterns of multimorbidity in primary care: A systematic review of observational studies. PLoS ONE 2014, 9, e102149. [Google Scholar] [CrossRef]
- Wetterling, T.; Junghanns, K. Does multimorbidity in older psychiatric patients lead to higher transfer rates between psychiatric and somatic departments? Z. Gerontol. Geriatr. 2019, 52, 568–574. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical MANUAL of Mental Disorders: DSM IV TR, 4th ed.; American Psychiatric Press: Washington, DC, USA, 2000. [Google Scholar]
- Inouye, S.K.; van Dyck, C.H.; Alessi, C.A.; Balkin, S.; Siegal, A.P.; Horwitz, R.I. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann. Intern. Med. 1990, 113, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Hestermann, U.; Backenstrass, M.; Gekle, I.; Hack, M.; Mundt, C.; Oster, P.; Thomas, C. Validation of a German version of the Confusion Assessment Method for delirium detection in a sample of acute geriatric patients with a high prevalence of dementia. Psychopathology 2009, 42, 270–276. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; Mittal, D.; Torres, R.; Kanary, K.; Norton, J.; Jimerson, N. Validation of the Delirium Rating Scale-revised-98: Comparison with the delirium rating scale and the cognitive test for delirium. J. Neuropsychiatry Clin. Neurosci. 2001, 3, 229–242. [Google Scholar] [CrossRef]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Salvi, F.; Miller, M.D.; Grilli, A.; Giorgi, R.; Towers, A.L.; Morichi, V.; Spazzafumo, L.; Mancinelli, L.; Espinosa, E.; Rappelli, A.; et al. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J. Am. Geriatr. Soc. 2008, 56, 1926–1931. [Google Scholar] [CrossRef]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Guy, W. Clinical global impression scale. In ECDEU Assessment Manual for Psychopharmacology; U.S. Department of Health, Education, and Welfare: Rockville, MD, USA, 1976. [Google Scholar]
- Krzych, Ł.J.; Rachfalska, N.; Putowski, Z. Delirium superimposed on dementia in perioperative period and intensive care. J. Clin. Med. 2020, 9, 3279. [Google Scholar] [CrossRef]
- Fick, D.M.; Steis, M.R.; Waller, J.L.; Inouye, S.K. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J. Hosp. Med. 2013, 8, 500–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.; Kreisel, S.H.; Oster, P.; Driessen, M.; Arolt, V.; Inouye, S.K. Diagnosing delirium in older hospitalized adults with dementia: Adapting the confusion assessment method to international classification of diseases, tenth revision, diagnostic criteria. J. Am. Geriatr. Soc. 2012, 60, 1471–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, A.L.; Tommet, D.; D’Aquila, M.; Schmitt, E.; Marcantonio, E.R.; Helfand, B.; Inouye, S.K.; Jones, R.N.; BASIL Study Group. Harmonization of delirium severity instruments: A comparison of the DRS-R-98, MDAS, and CAM-S using item response theory. BMC Med. Res. Methodol. 2018, 18, 92. [Google Scholar] [CrossRef] [Green Version]
- Persico, I.; Cesari, M.; Morandi, A.; Haas, J.; Mazzola, P.; Zambon, A.; Annoni, G.; Bellelli, G. Frailty and delirium in older adults: A systematic review and meta-analysis of the literature. J. Am. Geriatr. Soc. 2018, 66, 2022–2030. [Google Scholar] [CrossRef]
- Morandi, A.; Davis, D.; Fick, D.M.; Turco, R.; Boustani, M.; Lucchi, E.; Guerini, F.; Morghen, S.; Torpilliesi, T.; Gentile, S.; et al. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation inpatients. J. Am. Med. Dir. Assoc. 2014, 15, 349–354. [Google Scholar] [CrossRef]
- Lawson, R.A.; McDonald, C.; Burn, D.J. Defining delirium in idiopathic Parkinson’s disease: A systematic review. Park. Relat. Disord. 2019, 64, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.; Lawson, R.A.; Price, A.; Taylor, J.P. Challenges in diagnosis and management of delirium in Lewy body disease. Acta Psychiatr. Scand. 2022, 147, 475–480. [Google Scholar] [CrossRef]
- Wang, S.; Lindroth, H.; Chan, C.; Greene, R.; Serrano-Andrews, P.; Khan, S.; Rios, G.; Jabbari, S.; Lim, J.; Saykin, A.J.; et al. A systematic review of delirium biomarkers and their alignment with the NIA-AA research framework. J. Am. Geriatr. Soc. 2021, 69, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.G.; Vasunilashorn, S.M.; Libermann, T.; Marcantonio, E.R.; Inouye, S.K. Delirium and Alzheimer disease: A proposed model for shared pathophysiology. Int. J. Geriatr. Psychiatry 2019, 34, 781–789. [Google Scholar] [CrossRef] [PubMed]
| Other Diagnoses | Dementia Alone | Delirium Alone | Delirium Super- Imposed Dementia | Statistics ANOVA | |
|---|---|---|---|---|---|
| n | 197 | 105 | 46 | 218 | |
| Age (range 66–101) | 76.6 ± 7.5 | 80.1 ± 7.2 | 77.5 ± 8.0 | 76.6 ± 7.5 | F = 20.8 df 3 p < 0.001 |
| Gender [male] | 31.5% | 30.5% | 34.8% | 33.9% | Chi2 0.61 df 3 p = 0.895 |
| CDR (max. 3) | 0.1 ± 0.2 | 1.6 ± 0.6 | 0.2 ± 0.3 | 2.2 ± 0.8 | F = 508.8 df 3 p < 0.001 |
| CAM-S (max. 7) | 2.2 ± 1.6 | 3.4 ± 1.3 | 5.5 ± 1.4 | 5.9 ± 1.7 | F = 211.8 df 3 p < 0.001 |
| DRS-98 (max. 16) | 5.2 ± 2.4 | 7.8 ± 2.0 | 11.1 ± 2.4 | 11.4 ± 2.5 | F = 250.7 df 3 p < 0.001 |
| CIRS 13 (Max. 52) | 12.7 ± 3.5 | 13.3 ± 3.8 | 13.4 ± 4.0 | 12.9 ± 3.8 | F = 36.9 df 3 p = 0.890 |
| cCT findings | |||||
| Infarcts | 18 (22.0%) | 18 (17.1%) | 7 (15.2%) | 39 (17.9%) | Chi2 7.21 df 3 p = 0.066 |
| WMH | 104 (52.8%) | 68 (64,8%) | 29 (63.0%) | 123 (56.4%) | Chi2 4.71 df 3 p = 0.194 |
| Dementia Alone | Delirium Superimposed Dementia | Statistics | |
|---|---|---|---|
| n | 105 | 218 | |
| Age (range 66–101) | 80.1 ± 7.2 | 76.6 ± 7.5 | ANOVA F = 5.97 df 1 p = 0.015 |
| Gender [male] | 30.2 | 33.9% | Odds ratio 0.85 (95% CI 0.51…1.41) |
| CDR (max. 3) | 1.6 ± 0.6 | 2.2 ± 0.8 | ANOVA F = 44.9 df = 1 p < 0.001 |
| Multimorbidity | |||
| Number of ICD-10 diagnoses | 10.5 ± 3.3 | 11.1 ± 3.7 | ANOVA F= 1.93 df = 1 p = 0.165 |
| CIRS-13 (max. 52) | 13.3 ± 3.8 | 12.9 ± 3.8 | ANOVA F = 0.81 df = 1 p = 0.370 |
| MNA- SF (max. 14) | 7.3 ± 1.6 | 6.5 ± 1.7 | ANOVA F = 18.3 df = 1 p < 0.001 |
| Disability | |||
| Visual impairment | 8 (7.6%) | 19 (8.7%) | Odds ratio 1.16 (95% CI 0.49…2.74) |
| Hearing loss | 16 (15.2%) | 46 (21.1%) | Odds ratio 1.49 (95% CI 0.80…2.78) |
| Restricted mobility | 30 (28.6%) | 58 (26.6%) | Odds ratio 0.91 (95% CI 0.54…1.52) |
| cCT findings | |||
| Infarcts | 18 (17.1%) | 39 (17.9%) | Odds ratio 1.05 (95% CI 0.57…1.95) |
| WMH | 68 (64.8%) | 123 (56.4%) | Odds ratio 0.73 (95% CI 0.46…1.19) |
| cCT-Finding | Atrophy Only | Infarction | WMH | Statistics |
|---|---|---|---|---|
| n | 56 | 39 | 123 | |
| Age [years] | 79.9 ± 6.6 | 82.8 ± 7.4 | 83.0 ± 7.0 | F = 4.00 df 2 p = 0.020 |
| Gender [% male] | 41.1% | 38.5% | 29.3% | Chi2 2.82 df 2 p = 0.244 |
| CDR (max. 3) | 2.3 ± 0.7 | 2.3 ± 0.7 | 2.1 ± 0.8 | F = 3.25 df 2 p = 0.040 |
| CAM-S (max. 7) | 6.0 ± 1.8 | 5.9 ± 1.5 | 5.9 ± 1.7 | F = 0.04 df 2 p= 0.961 |
| DRS-98 (max. 16) | 11.4 ± 2.7 | 12.1 ± 2.5 | 11.2 ± 2.3 | F = 1.96 df 2 p = 0.143 |
| Multimorbidity | ||||
| Number of ICD-10 diagnoses | 10.7 ± 4.0 | 11.6 ± 3.3 | 11.2 ± 3.7 | F = 0.83 df 2 p = 0.438 |
| CIRS-13 (max. 52) | 12.8 ± 3.7 | 14.1 ± 3.5 | 12.5 ± 4.0 | F = 2,78 df 2 p = 0.064 |
| MNA-SF (max. 14) | 6.4 ± 1.5 | 6.6 ± 1.7 | 6.5 ± 1.7 | F = 0.19 df 2 p = 0.829 |
| Disability | ||||
| Visual impairment | 2 (3.6%) | 8 (20.5%) | 9 (7.3%) | Chi2 8.99 df 2 p = 0.011 |
| Hearing loss | 8 (14.3%) | 9 (23.1%) | 29 (23.6%) | Chi2 2.11 df 2 p = 0.349 |
| Restricted mobility | 19 (33.9%) | 14 (35.9%) | 25 (20.3%) | Chi2 5.75 df 2 p = 0.057 |
| Dementia Alone | Delirium Superimposed Dementia | Statistics | |
|---|---|---|---|
| Elevated Hba1c (>6.5%) | 48 (45.7%) | 73 (33.5%) | Odds ratio 0.60 (95% CI 0.37 …0.96) |
| Leukocytosis (>9000/µL) | 15 (14.3%) | 37 (17.0%) | Odds ratio 1,23 (95% CI 0.64 …2.35) |
| Anemia (Hb females < 12 g/L, males < 14 g/L) | 60 (57.1%) | 121 (55.5%) | Odds ratio 0.94 (95% CI 0.59 …1.50) |
| Hematocrit (%) | 15 (14.3%) | 41 (18.8%) | Odds ratio 1.39 (95% CI 0.73 …2.65) |
| Hyponatremia (<135 mmol/L) | 7 (6.7%) | 17 (7.8%) | Odds ratio 1.18 (95% CI 0.48 …2.95) |
| Elevated creatinine (>90 mg/L) | 47 (44.8%) | 92 (42.2%) | Odds ratio 0.90 (95% CI 0.56 …1.44) |
| TSH (<0.25 µU/mL) | 9 (8.6%) | 20 (9.2%) | Odds ratio 1.08 (95% CI 0.47 …2.46) |
| Current Infection | 29 (27.6%) | 86 (39.4%) | Odds ratio 1.71 (95% CI 1.03 …2.83) |
| Dehydration | 22 (21.0%) | 57 (26.1%) | Odds ratio 1.33 (95% CI 0.76 …2.34) |
| Medication | |||
| Psychotropic drugs | 1.7 ± 1.1 | 1.9 ± 1.0 | ANOVA F 2.08 df 2 p = 0.150 |
| Other medication | 4.8 ± 2.9 | 4.5 ± 2.6 | ANOVA F 0.99 df 2 p = 0.320 |
| cCT Finding | No Or Atrophy | Infarction | WMH | Statistics |
|---|---|---|---|---|
| Elevated Hba1c (>6.5%) | 19 (33.9%) | 10 (25.6%) | 44 (35.8%) | Chi2 1.37 df 2 p = 0.504 |
| Leukocytosis (>9000/µL) | 6 (10.7%) | 6 (15.4%) | 25 (20.3%) | Chi2 2.61 df 2 p = 0.272 |
| Anemia (Hb females < 12 g/ L, males < 14 g/L) | 36 (64.3%) | 23 (59.0%) | 62 (50.4%) | Chi2 3.23 df 2 p = 0.199 |
| Hematocrit (%) | 7 (12.5%) | 8 (20.5%) | 26 (21.1%) | Chi2 1.97 df 2 p = 0.373 |
| Hyponatremia (>135 mmol/L) | 3 (5.4%) | 4 (10.3%) | 10 (8.1%) | Chi2 0.81 df 2 p = 0.667 |
| Elevated creatinine (>90 mg/L) | 21 (37.5%) | 14 (35.9%) | 57 (46.3%) | Chi2 2.00 df 2 p = 0.365 |
| TSH (<0.25 µU/mL) | 5 (8.9%) | 1 (2.6%) | 14 (11.4%) | Chi2 2.77 df 2 p = 0.250 |
| Current infection | 18 (32.1%) | 18 (46.2%) | 50 (40.7%) | Chi2 2.00 df 2 p = 0.357 |
| Dehydration | 13 (23.2%) | 9 (15.8%) | 35 (28.5%) | Chi2 0.78 df 2 p = 0.677 |
| Restricted mobility | 19 (33.9%) | 14 (35.9%) | 25 (20.3%) | Chi2 5.75 df 2 p = 0.057 |
| Medication | ||||
| Psychotropic drugs (range 0–4) | 2.3 ± 1.0 | 1.7 ± 1.0 | 1.8 ± 1.0 | ANOVA F = 6.10 df 2 p = 0.003 |
| Other medication (range 0–15) | 3.5 ± 2.1 | 4.5 ± 2.8 | 5.0 ± 2.7 | ANOVA F = 6.10 df 2 p = 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wetterling, T.; Junghanns, K. Contribution of Different Brain Disorders and Multimorbidity to Delirium Superimposed Dementia (DSD). Geriatrics 2023, 8, 64. https://doi.org/10.3390/geriatrics8030064
Wetterling T, Junghanns K. Contribution of Different Brain Disorders and Multimorbidity to Delirium Superimposed Dementia (DSD). Geriatrics. 2023; 8(3):64. https://doi.org/10.3390/geriatrics8030064
Chicago/Turabian StyleWetterling, Tilman, and Klaus Junghanns. 2023. "Contribution of Different Brain Disorders and Multimorbidity to Delirium Superimposed Dementia (DSD)" Geriatrics 8, no. 3: 64. https://doi.org/10.3390/geriatrics8030064
APA StyleWetterling, T., & Junghanns, K. (2023). Contribution of Different Brain Disorders and Multimorbidity to Delirium Superimposed Dementia (DSD). Geriatrics, 8(3), 64. https://doi.org/10.3390/geriatrics8030064






