The Risk of Drug Interactions in Older Primary Care Patients after Hospital Discharge: The Role of Drug Reconciliation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
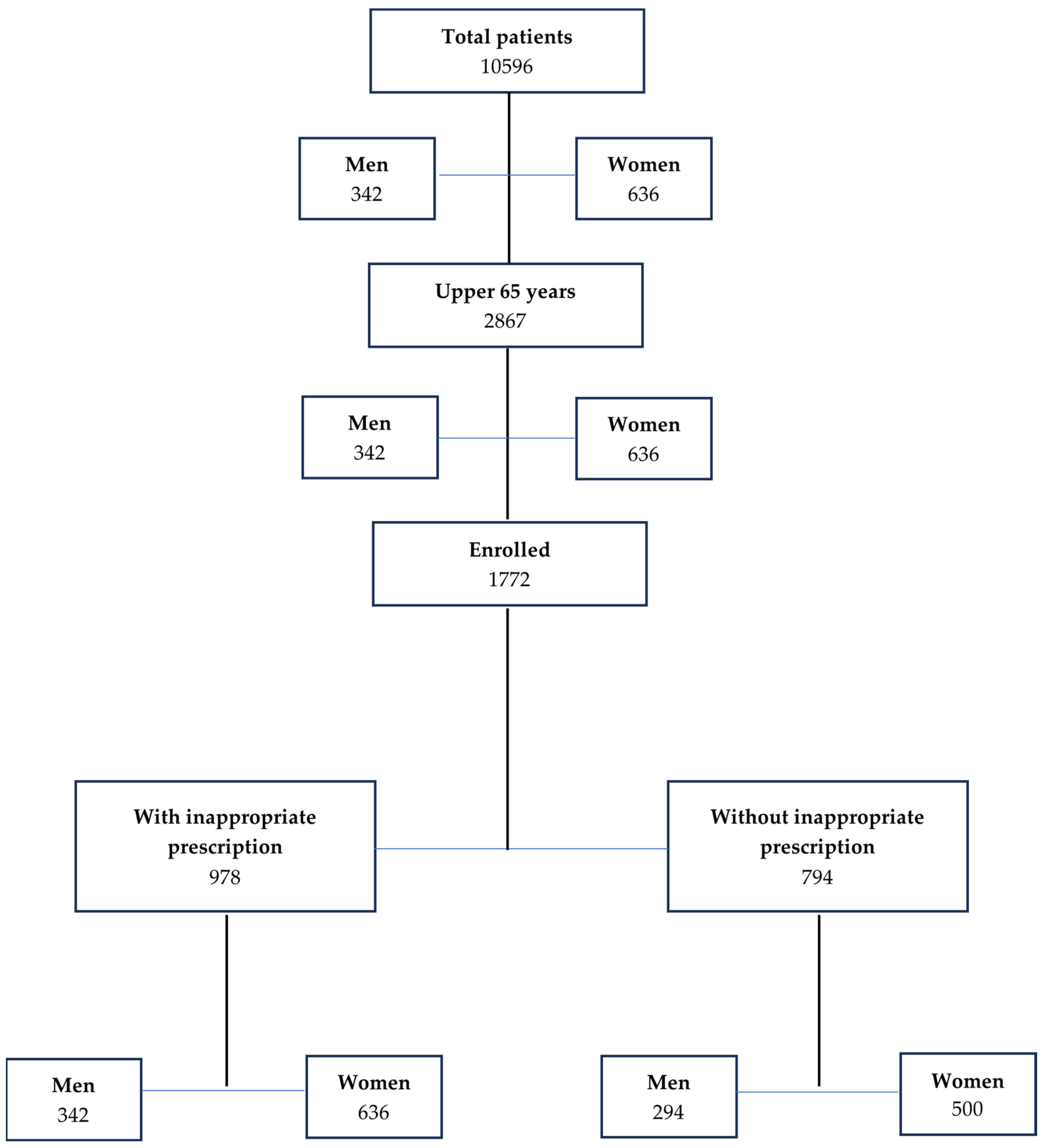
2.2. Protocol
2.3. Inclusion and Exclusion Criteria
2.4. Endpoints
2.5. Ethical Considerations
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Inappropriate Drug Prescription
3.3. DIPS Score
3.4. Risk Evaluation
4. Discussion
4.1. Proton Pump Inhibitors
4.2. Statins
4.3. Clopidogrel
4.4. Antimicrobial Drugs
4.5. SSRI
4.6. NSAIDs and Pain Killers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wastesson, J.W.; Morin, L.; Tan, E.C.K.; Johnell, K. An update on the clinical consequences of polypharmacy in older adults: A narrative review. Expert Opin. Drug Saf. 2018, 17, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.W. Probable adverse drug reactions in a rural geriatric nursing home population: A four-year study. J. Am. Geriatr. Soc. 1996, 44, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Lavan, A.H.; Gallagher, P. Predicting risk of adverse drug reactions in older adults. Ther. Adv. Drug Saf. 2016, 7, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; Siniscalchi, A.; Palleria, C.; Mumoli, L.; Staltari, O.; Squillace, A.; Maida, F.; Russo, E.; Gratteri, S.; De Sarro, G.; et al. Adverse drug reactions related to drug administration in hospitalized patients. Curr. Drug Saf. 2017, 12, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Rochon, P.A.; Gurwitz, J.H. Optimising drug treatment for elderly people: The prescribing cascade. BMJ 1997, 315, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic drug-drug interaction and their implication in clinical management. J. Res. Med. Sci. 2013, 18, 601–610. [Google Scholar]
- Di Mizio, G.; Marcianò, G.; Palleria, C.; Muraca, L.; Rania, V.; Roberti, R.; Spaziano, G.; Piscopo, A.; Ciconte, V.; Di Nunno, N.; et al. Drug–Drug Interactions in Vestibular Diseases, Clinical Problems, and Medico-Legal Implications. Int. J. Environ. Res. Public Health 2021, 18, 12936. [Google Scholar] [CrossRef]
- Rajska-Neumann, A.; Wieczorowska-Tobis, K. Polypharmacy and potential inappropriateness of pharmaco-logical treatment among community-dwelling elderly patients. Arch. Gerontol. Geriatr. 2007, 44 (Suppl. S1), 303–309. [Google Scholar] [CrossRef]
- Zhan, C.; Sangl, J.; Bierman, A.S.; Miller, M.R.; Friedman, B.; Wickizer, S.W.; Meyer, G.S. Potentially inappropriate medication use in the community-dwelling elderly: Findings from the 1996 Medical Expenditure Panel Survey. JAMA 2001, 286, 2823–2829. [Google Scholar] [CrossRef]
- Simon, S.R.; Chan, K.A.; Soumerai, S.B.; Wagner, A.K.; Andrade, S.E.; Feldstein, A.C.; Lafata, J.E.; Davis, R.L.; Gurwitz, J.H. Potentially inappropriate medication use by elderly persons in U.S. Health Maintenance Organizations, 2000–2001. J. Am. Geriatr. Soc. 2005, 53, 227–232. [Google Scholar] [CrossRef]
- Curtis, L.H.; Østbye, T.; Sendersky, V.; Hutchison, S.; Dans, P.E.; Wright, A.; Woosley, R.L.; Schulman, K.A. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch. Intern. Med. 2004, 164, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Roughead, E.E.; Anderson, B.; Gilbert, A.L. Potentially inappropriate prescribing among Australian veterans and war widows/widowers. Intern. Med. J. 2007, 37, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Breuker, C.; Macioce, V.; Mura, T.; Castet-Nicolas, A.; Audurier, Y.; Boegner, C.; Jalabert, A.; Villiet, M.; Avignon, A.; Sultan, A. Medication Errors at Hospital Admission and Discharge. J. Patient Saf. 2017, 17, e645–e652. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, L.; Alexandra, J.F.; Tesmoingt, C.; Eerdekens, C.; Macrez, A.; Papo, T.; Arnaud, P.; Papy, E. Medication reconciliation: A prospective study in an internal medicine unit. Drugs Aging 2014, 31, 387–393. [Google Scholar] [CrossRef]
- Chiewchantanakit, D.; Meakchai, A.; Pituchaturont, N.; Dilokthornsakul, P.; Dhippayom, T. The effectiveness of medication reconciliation to prevent medication error: A systematic review and meta-analysis. Res. Soc. Adm. Pharm. 2020, 16, 886–894. [Google Scholar] [CrossRef]
- Gallelli, L.; Cione, E.; Siniscalchi, A.; Vasta, G.; Guerra, A.; Scaramuzzino, A.; Longo, L.; Muraca, L.; De Sarro, G.; “G & SP” Working Group; et al. Is there a Link between Non Melanoma Skin Cancer and Hydrochlorothiazide? Curr. Drug Saf. 2022, 17, 211–216. [Google Scholar] [CrossRef]
- Rende, P.; Paletta, L.; Gallelli, G.; Raffaele, G.; Natale, V.; Brissa, N.; Costa, C.; Gratteri, S.; Giofrè, C.; Gallelli, L. Retrospective evaluation of adverse drug reactions induced by antihypertensive treatment. J. Pharmacol. Pharmacother. 2013, 4, 47–50. [Google Scholar] [CrossRef]
- Gallelli, L.; Ferreri, G.; Colosimo, M.; Pirritano, D.; Flocco, M.A.; Pelaia, G.; Maselli, R.; De Sarro, G.B. Retrospective analysis of adverse drug reactions to bronchodilators observed in two pulmonary divisions of Catanzaro, Italy. Pharmacol. Res. 2003, 47, 493–499. [Google Scholar] [CrossRef]
- Gallelli, L.; Colosimo, M.; Pirritano, D.; Ferraro, M.; De Fazio, S.; Marigliano, N.M.; De Sarro, G. Retrospective evaluation of adverse drug reactions induced by nonsteroidal anti-inflammatory drugs. Clin. Drug Investig. 2007, 27, 115–122. [Google Scholar] [CrossRef]
- Horn, J.R.; Hansten, P.D.; Chan, L.N. Proposal for a new tool to evaluate drug interaction cases. Ann. Pharmacother. 2007, 41, 674–680. [Google Scholar] [CrossRef]
- Garrido-Garrido, E.M.; García-Garrido, I.; García-López-Durán, J.C.; García-Jiménez, F.; Ortega-López, I.; Bueno-Cavanillas, A. Estudio de pacientes polimedicados mayores de 65 años en un centro de asistencia primaria urbano. Rev. Calid. Asist. 2011, 26, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Kunakorntham, P.; Pattanaprateep, O.; Dejthevaporn, C.; Thammasudjarit, R.; Thakkinstian, A. Detection of statin-induced rhabdomyolysis and muscular related adverse events through data mining technique. BMC Med. Inform. Decis. Mak. 2022, 22, 233. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.E.; Russo, V.; Walsh, C.; Menditto, E.; Bennett, K.; Cahir, C. Prevalence and Factors Associated with Potential Drug-Drug Interactions in Older Community-Dwelling Adults: A Prospective Cohort Study. Drugs Aging 2021, 38, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Blom, D.J.; Hala, T.; Bolognese, M.; Lillestol, M.J.; Toth, P.D.; Burgess, L.; Ceska, R.; Roth, E.; Koren, M.J.; Ballantyne, C.M.; et al. A 52-Week Placebo-Controlled Trial of Evolocumab in Hyperlipidemia. N. Engl. J. Med. 2014, 370, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Montastruc, J.-L. Rhabdomyolysis and statins A pharmacovigilance comparative study between statins. Br. J. Clin. Pharmacol. 2023, 89, 2636–2638. [Google Scholar] [CrossRef] [PubMed]
- Cuisset, T.; Frere, C.; Quilici, J.; Poyet, R.; Gaborit, B.; Bali, L.; Brissy, O.; Morange, P.E.; Alessi, M.C.; Bonnet, J.L. Comparison of Omeprazole and Pantoprazole Influence on a High 150-mg Clopidogrel Maintenance Dose. The PACA (Proton Pump Inhibitors And Clopidogrel Association) Prospective Randomized Study. J. Am. Coll. Cardiol. 2009, 54, 1149–1153. [Google Scholar] [CrossRef]
- Zou, J.J.; Chen, S.L.; Tan, J.; Lin, G.; Zhao, Y.Y.; Xu, H.M.; Lin, S.; Zhang, J.; Fan, H.W.; Xie, H.G. Increased risk for developing major adverse cardiovascular events in stented Chinese patients treated with dual antiplatelet therapy after concomitant use of the proton pump inhibitor. PLoS ONE 2014, 9, e84985. [Google Scholar] [CrossRef]
- Kuscu, F.; Ulu, A.; Inal, A.S.; Suntur, B.M.; Aydemir, H.; Gul, S.; Ecemis, K.; Komur, S.; Kurtaran, B.; Kuscu, O.O.; et al. Potential drug–drug interactions with antimicrobials in hospitalized patients: A multicenter point-prevalence study. Med. Sci. Monit. 2018, 24, 4240–4247. [Google Scholar] [CrossRef]
- Bakker, T.; Dongelmans, D.A.; Nabovati, E.; Eslami, S.; de Keizer, N.F.; Abu-Hanna, A.; Klopotowska, J.E. Heterogeneity in the Identification of Potential Drug-Drug Interactions in the Intensive Care Unit: A Systematic Review, Critical Appraisal, and Reporting Recommendations. J. Clin. Pharmacol. 2022, 62, 706–720. [Google Scholar] [CrossRef]
- Bolhuis, M.S.; Panday, P.N.; Pranger, A.D.; Kosterink, J.G.W.; Alffenaar, J.W.C. Pharmacokinetic drug interactions of antimicrobial drugs: A systematic review on oxazolidinones, rifamycines, macrolides, fluoroquinolones, and beta-lactams. Pharmaceutics 2011, 3, 865–913. [Google Scholar] [CrossRef]
- Cattaneo, D.; Gervasoni, C.; Corona, A. The Issue of Pharmacokinetic-Driven Drug-Drug Interactions of Antibiotics: A Narrative Review. Antibiotics 2022, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Glen, H.; Mason, S.; Patel, H.; Macleod, K.; Brunton, V.G. E7080, a multi-targeted tyrosine kinase inhibitor suppresses tumor cell migration and invasion. BMC Cancer 2011, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Vavrová, K.; Indra, R.; Pompach, P.; Heger, Z.; Hodek, P. The impact of individual human cytochrome P450 enzymes on oxidative metabolism of anticancer drug lenvatinib. Biomed. Pharmacother. 2022, 145, 112391. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R.; Morganroth, J. Update on Cardiovascular Safety of Tyrosine Kinase Inhibitors: With a Special Focus on QT Interval, Left Ventricular Dysfunction and Overall Risk/Benefit. Drug Saf. 2015, 38, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.M.; Philbrick, A.M. Avoiding serotonin syndrome: The nature of the interaction between tramadol and selective serotonin reuptake inhibitors. Ann. Pharmacother. 2012, 46, 1712–1716. [Google Scholar] [CrossRef]
- Anglin, R.; Yuan, Y.; Moayyedi, P.; Tse, F.; Armstrong, D.; Leontiadis, G.I. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: A systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 811–819. [Google Scholar] [CrossRef]
- Haghbin, H.; Zakirkhodjaev, N.; Husain, F.F.; Lee-Smith, W.; Aziz, M. Risk of Gastrointestinal Bleeding with Concurrent Use of NSAID and SSRI: A Systematic Review and Network Meta-Analysis. Dig. Dis. Sci. 2023, 68, 1975–1982. [Google Scholar] [CrossRef]
- Zucker, I.; Prendergast, B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 2020, 11, 32. [Google Scholar] [CrossRef]
| Disease | Percentage |
|---|---|
| Cardiovascular diseases (Blood hypertension, atrial fibrillation) | 82.3 |
| Osteoarthritis | 79.5 |
| Diabetes mellitus type 2 | 26.2 |
| Depression | 18.7 |
| Impaired cognition, dementia | 16.8 |
| Gastroesophageal reflux disease | 13.3 |
| Urological diseases | 12.8 |
| COPD | 8.4 |
| Rheumatological diseases | 6.9 |
| Osteoporosis or osteopenia | 4.8 |
| Asthma | 1.2 |
| Hypothyroidism | 1.1 |
| Other | 1.6 |
| Characteristics | With PIP (n: 794) | Without PIP (n: 552) |
|---|---|---|
| Age (mean ± SD) | 76.3 ± 7.3 | 76.8 ± 8.5 |
| Men (%) | 41.3 | 46.6 |
| Women (%) | 58.7 *§ | 53.4 * |
| Current smokers (%) | 5.2 | 4.8 |
| Overweight (%) | 53.6 | 52.8 |
| Chronic diseases (mean ± SD) | 4 ± 2 | 5 ± 2 |
| Drugs | Percentage | Number |
|---|---|---|
| omeprazole and esomeprazole | 25 | 199 |
| atorvastatin and simvastatin | 20 | 159 |
| Clopidogrel | 12.9 | 102 |
| ciprofloxacin and levofloxacin | 11 | 87 |
| SSRIs | 10.1 | 80 |
| Opioids | 9 | 71 |
| SNRIs | 6 | 48 |
| Benzodiazepines | 3 | 24 |
| NSAIDs | 3 | 24 |
| Drugs Prescribed during Hospital Discharge | Drugs in Therapy |
|---|---|
| omeprazole and esomeprazole | citalopram (28), escitalopram (26), venlafaxine (25), tramadol (21), trazodone (12), aripiprazole (11), tizanidine (10), alfuzosin (9), domperidone (9), ciprofloxacin (9), amiodarone (9), vardenafil (8), levofloxacin (7), haloperidol (6), clopidogrel (5), moxifloxacin (4) |
| atorvastatin or simvastatin | esomeprazole (59), amlodipine (16), Sacubitril Valsartan (14), sitagliptin (12), ranolazine (9), amiodarone (9), tadalafil (8), dronedarone (6), warfarin (6), sildenafil (6), clopidogrel (3), diltiazem (3), ticagrelor (3), carbamazepine (2), everolimus (2), domperidone (1) |
| Clopidogrel | omeprazole (22), esomeprazole (18), lansoprazole (16), rosuvastatin (12), repaglinide (9), fluoxetine (6), paroxetine (5), tramadol (5), tapentadol (4), venlafaxine (3), amiodarone (2) |
| ciprofloxacin and levofloxacin | escitalopram (14), duloxetine (9), venlafaxine (6), tramadol (4), |
| omeprazole (10), metformin (9), simvastatin (7), betamethasone (3), paroxetine (3), fluoxetine (3), warfarin (2), furosemide (2), propafenone (1), ranolazine (1), zolpidem (1) | |
| diclofenac (6), ibuprofen (4), dutasteride (2) | |
| fluoxetine and paroxetine | rivaroxaban (6), apixaban (4), dabigatran (4), diclofenac (6), clopidogrel (6), tizanidine (4), tramadol (4), triazolam (3), frovatriptan (3), furosemide (2), trazodone (2), oxycodone (2), triazolam (2), amiodarone (2), almotriptan (1), eletriptan (1) |
| citalopram and escitalopram | omeprazole (7), tramadol (3), tizanidine (3), apixaban (2), trazodone (2), diclofenac (2), ibuprofen (2), ketoprofen (2), amitriptyline (2), buprenorphine (2), almotriptan (1) |
| Tramadol | omeprazole (7), gabapentin (7), paroxetine (6), amitriptyline (6), duloxetine (4), fluoxetine (2), oxycodone (2), alprazolam (1), diazepam (1) |
| Tapentadol | citalopram (6), escitalopram (3), paroxetine (3), oxycodone (3), almotriptan (2), trazodone (2), sertraline (2), risperidone (2) |
| Oxycodone | escitalopram (6), paroxetine (3), Triazolam (2), gabapentin (1) |
| Venlafaxine | omeprazole (9), esomeprazole (9) flecainide (6), ciprofloxacin (5), azithromycin (4), zolmitriptan (2) buprenorphine (1) |
| Duloxetine | trazodone (3), clobazam (2), rizatriptan (2), naproxen (2), etoricoxib (1) |
| Diazepam | omeprazole (16), tramadol (4), tapentadol (2), olanzapine (2) |
| alprazolam and triazolam | tramadol (2) |
| diclofenac and ketorolac | enoxaparin (8), venlafaxine (5), valsartan (5), furosemide (4), levofloxacin (2) |
| Drug 1 | Drug 2 | ADRs | Mechanism | Action | N Patients | DIPS Score |
|---|---|---|---|---|---|---|
| citalopram 20 mg once per day | lenvatinib 10 mg once per day | Increase in heart rate | QT interval prolongation | Change citalopram to duloxetine | 2 | 6 |
| escitalopram 20 mg once per day | lenvatinib 10 mg once per day | Increase in heart rate | QT interval prolongation | Change escitalopram to duloxetine | 1 | 6 |
| levofloxacin 500 mg once per day | lenvatinib 10 mg once day | Increase in heart rate | QT interval prolongation | Change levofloxacin to piperacillin/tazobactam | 1 | 6 |
| clopidogrel 75 mg once per day | omeprazole 40 mg once per day | Change in clopidogrel activity | Reduced liver activation of clopidogrel | Change omeprazole to pantoprazole | 7 | 6 |
| clopidogrel 75 mg once per day | esomeprazole 40 mg once per day | Change in clopidogrel activity | Reduced liver activation of clopidogrel | Reduce the dosage of esomeprazole to 10 mg | 1 | 6 |
| clopidogrel 75 mg once per day | fluoxetine 20 mg daily | Change in clopidogrel activity | Reduced liver activation of clopidogrel | Change fluoxetine to sertraline | 5 | 6 |
| omeprazole 20 mg daily | Methotrexate | Increased methotrexate toxicity | Reduced renal secretion of methotrexate | Stop omeprazole three days before the administration of methotrexate | 2 | 7 |
| atorvastatin 20 mg daily | sildenafil 20 mg | Muscular pain | CYP3A4 inhibition | Stop sildenafil change to vardenafil | 4 | 6 |
| atorvastatin 20 mg daily | clarithromycin 500 mg every 12 h | Muscular pain | CYP3A4 inhibition | Change clarithromycin to azithromycin | 1 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vocca, C.; Siniscalchi, A.; Rania, V.; Galati, C.; Marcianò, G.; Palleria, C.; Catarisano, L.; Gareri, I.; Leuzzi, M.; Muraca, L.; et al. The Risk of Drug Interactions in Older Primary Care Patients after Hospital Discharge: The Role of Drug Reconciliation. Geriatrics 2023, 8, 122. https://doi.org/10.3390/geriatrics8060122
Vocca C, Siniscalchi A, Rania V, Galati C, Marcianò G, Palleria C, Catarisano L, Gareri I, Leuzzi M, Muraca L, et al. The Risk of Drug Interactions in Older Primary Care Patients after Hospital Discharge: The Role of Drug Reconciliation. Geriatrics. 2023; 8(6):122. https://doi.org/10.3390/geriatrics8060122
Chicago/Turabian StyleVocca, Cristina, Antonio Siniscalchi, Vincenzo Rania, Cecilia Galati, Gianmarco Marcianò, Caterina Palleria, Luca Catarisano, Ilaria Gareri, Marco Leuzzi, Lucia Muraca, and et al. 2023. "The Risk of Drug Interactions in Older Primary Care Patients after Hospital Discharge: The Role of Drug Reconciliation" Geriatrics 8, no. 6: 122. https://doi.org/10.3390/geriatrics8060122
APA StyleVocca, C., Siniscalchi, A., Rania, V., Galati, C., Marcianò, G., Palleria, C., Catarisano, L., Gareri, I., Leuzzi, M., Muraca, L., Citraro, R., Nanci, G., Scuteri, A., Bianco, R. C., Fera, I., Greco, A., Leuzzi, G., De Sarro, G., D’Agostino, B., & Gallelli, L. (2023). The Risk of Drug Interactions in Older Primary Care Patients after Hospital Discharge: The Role of Drug Reconciliation. Geriatrics, 8(6), 122. https://doi.org/10.3390/geriatrics8060122








