Diaphragm Ultrasound in Different Clinical Scenarios: A Review with a Focus on Older Patients
Abstract
1. Introduction
2. Methods
3. Ultrasound Imaging of the Diaphragm
3.1. General Historical Background
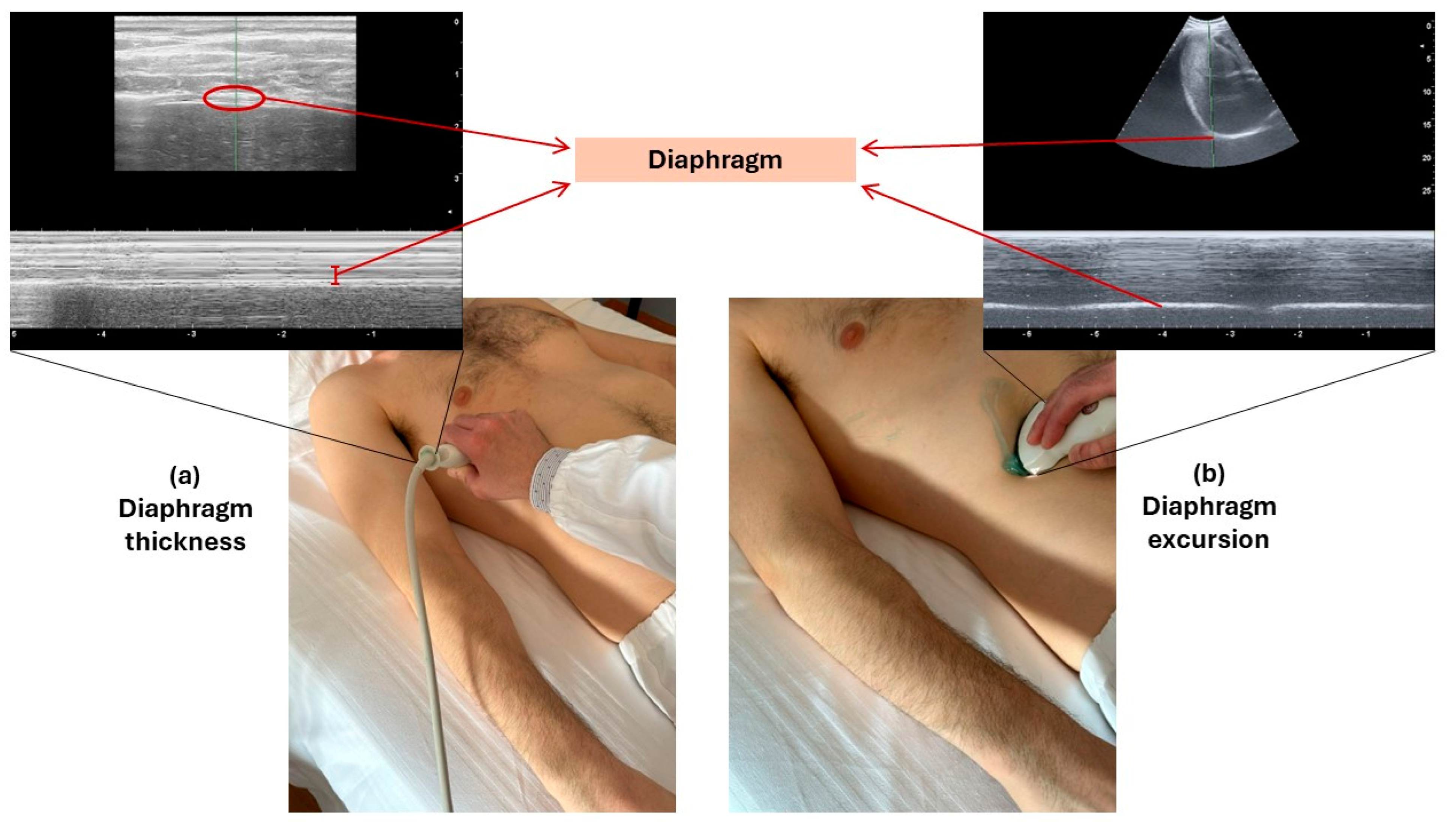
3.2. Technique of Diaphragm Assessment by Ultrasound
3.3. Reference Values of Diaphragm Ultrasound Parameters
3.4. Advantages and Disadvantages of Diaphragm Ultrasound
4. Diaphragm Ultrasound in Specific Clinical Situations or Diseases
4.1. Respiratory Failure Requiring Ventilatory Support
4.2. COPD
4.3. COVID-19, Other Pneumonia and Related Conditions
4.4. Congestive Heart Failure and Related Conditions
4.5. Other Conditions
5. Diaphragm Ultrasound in Geriatric Patients with Frailty and Sarcopenia
5.1. Structural and Functional Changes of the Diaphragm in Sarcopenia
5.2. Diaphragm Ultrasound Studies in Sarcopenic Patients
6. Possible Use of Diaphragm Ultrasound in Different Clinical Settings
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laghi, F.; Tobin, M.J. Disorders of the respiratory muscles. Am. J. Respir. Crit. Care Med. 2003, 168, 10–48. [Google Scholar] [CrossRef]
- Nason, M.K.; Walker, C.M.; McNeeley, M.F.; Burivong, W.; Fligner, C.L.; Goodwin, J.D. Imaging of the diaphragm: Anatomy and function. Radiographics 2012, 32, E51–E70. [Google Scholar] [CrossRef] [PubMed]
- McCool, F.D.; Tzelepis, G.E. Dysfunction of the diaphragm. N. Engl. J. Med. 2012, 366, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Greising, S.M.; Ottenheijm, C.A.C.; O’Halloran, K.D.; Barreiro, E. Diaphragm plasticity in aging and disease: Therapies for muscle weakness go from strength to strength. J. Appl. Physiol. 2018, 125, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R. Dynamic chest radiography: Flat-panel detector (FPD) based functional X-ray imaging. Radiol. Phys. Technol. 2016, 9, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wei, J.; Huang, H.; Gaebler, C.P.; Yuan, A.; Deasy, J.O. Automatic assessment of average diaphragm motion trajectory from 4DCT images through machine learning. Biomed. Phys. Eng. Express 2015, 1, 045015. [Google Scholar] [CrossRef] [PubMed]
- Takazakura, R.; Takahashi, M.; Nitta, N.; Murata, K. Diaphragmatic motion in the sitting and supine positions: Healthy subject study using a vertically open magnetic resonance system. J. Magn. Reson. Imaging 2004, 19, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Leal, B.E.; Gonçalves, M.A.; Lisboa, L.G.; Schmitz Linné, L.M.; de Sousa Tavares, M.G.; Pereira Yamaguti, W.; Paulin, E. Validity and reliability of fluoroscopy for digital radiography: A new way to evaluate diaphragmatic mobility. BMC Pulm. Med. 2017, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Cicero, G.; Mazziotti, S.; Blandino, A.; Granata, F.; Gaeta, M. Magnetic Resonance Imaging of the Diaphragm: From Normal to Pathologic Findings. J. Clin. Imaging Sci. 2020, 10, 1. [Google Scholar] [CrossRef]
- Laghi, F.; Tobin, M. Monitoring respiratory muscle function. In Cardiopulmonary Monitoring—Basic Physiology, Tools, and Bedside Management, 1st ed.; Magder, S., Malhotra, A., Hibbert, K.A., Hardin, C.C., Eds.; Springer: London, UK, 2021; pp. 533–584. [Google Scholar]
- Shaikh, H.; Laghi, F. Role of Diaphragm Ultrasound When NIV Fails in COPD Exacerbations. Respir. Care 2019, 64, 1600–1602. [Google Scholar] [CrossRef]
- Sarwal, A.; Walker, F.O.; Cartwright, M.S. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve 2013, 47, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Cohen, W.H. (Ed.) Evaluation of the diaphragm by a subcostal B-scan technique. In Proceedings of the First World Congress on Ultrasound Diagnostics in Medicine and SIDUO III, UltrasonoGraphia Media 1969, Vienna, Austria, 2–7 June 1969. [Google Scholar]
- Wait, J.L.; Nahormeck, P.A.; Yost, Y.T.; Rochester, D.P. Diaphragmatic thickness-lung volume relationship in vivo. J. Appl. Physiol. 1989, 67, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Laveneziana, P.; Albuquerque, A.; Aliverti, A.; Babb, T.; Barreiro, E.; Dres, M.; Dubé, D.P.; Fauroux, B.; Gea, J.; Guenette, J.A.; et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir. J. 2019, 53, 1801214. [Google Scholar] [CrossRef] [PubMed]
- Umbrello, M.; Formenti, P. Ultrasonographic Assessment of Diaphragm Function in Critically Ill Subjects. Respir. Care 2016, 61, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Zambon, M.; Greco, M.; Bocchino, S.; Cabrini, L.; Beccaria, P.F.; Zangrillo, A. Assessment of diaphragmatic dysfunction in the critically ill patients with ultrasound: A systematic review. Intensive Care Med. 2017, 43, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Miyazaki, S.; Tamaki, A.; Yoshimura, Y.; Arai, H.; Fujiwara, D.; Katsura, H.; Kawagoshi, A.; Kozu, R.; Maeda, K.; et al. Respiratory sarcopenia: A position paper by four professional organizations. Geriatr. Gerontol. Int. 2023, 23, 5–15. [Google Scholar] [CrossRef]
- Ticinesi, A.; Meschi, T.; Narici, M.V.; Lauretani, F.; Maggio, M. Muscle Ultrasound and Sarcopenia in Older Individuals: A Clinical Perspective. J. Am. Med. Dir. Assoc. 2017, 18, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.; Abo, S.; Whish-Wilson, G.A.; D’Souza, A.; Beach, L.J.; Mathur, S.; Mayer, K.P.; Ntoumenopoulos, G.; Baldwin, C.; El-Ansary, D.; et al. Methodological and clinimetric evaluation of inspiratory muscle ultrasound in the critical care setting: A systematic review and meta-analysis. Crit. Care Med. 2023, 51, e24–e36. [Google Scholar] [CrossRef]
- Bellissimo, C.E.; Morris, I.S.; Wong, J.; Goligher, E.C. Measuring diaphragm thickness and function using point-of-care ultrasound. J. Vis. Exp. 2023, 201, e65431. [Google Scholar] [CrossRef]
- Goligher, E.C.; Laghi, F.; Detsky, M.E.; Farias, P.; Murray, A.; Brace, D.; Brochard, L.J.; Sebastien-Bolz, S.; Rubenfeld, G.D.; Kavanagh, B.P.; et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: Feasibility, reproducibility and validity. Intensive Care Med. 2015, 41, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, S.; Mancini, D.; Laudisio, A.; Antonelli Incalzi, R. Reproducibility of diaphragmatic thickness measured by M-mode ultrasonography in health volunteers. Respir. Physiol. Neurobiol. 2019, 260, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Spiesshoefer, J.; Herkenrath, S.; Henke, C.; Langenbruch, L.; Schneppe, M.; Randerath, W.; Young, P.; Brix, T.; Boentert, M. Evaluation of respiratory muscle strength and diaphragm ultrasound: Normative values, theoretical considerations, and practical recommendations. Respiration 2020, 99, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, I.; Picciafuochi, F.; Bartolucci, M.; Matteini, S.; Virgili, G.; Adembri, C. Evaluation of diaphragm thickening by diaphragm ultrasonography: A reproducibility and a repeatability study. J. Ultrasound 2021, 24, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Boussuges, A.; Gole, Y.; Balnc, P. Diaphragmatic motion studied by M-mode ultrasonography. Methods, reproducibility, and normal values. Chest 2009, 135, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Noh, D.K.; Lee, J.J.; You, J.H. Diaphragm breathing movement measurement using ultrasound and radiographic imaging: A concurrent validity. Biomed. Mater. Eng. 2014, 24, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Grams, S.T.; von Saltiél, R.; Mayer, A.F.; Schivinski, C.I.S.; Nobre, L.F.S.; Nóbrega, I.S.; Jacomino, M.E.M.L.P.; Paulin, E. Assessment of the reproducibility of the indirect ultrasound method of measuring diaphragm mobility. Clin. Physiol. Funct. Imaging 2014, 34, 18–25. [Google Scholar] [CrossRef]
- Fayssoil, A.; Behin, A.; Ogna, A.; Mompoint, D.; Amthor, E.; Clair, B.; Laforet, P.; Mansart, A.; Prigent, H.; Orlikowski, D.; et al. Diaphragm: Pathophysiology and Ultrasound Imaging in Neuromuscular Disorders. J. Neuromuscul. Dis. 2018, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, D.K.; Gandevia, S.C.; Gorman, R.B.; Southon, F.C. Dynamic changes in the zone of apposition and diaphragm length during maximal respiratory efforts. Thorax 1994, 49, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Aarab, Y.; Flatres, A.; Garnier, F.; Capdevila, M.; Raynaud, F.; Lacampagne, A.; Chapeau, D.; Klouche, K.; Etienne, P.; Jaber, S.; et al. Shear Wave Elastography, a New Tool for Diaphragmatic Qualitative Assessment: A Translational Study. Am. J. Respir. Crit. Care Med. 2021, 204, 797–806. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; El Kaffas, A.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Nouvenne, A.; Zanichelli, I.; Cerundolo, N.; Milanese, G.; Sverzellati, N.; Rendo, M.; Ridolo, E.; Scarlata, S.; Meschi, T.; Ticinesi, A. Thoracic Ultrasound Strain Elastosonography as a Noninvasive Biomarker of Chronic Obstructive Pulmonary Disease-Associated Lung Injury: A Feasibility Study. Respiration 2022, 101, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Wu, Z.Z.; Tao, F.Y.; Zhu, S.T.; Chen, S.P.; Cai, C.; Liang, Z.H.; Shi, B.B.; Chen, B.; Xie, X.P. Ultrasound Shear Wave Elastography for Evaluation of Diaphragm Stiffness in Patients with Stable COPD: A Pilot Trial. J. Ultrasound Med. 2021, 40, 2655–2663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, C.; Yan, L.; Zhang, L.; Wan, Y.; Wang, Q.; Wang, P.; Xu, J. Shear wave elastography of the diaphragm in acute exacerbation of chronic obstructive pulmonary disease: A prospective observational study. Medicine 2023, 102, e33329. [Google Scholar] [CrossRef] [PubMed]
- Flatres, A.; Aarab, Y.; Nougaret, S.; Garnier, F.; Larcher, R.; Amalric, M.; Klouche, K.; Etienne, P.; Subra, G.; Jaber, S.; et al. Real-time shear wave ultrasound elastography: A new tool for the evaluation of diaphragm and limb muscle stiffness in critically ill patients. Crit. Care 2020, 24, 34. [Google Scholar] [CrossRef] [PubMed]
- Fossé, Q.; Poulard, T.; Niérat, M.C.; Virolle, S.; Morawiec, E.; Hogrel, J.Y.; Similowski, T.; Demoule, A.; Genisson, J.L.; Bachasson, D.; et al. Ultrasound shear wave elastography for assessing diaphragm function in mechanically ventilated patients: A breath-by-breath analysis. Crit. Care 2020, 24, 669. [Google Scholar] [CrossRef]
- Ueki, J.; De Bruin, P.F.; Pride, N.B. In vivo assessment of diaphragm contraction by ultrasound in normal subjects. Thorax 1995, 50, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Kabil, A.E.; Sobh, E.; Elsaeed, M.; Hassanin, H.E.; Yousef, I.H.; Eltrawy, H.H.; Ewis, A.M.; Aboseif, A.; Albalsha, A.M.; Elsawy, S.; et al. Diaphragmatic excursion by ultrasound: Reference values for the normal population; a cross-sectional study in Egypt. Multidiscip. Respir. Med. 2022, 17, 842. [Google Scholar] [CrossRef]
- Harper, C.J.; Shahgholi, L.; Cieslak, K.; Hellyer, N.J.; Strommen, J.A.; Boon, A.J. Variability in diaphragm motion during normal breathing, assessed with B-mode ultrasound. J. Orthop. Sports Phys. Ther. 2013, 43, 927–931. [Google Scholar] [CrossRef]
- Boon, A.J.; Harper, C.J.; Shahgholi Ghahfarokhi, L.; Strommen, J.A.; Watson, J.C.; Sorenson, E.J. Two-dimensional ultrasound imaging of the diaphragm: Quantitative values in normal subjects. Muscle Nerve 2013, 47, 884–889. [Google Scholar] [CrossRef]
- Carrillo-Esper, R.; Pérez-Calatayud, A.A.; Arch-Tirado, E.; Díaz-Carrillo, M.A.; Garrido-Aguirre, E.; Tapia-Velazco, R.; Peña-Pérez, C.A.; Espinoza-de los Monteros, I.; Meza-Márquez, J.M.; Flores-Rivera, O.I.; et al. Standardization of sonographic diaphragm thickness evaluations in healthy volunteers. Respir. Care 2016, 61, 920–924. [Google Scholar] [CrossRef]
- Glau, C.L.; Lin, E.E.; Conlon, T.W.; Himebauch, A.S.; Keim, G.P.; Nishisaki, A. Ultrasound assessment of diaphragm thickness, contractility, and strain in healthy pediatric patients. Pediatr. Pulmonol. 2024, 59, 433–441. [Google Scholar] [CrossRef]
- Le Neindre, A.; Philippart, F.; Luperto, M.; Wormser, J.; Morel-Sapene, J.; Aho, S.L.; Mongodi, S.; Mojoli, F.; Bouhemad, B. Diagnostic accuracy of diaphragm ultrasound to predict weaning outcome: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2021, 117, 103890. [Google Scholar] [CrossRef]
- Laghi, F.A.; Saad, M.; Shaikh, H. Ultrasound and non-ultrasound imaging techniques in the assessment of diaphragmatic dysfunction. BMC Pulm. Med. 2021, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Duyndam, A.; Smit, J.; Heunks, L.; Molinger, J.; IJland, M.; van Rosmalen, J.; van Dijk, M.; Tibboel, D.; Ista, E. Reference values of diaphragmatic dimensions in healthy children aged 0–8 years. Eur. J. Pediatr. 2023, 182, 2577–2589. [Google Scholar] [CrossRef]
- Fayssoil, A.; Nguyen, L.S.; Ogna, A.; Stojkovic, T.; Meng, P.; Mompoint, D.; Carlier, R.; Prigent, H.; Clair, B.; Behin, A.; et al. Diaphragm stiff ultrasound: Normal values, relationship with sniff nasal pressure and accuracy for predicting respiratory involvement in patients with neuromuscular disorders. PLoS ONE 2019, 14, e0214288. [Google Scholar] [CrossRef]
- Boussuges, A.; Rives, S.; Finance, J.; Chaumet, G.; Vallée, N.; Risso, J.J.; Brégeon, F. Ultrasound assessment of diaphragm thickness and thickening: Reference values and limits of normality when in a seated position. Front. Med. 2021, 8, 742703. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Minami, T.; Yoshino, S.; Emoto, K.; Mabuchi, S.; Hanazawa, R.; Hirakawa, A.; Hashimoto, M. Diaphragm ultrasonography: Reference values and influencing factors for thickness, thickening fraction, and excursion in the seated position. Lung 2024, 202, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Vieira Santana, P.; Zumpano Cardenas, L.; Pereira de Albuquerque, A.L.; Ribeiro de Carvalho, C.R.; Caruso, P. Diaphragmatic ultrasound: A review of its methodological aspects and clinical uses. J. Bras. Pneumol. 2020, 46, e2020064. [Google Scholar] [CrossRef]
- Sferrazza Papa, G.F.; Pellegrino, G.M.; Di Marco, F.; Imeri, G.; Brochard, L.; Goligher, E.; Centanni, S. A review of the ultrasound assessment of diaphragmatic function in clinical practice. Respiration 2016, 91, 403–411. [Google Scholar] [CrossRef]
- Testa, A.; Soldati, G.; Giannuzzi, R.; Berardi, S.; Portale, G.; Gentiloni Silveri, N. Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med. Biol. 2011, 37, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Scarlata, S.; Nouvenne, A.; Lauretani, F.; Antonelli Incalzi, R.; Ungar, A.; GRETA (Gruppo di Ricerca sull’Ecografia Toracica nell’Anziano) Group of the Italian Society of Gerontology and Geriatrics (SIGG). The Geriatric Patient: The Ideal One for Chest Ultrasonography? A Review From the Chest Ultrasound in the Elderly Study Group (GRETA) of the Italian Society of Gerontology and Geriatrics (SIGG). J. Am. Med. Dir. Assoc. 2020, 21, 447–454.e6. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.E.; Paratz, J.D.; Bersten, A.D. Diaphragm and peripheral muscle thickness on ultrasound: Intra-rater reliability and variability of a methodology using non-standard recumbent positions. Respirology 2011, 16, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Houston, J.G.; Angus, R.M.; Cowan, M.D.; McMillan, N.C.; Thomson, N.C. Ultrasound assessment of normal hemidiaphragmatic movement: Relation to inspiratory volume. Thorax 1994, 49, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Osadnik, C.R.; Brighton, L.J.; Burtin, C.; Cesari, M.; Lahousse, L.; Man, W.D.C.; Marengoni, A.; Sajnic, A.; Singer, J.P.; Ter Beek, L.; et al. European Respiratory Society statement on frailty in adults with chronic lung disease. Eur. Respir. J. 2023, 62, 2300442. [Google Scholar] [CrossRef] [PubMed]
- Haaksma, M.E.; Smit, J.M.; Boussuges, A.; Demoule, A.; Dres, M.; Ferrari, G.; Formenti, P.; Goligher, E.C.; Heunks, L.; Lim, E.H.T.; et al. EXpert consensus On Diaphragm UltraSonography in the critically ill (EXODUS): A Delphi consensus statement on the measurement of diaphragm ultrasound-derived parameters in a critical care setting. Crit. Care 2022, 26, 99. [Google Scholar] [CrossRef] [PubMed]
- Demoule, A.; Jung, B.; Prodanovic, H.; Molinari, N.; Chanques, G.; Coirault, C.; Matecki, S.; Duguet, A.; Similowski, T.; Jaber, S. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am. J. Respir. Crit. Care Med. 2013, 188, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Álvarez, A.M.; Tenza-Lozano, E.M.; Latour-Pérez, J. Diaphragm and Lung Ultrasound to Predict Weaning Outcome: Systematic Review and Meta-Analysis. Chest 2017, 152, 1140–1150. [Google Scholar] [CrossRef]
- Santangelo, E.; Mongodi, S.; Bouhemad, B.; Mojoli, F. The weaning from mechanical ventilation: A comprehensive ultrasound approach. Curr. Opin. Crit. Care 2022, 28, 322–330. [Google Scholar] [CrossRef]
- Kilaru, D.; Panebianco, N.; Baston, C. Diaphragm Ultrasound in Weaning From Mechanical Ventilation. Chest 2021, 159, 1166–1172. [Google Scholar] [CrossRef]
- Mahmoodpoor, A.; Fouladi, S.; Ramouz, A.; Shadvar, K.; Ostadi, Z.; Soleimanpour, H. Diaphragm ultrasound to predict weaning outcome: Systematic review and meta-analysis. Anaesthesiol. Intensive Ther. 2022, 54, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Oto, J.; Ueno, Y.; Nakataki, E.; Itagaki, T.; Nishimura, M. Change in diaphragm and intercostal muscle thickness in mechanically ventilated patients: A prospective observational study. J. Intensive Care 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.B.; Lim, J.K.B.; Glau, C.; Conlon, T.; James, R.; Lee, J.H. A narrative review of diaphragmatic ultrasound in pediatric critical care. Pediatr. Pulmonol. 2021, 56, 2471–2483. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Moury, P.H.; Mahul, M.; de Jong, A.; Galia, F.; Prades, A.; Albaladejo, P.; Chanques, G.; Molinari, N.; Jaber, S. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016, 42, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Supinski, G.S.; Morris, P.E.; Dhar, S.; Callahan, L.A. Diaphragm dysfunction in critical illness. Chest 2018, 153, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Muller, M.; Putegnat, J.B.; Steyer, J.; Barrau, S.; Boissier, F.; Bourdin, G.; Mekontso-Dessap, A.; Levrat, A.; Pommier, C.; et al. Inability of diaphragm ultrasound to predict extubation failure. A multicenter study. Chest 2019, 155, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Baptistella, A.R.; Sarmento, F.J.; Ribeiro da Silva, K.; Baptistella, S.F.; Taglietti, M.; Zuquello, R.Á.; Nunes Filho, J.R. Predictive factors of weaning from mechanical ventilation and extubation outcome: A systematic review. J. Crit. Care 2018, 48, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Béduneau, G.; Pham, T.; Schortgen, F.; Piquilloud, L.; Zogheib, E.; Jonas, M.; Grelon, F.; Runge, I.; Terzi, N.; Grangé, S.; et al. Epidemiology of Weaning Outcome according to a New Definition. The WIND Study. Am. J. Respir. Crit. Care Med. 2017, 195, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Sarwal, A.; Liu, A.; Cartwright, M.S.; Dhar, S.; Morris, P.E. Sonography for assessing dynamic diaphragm dysfunction in acute respiratory distress. J. Ultrasound Med. 2015, 34, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ma, H.; Zhong, W.; Wang, X.; Wu, Y.; Qin, T.; Wang, S.; Tan, N. Using M-mode ultrasonography to assess diaphragm dysfunction and predict the success of mechanical ventilation weaning in elderly patients. J. Thorac. Dis. 2017, 9, 3177–3186. [Google Scholar] [CrossRef]
- Kocyigit, H.; Gunalp, M.; Genc, S.; Oguz, A.B.; Koca, A.; Polat, O. Diaphragm dysfunction detected with ultrasound to predict noninvasive mechanical ventilation failure: A prospective cohort study. Am. J. Emerg. Med. 2021, 45, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Er, B.; Mizrak, B.; Aydemir, A.; Binay, S.; Doğu, C.; Kazanci, D.; Turan, S. Is diaphragm ultrasound better than rapid shallow breathing test for predicting weaning in critically ill elderly patients? Tuberk. Toraks 2023, 71, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Pirompanich, P.; Romsaiut, S. Use of diaphragm thickening fraction combined with rapid shallow breathing index for predicting success of weaning from mechanical ventilator in medical patients. J. Intensive Care 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Varón-Vega, F.; Hernández, Á.; López, M.; Cáceres, E.; Giraldo-Cadavid, L.F.; Uribe-Hernandez, A.M.; Crevoisier, S. Usefulness of diaphragmatic ultrasound in predicting extubation success. Med. Intensiv. 2021, 45, 226–233. [Google Scholar] [CrossRef] [PubMed]
- De Spiegeleer, A.; Kahya, H.; Sanchez-Rodriguez, D.; Piotrowicz, K.; Surquin, M.; Marco, E.; Detremerie, C.; Hussein, D.; Hope, S.; Dallmeier, D.; et al. Acute sarcopenia changes following hospitalization: Influence of pre-admission care dependency level. Age Ageing 2021, 50, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Martone, A.M.; Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.G.; Manca, G.M.; et al. The incidence of sarcopenia among hospitalized older patients: Results from the Glisten study. J. Cachexia Sarcopenia Muscle 2017, 8, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Fazzini, B.; Märkl, T.; Costas, C.; Blobner, M.; Schaller, S.J.; Prowle, J.; Puthucheary, Z.; Wackerhage, H. The rate and assessment of muscle wasting during critical illness: A systematic review and meta-analysis. Crit. Care 2023, 27, 2. [Google Scholar] [CrossRef] [PubMed]
- Er, B.; Simsek, M.; Yildirim, M.; Halacli, B.; Ocal, S.; Ersoy, E.O.; Demir, A.U.; Topeli, A. Association of baseline diaphragm, rectus femoris and vastus intermedius muscle thickness with weaning from mechanical ventilation. Respir. Med. 2021, 185, 106503. [Google Scholar] [CrossRef]
- Brunker, L.B.; Boncyk, C.S.; Rengel, K.F.; Hughes, C.G. Elderly Patients and Management in Intensive Care Units (ICU): Clinical Challenges. Clin. Interv. Aging 2023, 18, 93–112. [Google Scholar] [CrossRef]
- Similowski, T.; Yan, S.; Gauthier, A.P.; Macklem, P.T.; Bellemare, F. Contractile properties of the human diaphragm during chronic hyperinflation. N. Engl. J. Med. 1991, 25, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Boriek, A.M.; Lopez, M.A.; Velasco, C.; Bakir, A.A.; Frolov, A.; Wynd, S.; Babb, T.G.; Hanania, N.A.; Hoffmann, E.A.; Sharafkhaneh, A. Obesity modulates diaphragm curvature in subjects with and without COPD. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R620–R629. [Google Scholar] [CrossRef] [PubMed]
- Smargiassi, A.; Inchingolo, R.; Tagliaboschi, L.; Di Marco Berardino, A.; Valente, S.; Corbo, G.M. Ultrasonographic assessment of the diaphragm in chronic obstructive pulmonary disease patients: Relationships with pulmonary function and the influence of body composition-a pilot study. Respiration 2014, 87, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Hua-Rong, Z.; Liang, C.; Rong, L.; Yi-Fan, T.; Dou-Zi, S.; Yue, C.; Zu-Lin, L. Ultrasonographic evaluation of diaphragm function in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Medicine 2022, 101, e32560. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Q. Research progress on diaphragm ultrasound in chronic obstructive pulmonary diseases: A narrative review. Ultrasound Med. Biol. 2022, 48, 587–597. [Google Scholar] [CrossRef]
- Zhou, E.F.; Fu, S.N.; Huang, C.; Huang, X.P.; Wong, A.Y.L. Reliability and validity of ultrasonography in evaluating the thickness, excursion, stiffness, and strain rate of respiratory muscles in non-hospitalized individuals: A systematic review. BMC Oral Health 2023, 23, 959. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, N.; Sosnowski, N.; Pinkhasik, A.; Vonderbank, S.; Bastian, A. Sonographic evaluation of diaphragmatic dysfunction in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1925–1930. [Google Scholar]
- Qaiser, M.; Khan, N.; Jain, A. Ultrasonographic assessment of diaphragmatic excursion and its correlation with spirometry in chronic obstructive pulmonary disease patients. Int. J. Appl. Basic Med. Res. 2020, 10, 256–259. [Google Scholar]
- Chen, Y.; Li, J.; Zhu, Z.; Lyu, G. Lung ultrasound assessment of lung hyperinflation in patients with stable COPD: An effective diagnostic tool. Int. J. Chronic Obstr. Pulm. Dis. 2024, 19, 319–330. [Google Scholar] [CrossRef]
- Kang, H.W.; Kim, T.O.; Lee, B.R.; Yu, J.Y.; Chi, S.Y.; Ban, H.J.; Oh, I.J.; Kim, K.S.; Kwon, Y.S.; Kim, Y.I.; et al. Influence of diaphragmatic mobility on hypercapnia in patients with chronic obstructive pulmonary disease. J. Korean Med. Sci. 2011, 26, 1209–1213. [Google Scholar] [CrossRef]
- Zanforlin, A.; Smargiassi, A.; Inchingolo, R.; di Marco Berardino, A.; Valente, S.; Ramazzina, E. Ultrasound analysis of diaphragm kinetics and the diagnosis of airway obstruction: The role of the M-mode index of obstruction. Ultrasound Med. Biol. 2014, 40, 1065–1071. [Google Scholar] [CrossRef]
- Kim, T.; Huh, S.; Chung, J.H.; Kim, Y.S.; Yun, R.Y.; Park, O.; Lee, S.E. Clinical values of diaphragmatic movement in patients with chronic obstructive pulmonary diseases. BMC Pulm. Med. 2023, 23, 33. [Google Scholar] [CrossRef]
- Mizusawa, H.; Matsumoto, H.; Shiraishi, M.; Sugiya, R.; Takeda, Y.; Noguchi, M.; Kimura, T.; Ishikawa, A.; Nishiyama, O.; Higashimoto, Y. Evaluation of patients with chronic obstructive pulmonary disease by maximal inspiratory pressure and diaphragmatic excursion with ultrasound sonography. Respir. Investig. 2024, 62, 234–239. [Google Scholar] [CrossRef]
- Shiraishi, M.; Higashimoto, Y.; Sugiya, R.; Mizusawa, H.; Takeda, Y.; Fujita, S.; Nishiyama, O.; Kudo, S.; Kimura, T.; Chiba, Y.; et al. Diaphragmatic excursion correlates with exercise capacity and dynamic hyperinflation in COPD patients. ERJ Open Res. 2020, 6, 00589–02020. [Google Scholar] [CrossRef]
- Shiraishi, M.; Higashimoto, Y.; Sugiya, R.; Mizusawa, H.; Takeda, Y.; Fujita, S.; Nishiyama, O.; Kudo, S.; Kimura, T.; Chiba, Y.; et al. Diaphragmatic excursion is correlated with the improvement in exercise tolerance after pulmonary rehabilitation in patients with chronic obstructive pulmonary diseases. Respir. Res. 2021, 22, 271. [Google Scholar] [CrossRef]
- Evrin, T.; Korkut, S.; Sonmez, L.O.; Szarpak, L.; Katipoglu, B.; Smereka, J.; Guven, R.; Akpinar, E.E. Evaluating stable chronic obstructive pulmonary disease by ultrasound. Emerg. Med. Int. 2019, 2019, 5361620. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; Erbuth, A.; Boyko, M.; Vonderbank, S.; Gürleyen, H.; Gibis, N.; Bastian, A. Comparison of ultrasound measurements for diaphragmatic mobility, diaphragmatic thickness, and diaphragm thickening fraction with each other and with lung function in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 2217–2227. [Google Scholar] [CrossRef] [PubMed]
- Rittayamai, N.; Chuaychoo, B.; Tscheikuna, J.; Dres, M.; Goligher, E.C.; Brochard, L. Ultrasound evaluation of diaphragm force reserve in patients with chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2020, 17, 1222–1230. [Google Scholar] [CrossRef]
- Okura, K.; Iwakura, M.; Shibata, K.; Kawagoshi, A.; Sugawara, K.; Takahashi, H.; Satake, M.; Shioya, T. Diaphragm thickening assessed by ultrasonography is lower than healthy adults in patients with chronic obstructive pulmonary disease. Clin. Respir. J. 2020, 14, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Topcuoğlu, C.; Yümin, E.T.; Hizal, M.; Konuk, S. Examination of diaphragm thickness, mobility and thickening fraction in individuals with COPD of different severity. Turk. J. Med. Sci. 2022, 52, 1288–1298. [Google Scholar] [CrossRef]
- Okura, K.; Kawagoshi, A.; Iwakura, M.; Sugawara, K.; Takahashi, H.; Kashiwagura, T.; Homma, M.; Satake, M.; Shioya, T. Contractile capability of the diaphragm assessed by ultrasonography predicts nocturnal oxygen saturation in COPD. Respirology 2017, 22, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Baria, M.R.; Shahgholi, L.; Sorenson, E.J.; Harper, C.J.; Lim, K.G.; Strommen, J.A.; Mottram, C.D.; Boon, A.J. B-mode ultrasound assessment of diaphragm structure and function in patients with COPD. Chest 2014, 146, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ogan, N.; Aydemir, Y.; Evrin, T.; Ataç, G.K.; Baha, A.; Katipoğlu, B.; Süzen, B.; Akpinar, E.E. Diaphragmatic thickness in chronic obstructive pulmonary disease and relationship with clinical severity parameters. Turk. J. Med. Sci. 2019, 49, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- An, T.J.; Yoo, Y.J.; Lim, J.U.; Seo, W.; Park, C.K.; Rhee, C.K.; Yoon, H.K. Diaphragm ultrasound is an imaging biomarker that distinguishes exacerbation status from stable chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Lim, G.; Lee, Y.J.; Cho, Y.J.; Park, J.S.; Yoon, H.I.; Lee, J.H.; Lee, C.T. Ultrasound assessment of diaphragmatic function during acute exacerbation of chronic obstructive pulmonary disease: A pilot study. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2479–2484. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Sguazzotti, I.; Zanoni, M.; Messina, A.; Colombo, D.; Vignazia, G.L.; Vetrugno, L.; Garofalo, E.; Bruni, A.; Navalesi, P.; et al. Diaphragmatic ultrasound assessment in subjects with acute hypercapnic respiratory failure admitted to the Emergency Department. Respir. Care 2019, 64, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Antenora, F.; Fantini, R.; Iattoni, A.; Castaniere, I.; Sdanganelli, A.; Livrieri, F.; Tonelli, R.; Zona, S.; Monelli, M.; Clini, E.M.; et al. Prevalence and outcomes of diaphragmatic dysfunction assessed by ultrasound technology during acute exacerbation of COPD: A pilot study. Respirology 2017, 22, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Souza, H.; Rocha, T.; Pessoa, M.; Rattes, C.; Brandão, D.; Fregonezi, G.; Campos, S.; Aliverti, A.; Dornelas, A. Effects of inspiratory muscle training in elderly women on respiratory muscle strength, diaphragm thickness and mobility. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Ichiba, T.; Miyagawa, T.; Tsuda, T.; Kera, T.; Yasuda, O. Changes in diaphragm thickness and 5-min walking distance improvement after inspiratory muscle training in patients with chronic obstructive pulmonary disease: Clinical trial. Heliyon 2023, 9, e20079. [Google Scholar] [CrossRef]
- Sepúlveda-Loyola, W.; Osadnik, C.; Phu, S.; Morita, A.A.; Duque, G.; Probst, D.S. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 1164–1176. [Google Scholar] [CrossRef]
- Nouvenne, A.; Zani, M.D.; Milanese, G.; Parise, A.; Baciarello, M.; Bignami, E.G.; Odone, A.; Sverzellati, N.; Meschi, T.; Ticinesi, A. Lung Ultrasound in COVID-19 Pneumonia: Correlations with Chest CT on Hospital admission. Respiration 2020, 99, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Zanforlin, A.; Strapazzon, G.; Falk, M.; Gallina, V.; Viteritti, A.; Valzolgher, L.; La Guardia, M.; Ferro, F.; Pagani, L.; Vezzali, N. Lung Ultrasound in the Emergency Department for Early Identification of COVID-19 Pneumonia. Respiration 2021, 100, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Nouvenne, A.; Ticinesi, A.; Parise, A.; Prati, B.; Esposito, M.; Cocchi, V.; Crisafulli, E.; Volpi, A.; Rossi, S.; Bignami, E.G.; et al. Point-of-Care Chest Ultrasonography as a Diagnostic Resource for COVID-19 Outbreak in Nursing Homes. J. Am. Med. Dir. Assoc. 2020, 21, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; de Vries, H.J.; Vlaar, A.P.J.; van der Hoeven, J.; Boon, R.A.; Heunks, L.M.A.; Ottenheijm, C.A.C.; Dutch COVID-19 Diaphrgam Investigators. Diaphragm pathology in critically ill patients with COVID-19 and postmortem findings from 3 medical centers. JAMA Intern. Med. 2021, 181, 122–124. [Google Scholar] [CrossRef] [PubMed]
- López-Viñas, L.; Vega-Villar, J.; Rocío-Martín, E.; García-García, P.; De La Rosa Santiago, E.; Galván-Román, J.M.; Wix-Ramos, R. Diaphragm impairment in patients admitted for severe COVID-19. Eur. J. Transl. Myol. 2022, 32, 10460. [Google Scholar] [CrossRef]
- Formenti, P.; Umbrello, M.; Castagna, V.; Cenci, S.; Bichi, F.; Pozzi, T.; Bonifazi, M.; Coppola, S.; Chiumello, D. Respiratory and peripheral muscular ultrasound characteristics in ICU COVID 19 ARDS patients. J. Crit. Care 2022, 67, 14–20. [Google Scholar] [CrossRef]
- Steinberg, I.; Chiodaroli, E.; Gattarello, S.; Cappio Borlino, S.; Chiumello, D. Diaphragmatic ultrasound and esophageal pressure in COVID-19 pneumonia during helmet C-PAP. Intensive Care Med. 2022, 48, 1095–1096. [Google Scholar] [CrossRef] [PubMed]
- Lassola, S.; Miori, S.; Sanna, A.; Cucino, A.; Magnoni, S.; Umbrello, M. Central venous pressure swing outperforms diaphragm ultrasound as a measure of inspiratory effort during pressure support ventilation in COVID-19 patients. J. Clin. Monit. Comput. 2022, 36, 461–471. [Google Scholar] [CrossRef]
- Corradi, F.; Isirdi, A.; Malacarne, P.; Santori, G.; Barbieri, G.; Romei, C.; Bove, T.; Vetrugno, L.; Falcone, M.; Bertini, P.; et al. Low diaphragm muscle mass predicts adverse outcome in patients hospitalized for COVID-19 pneumonia: An exploratory pilot study. Minerva Anestesiol. 2021, 87, 432–438. [Google Scholar] [CrossRef]
- Pivetta, E.; Cara, I.; Paglietta, G.; Scategni, V.; Labarile, G.; Tizzani, M.; Porrino, G.; Locatelli, S.; Calzolari, G.; Morello, F.; et al. Diaphragmatica point-of-care ultrasound in COVID-19 patients in the Emergency Department-a proof-of-concept study. J. Clin. Med. 2021, 10, 5291. [Google Scholar] [CrossRef]
- Helmy, M.A.; Milad, L.M.; Hasanin, A.; Mostafa, M. The novel use of diaphragmatic excursion on hospital admission to predict the need for ventilatory support in patients with coronavirus disease 2019. Anaesth. Crit. Care Pain Med. 2021, 40, 100976. [Google Scholar] [CrossRef] [PubMed]
- Lázaro-Sierra, J.; Doz Arcas, M.; Clavería Marco, P.; Rosell Abos, M.T.; Santolaria López, M.A.; Pérez Gimenez, L.; Lanzuela Benedicto, T.; Zuil Martin, M.; Boldova Loscertales, A.; García Saez, S.; et al. Prognostic value of diaphragmatic ultrasound in patients admitted for COVID-19 pneumonia. Open Respir. Arch. 2023, 6, 100290. [Google Scholar] [CrossRef] [PubMed]
- Hadda, V.; Raja, A.; Suri, T.M.; Khan, M.A.; Mittal, S.; Madan, K.; Mohan, A. Temporal evolution of diaphragm thickness and diaphragm excursion among subjects hospitalized with COVID-19: A prospective observational study. Respir. Med. Res. 2023, 83, 100960. [Google Scholar] [CrossRef] [PubMed]
- Corradi, F.; Vetrugno, L.; Orso, D.; Bove, T.; Schreiber, A.; Boero, E.; Santori, G.; Isirdi, A.; Barbieri, G.; Forfori, F. Diaphragmatic thickening fraction as a potential predictor of response to continuous positive airway pressure ventilation in Covid-19 pneumonia: A single-center pilot study. Respir. Physiol. Neurobiol. 2021, 284, 103585. [Google Scholar] [CrossRef] [PubMed]
- Dal, H.C.; Dolek, B.A.; Beyoğlu, M.A.; Acar, D.; Gozukara, M.G.; Turan, S. Evaluation of diaphragm thickness to predict intubation requirement and mortality in critical COVID-19 patients. Saudi Med. J. 2022, 43, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Haaksma, M.E.; Smit, J.M.; Kramer, R.; Heldeweg, M.L.A.; Veldhuis, L.I.; Lieveld, A.; Pikerie, D.; Mousa, A.; Girbes, A.R.J.; Heunks, L.; et al. Evolution of respiratory muscles thickness in mechanically ventilated patients with COVID-19. Respir. Care 2022, 67, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Umbrello, M.; Guglielmetti, L.; Formenti, P.; Antonucci, E.; Cereghini, S.; Filardo, C.; Montanari, G.; Muttini, S. Qualitative and quantitative muscle ultrasound changes in patients with COVID-19-related ARDS. Nutrition 2021, 92, 111499. [Google Scholar] [CrossRef] [PubMed]
- Dams, K.; De Meyer, G.R.; Jacobs, R.; Schepens, T.; Perkisas, S.; Moorkens, G.; Jorens, P. Combined ultrasound of m. quadriceps and diaphragm to determine the occurrence of sarcopenia and prolonged ventilation in a COVID-19 ICU cohort: The COVID-SARCUS trial. Nutrition 2024, 117, 112250. [Google Scholar] [CrossRef] [PubMed]
- Vetrugno, L.; Orso, D.; Corradi, F.; Zani, G.; Spadaro, S.; Meroi, F.; D’Andrea, N.; Bove, T.; Cammarota, G.; De Robertis, E.; et al. Diaphragm ultrasound evaluation during weaning from mechanical ventilation in COVID-19 patients: A pragmatic, cross-section, multicenter study. Respir. Res. 2022, 23, 210. [Google Scholar] [CrossRef]
- Helmy, M.A.; Milad, L.M.; Osman, S.H.; Ali, M.A.; Hasanin, A. Diaphragmatic excursion: A possible key player for predicting successful weaning in patients with severe COVID-19. Anaesth. Crit. Care Pain Med. 2021, 40, 100875. [Google Scholar] [CrossRef]
- Gagliardi, V.; Butturini, A.; Ferraro, G.; Gagliardi, G. Diaphragm ultrasound: A valuable predictor of the outcome of extubation. An observational pilot study in Covid-19 related ARDS. Arch. Clin. Biomed. Res. 2022, 6, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Romero-Morales, C.; Falla, D.; Pecos-Martín, D.; García-Pérez-de-Sevilla, G.; García-Bermejo, P.; Navarro-Flores, E.; López-López, D. Ultrasound imaging assessment of the diaphragm and abdominal muscles in people with a recent history of moderate Covid-19 infection and healthy participants: A cross-sectional pilot study. PLoS ONE 2023, 18, e0281098. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Seisdedos, M.N.; Valcárcel-Linares, D.; Gómez-González, M.T.; Lázaro-Navas, I.; López-González, L.; Pecos-Martín, D.; Rodríguez-Costa, I. Inspiratory muscle strength and function in mechanically ventilated COVID-19 survivors 3 and 6 months after intensive care unit discharge. ERJ Open Res. 2023, 9, 00329–02022. [Google Scholar] [CrossRef]
- Regmi, B.; Friedrich, J.; Jörn, B.; Senol, M.; Giannoni, A.; Boentert, M.; Daher, A.; Dreher, M.; Spiesshofer, J. Diaphragm muscle weakness might explain exertional dyspnea 15 months after hospitalization for COVID-19. Am. J. Respir. Crit. Care Med. 2023, 207, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Boussuges, A.; Habert, P.; Chaumet, G.; Rouibah, R.; Delorme, L.; Menard, A.; Million, M.; Bartoli, A.; Guedj, E.; Gouitaa, M.; et al. Diaphragm ultrasound dysfunction after severe COVID-19: An ultrasound study. Front. Med. 2022, 9, 949281. [Google Scholar] [CrossRef] [PubMed]
- Eman, G.; Synn, S.; Galen, B.; Shah, R.; Nauka, P.; Hope, A.A.; Congdon, S.; Islam, M. Thoracic ultrasound in COVID-19: Use of lung and diaphragm ultrasound in evaluating dyspnea in survivors of acute respiratory distress syndrome from COVID-19 pneumonia in a post-ICU clinic. Lung 2023, 201, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Farr, E.; Wolfe, A.R.; Deshmukh, S.; Rydberg, L.; Soriano, R.; Walter, J.M.; Boon, A.J.; Wolfe, L.F.; Franz, C.K. Diaphragm dysfunction in severe COVID-19 as determined by neuromuscular ultrasound. Ann. Clin. Transl. Neurol. 2021, 8, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Spiesshofer, J.; Friedrich, J.; Regmi, B.; Geppert, J.; Jörn, B.; Kersten, A.; Giannoni, A.; Boentert, M.; Marx, G.; Marx, N.; et al. Diaphragm dysfunction as a potential determinant of dyspnea on exertion in patients 1 year after COVID-19-related ARDS. Respir. Res. 2022, 23, 187. [Google Scholar] [CrossRef] [PubMed]
- Veldman, C.; de Boer, W.S.; Kerstjens, H.A.M.; Edens, M.A.; van den Berg, J.W.K. Sonographic follow-up of diaphragm function in COVID-19: An exploratory study. ERJ Open Res. 2023, 9, 00623–02022. [Google Scholar] [CrossRef]
- Vieira da Costa, K.; Cordeiro de Souza, I.T.; Dos Santos Felix, J.V.; Furtado Brandão, C.B.; de Souza Fernandes, V.M.; Lugon Favero, A.B.; de Aquino Gouveia, M.L.; Tavares de Lima, D.; de Morais Lima, J.H.; Pedrosa, R.; et al. Efficacy of a rehabilitation protocol on pulmonary and respiratory muscle function and ultrasound evaluation of diaphragm and quadriceps femoris in patients with post-COVID-19 syndrome: A series of cases. Monaldi Arch. Chest Dis. 2023, 93, 2206. [Google Scholar] [CrossRef]
- Tana, C.; Moffa, L.; Falasca, K.; Vecchiet, J.; Tana, M.; Mantini, C.; Ricci, F.; Ticinesi, A.; Meschi, T.; Cipollone, F.; et al. Approach to COVID-19 in older adults and indications for improving the outcomes. Ann. Med. 2023, 55, 2265298. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, K.; Gaşowski, J.; Michel, J.P.; Veronese, N. Post-COVID-19 acute sarcopenia: Physiopathology and management. Aging Clin. Exp. Res. 2021, 33, 2887–2898. [Google Scholar] [CrossRef] [PubMed]
- Montes-Ibarra, M.; Oliveira, C.L.P.; Orsso, C.E.; Landi, F.; Marzetti, E.; Prado, C.M. The Impact of Long COVID-19 on Muscle Health. Clin. Geriatr. Med. 2022, 38, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.C.; Zarcone, C.; Tassistro, E.; Rebora, P.; Rossi, E.; Luppi, F.; Foti, G.; Squillace, N.; Lettino, M.; Strepparava, M.G.; et al. Frailty and long-COVID: Is COVID-19 responsible for a transition in frailty status among older adults who survived hospitalization for COVID-19? Aging Clin. Exp. Res. 2023, 35, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Covino, M.; Russo, A.; Salini, S.; De Matteis, G.; Simeoni, B.; Pirone, F.; Massaro, C.; Recupero, C.; Landi, F.; Gasbarrini, A.; et al. Long-Term Effects of Hospitalization for COVID-19 on Frailty and Quality of Life in Older Adults ≥ 80 Years. J. Clin. Med. 2022, 11, 5787. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.G.; Verdi, E.B.; Süslü, S.N.; Öz, M.; Erol, S.; Çiftçi, F.; Çiledağ, A.; Kaya, A. Can diaphragm excursion predict prognosis in patients with severe pneumonia? Tuberk. Toraks. 2021, 69, 51–519. [Google Scholar]
- Chu, S.E.; Lu, J.X.; Chang, S.C.; Hsu, K.H.; Goh, Z.N.L.; Seak, C.K.; Seak, J.C.Y.; Ng, C.J.; Seak, C.J. Point-of-care application of diaphragmatic ultrasonography in the emergency department for the prediction of development of respiratory failure in community-acquired pneumonia: A pilot study. Front. Med. 2022, 9, 960847. [Google Scholar] [CrossRef]
- Şik, N.; Çitlenbik, H.; Öztürk, A.; Yilmaz, D.; Duman, M. Point of care diaphragm ultrasound: An objective tool to predict the severity of pneumonia and outcomes in children. Pediatr. Pulmonol. 2021, 56, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Lima, A.R.; Felippe Martinez, P.; Luiz Damatto, R.; Mariano Cezar, M.D.; Mendes Guizoni, D.; Bonomo, C.; Assis Oliveira, S., Jr.; Dal-Pai Silva, M.; Mamede Zornoff, L.A.; Okoshi, K.; et al. Heart-failure induced diaphragm myopathy. Cell. Physiol. Biochem. 2014, 34, 333–345. [Google Scholar] [CrossRef]
- Arutyunov, A.G.; Ilyina, K.V.; Arutyunov, G.P.; Kolesnikova, E.A.; Pchelin, V.V.; Kulagina, N.P.; Tokmin, D.S.; Tulyakova, E.V. Morphofunctional features of the diaphragm in patients with chronic heart failure. Kardiologiia 2019, 59, 12–21. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kinugasa, Y.; Sugihara, S.; Mukai-Yatagai, N.; Kato, M. Ultrasonographic assessment of organs other than the heart in patients with heart failure. J. Med. Ultrason. 2019, 46, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Caruana, L.; Petrie, M.C.; McMurray, J.J.; MacFarlane, N.G. Altered diaphragm position and function in patients with chronic heart failure. Eur. J. Heart Fail. 2001, 3, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, S.; Di Matteo, E.; Finamore, P.; Perri, G.; Mancini, D.; Sogaro, L.; Grandi, T.; Brando, E.; Travaglino, F.; Sambuco, F.; et al. Diaphragmatic ultrasound evaluation in acute heart failure: Clinical and functional associations. Intern. Emerg. Med. 2024, 19, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, M.; Kinugasa, Y.; Sota, T.; Yamada, K.; Ishisugi, T.; Hirai, M.; Yanagihara, K.; Haruki, N.; Matsubara, K.; Kato, M.; et al. Diaphragm muscle dysfunction in patients with heart failure. J. Card. Fail. 2018, 24, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Kinugasa, Y.; Sota, T.; Miyagi, M.; Sugihara, S.; Kato, M.; Yamamoto, K. Inspiratory muscle weakness is associated with exercise intolerance in patients with heart failure with preserved ejection fraction: A preliminary study. J. Card. Fail. 2016, 22, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, K.; Miyagi, M.; Sota, T.; Yamada, K.; Ishigushi, T.; Hirai, M.; Yanagihara, K.; Haruki, N.; Matsubara, K.; Kato, M.; et al. Dynapenia and diaphragm muscle dysfunction in patients with heart failure. Eur. J. Prev. Cardiol. 2018, 25, 1785–1786. [Google Scholar] [CrossRef] [PubMed]
- Spiesshofer, J.; Henke, C.; Kabitz, H.J.; Bengel, P.; Schütt, K.; Nofer, J.R.; Spieker, M.; Orwat, S.; Diller, G.P.; Strecker, J.K.; et al. Heart failure results in inspiratory muscle dysfunction irrespective of left ventricular ejection fraction. Respiration 2021, 100, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Andriopoulou, M.; Dimaki, N.; Kallistratos, M.S.; Chamodraka, E.; Jahaj, E.; Vassiliou, A.G.; Giokas, G.; Kotanidou, A.; Manolis, A.J.; Piepoli, M.F.; et al. Skeletal muscle alterations and exercise intolerance in heart failure with preserved ejection fraction patients: Ultrasonography assessment of diaphragm and quadriceps. Eur. J. Heart Fail. 2022, 24, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yin, Q.; Wang, S.Y.; Wang, Y.Y.; Xiao, J.J.; Tang, T.T.; Ni, W.J.; Ren, L.Q.; Liu, H.; Zhang, X.L.; et al. Ultrasound-assessed diaphragm dysfunction predicts clinical outcomes in hemodialysis patients. Sci. Rep. 2022, 12, 16550. [Google Scholar] [CrossRef]
- Hobson-Webb, L.D.; Simmons, S. Ultrasound in the diagnosis and monitoring of amyotrophic lateral sclerosis: A review. Muscle Nerve 2019, 60, 114–123. [Google Scholar] [CrossRef]
- Sartucci, F.; Pelagatti, A.; Santin, M.; Bocci, T.; Dolciotti, C.; Bongioanni, P. Diaphragm ultrasonography in amyotrophic lateral sclerosis: A diagnostic tool to assess ventilatory dysfunction and disease severity. Neurol. Sci. 2019, 40, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulos, K.; Lykouras, D.; Veltsista, D.; Skaramagkas, V.; Karkoulias, K.; Tzouvelekis, A.; Chroni, E. The utility of diaphragm ultrasound thickening indices for assessing respiratory decompensation in amyotrophic lateral sclerosis. Muscle Nerve 2023, 68, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.; Alves, P.; Pimentel, B.; Swash, M.; de Carvalho, M. Ultrasound for assessment of diaphragm in ALS. Clin. Neurophysiol. 2016, 127, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Ma, J.; Pang, X.; Huang, S.; Zhang, J.; Wang, J.; Chang, X.; Guo, J.; Zhang, W. Diaphragm ultrasound in the diagnosis of respiratory dysfunction in patients with amyotrophic lateral sclerosis. Rev. Neurol. 2021, 177, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Fantini, R.; Mandrioli, J.; Zona, S.; Antenora, F.; Iattoni, A.; Monelli, M.; Fini, N.; Tonelli, R.; Clini, E.; Marchioni, A. Ultrasound assessment of diaphragmatic function in patients with amyotrophic lateral sclerosis. Respirology 2016, 21, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Fantini, R.; Tonelli, R.; Castaniere, I.; Tabbì, L.; Pellegrino, M.R.; Cerri, S.; Livrieri, F.; Giaroni, F.; Monelli, M.; Ruggieri, V.; et al. Serial ultrasound assessment of diaphragmatic function and clinical outcome in patients with amyotrophic lateral sclerosis. BMC Pulm. Med. 2019, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Summerhill, E.M.; El-Sameed, Y.A.; Glidden, T.J.; McCool, F.D. Monitoring recovery from diaphragm paralysis with ultrasound. Chest 2008, 133, 737–743. [Google Scholar] [CrossRef]
- Yajima, W.; Yoshida, T.; Kondo, T.; Uzura, M. Respiratory failure due to diaphragm paralysis after brachial plexus injury diagnosed by point-of-care ultrasound. BMJ Case Rep. 2022, 15, e246923. [Google Scholar] [CrossRef] [PubMed]
- Caleffi-Pereira, M.; Pletsch-Assunção, R.; Zumpano Cardenas, L.; Vieira Santana, P.; Ferreira, J.G.; Iamonti, V.C.; Caruso, P.; Fernandez, A.; Ribeiro de Carvalho, C.R.; Pereira Albuquerque, A.L. Unilateral diaphragm paralysis: A dysfunction restricted not just to one hemidiaphragm. BMC Pulm. Med. 2018, 18, 126. [Google Scholar] [CrossRef]
- Kim, M.; Lee, K.; Cho, J.; Lee, W. Diaphragm thickness and inspiratory muscle functions in chronic stroke patients. Med. Sci. Monit. 2017, 23, 1247–1253. [Google Scholar] [CrossRef]
- Liu, X.; Qu, Q.; Deng, P.; Zhao, Y.; Liu, C.; Fu, C.; Jia, J. Assessment of diaphragm in hemiplegic patients after stroke with ultrasound and its correlation of extremity motor and balance function. Brain Sci. 2022, 12, 882. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, S.; Liao, L.; He, J.; Tang, D.; Wu, W.; Wang, K. Diaphragmatic ultrasound can help evaluate pulmonary dysfunction in patients with stroke. Front. Neurol. 2023, 14, 1061003. [Google Scholar] [CrossRef] [PubMed]
- Park, G.Y.; Kim, S.R.; Kim, Y.W.; Jo, K.W.; Lee, E.J.; Kim, Y.M.; Im, S. Decreased diaphragm excursion in stroke patients with dysphagia as assessed by M-Mode sonography. Arch. Phys. Med. Rehabil. 2015, 96, 114–121. [Google Scholar] [CrossRef]
- Choi, Y.M.; Park, G.Y.; Yoo, Y.; Sohn, D.; Jang, Y.; Im, S. Reduced diaphragm excursion during reflexive citric acid cough test in subjects with subacute stroke. Respir. Care 2017, 62, 1571–1581. [Google Scholar] [CrossRef]
- Nucci, R.A.B.; Rodrigues de Souza, R.; Suemoto, C.K.; Busse, A.L.; Mesiano Maifrino, L.B.; Anaruma, C.A.; Pasqualucci, C.A.; Jacob-Filho, W. Diaphragm muscle structure in the elderly: Findings from an autopsy study. Acta Histochem. 2020, 122, 151487. [Google Scholar] [CrossRef]
- Greising, S.M.; Mantilla, C.B.; Gorman, B.A.; Ermilov, L.G.; Sieck, G.C. Diaphragm muscle sarcopenia in aging mice. Exp. Gerontol. 2013, 48, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Greising, S.M.; Mantilla, C.B.; Medina-Martínez, J.S.; Stowe, J.M.; Sieck, G.C. Functional impact of diaphragm muscle sarcopenia in both male and female mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L46–L52. [Google Scholar] [CrossRef]
- Greising, S.M.; Medina-Martínez, J.S.; Vasdev, A.K.; Sieck, G.C.; Mantilla, C.B. Analysis of muscle fiber clustering in the diaphragm of sarcopenic mice. Muscle Nerve 2015, 52, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.E.; Greising, S.M.; Mantilla, C.B.; Sieck, G.C. Functional impact of sarcopenia in respiratory muscles. Respir. Physiol. Neurobiol. 2016, 226, 137–146. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Mantilla, C.B.; Sieck, G.C. Impact of sarcopenia on diaphragm muscle fatigue. Exp. Physiol. 2019, 104, 1090–1099. [Google Scholar] [CrossRef]
- Viang, P.; Vasdev, A.; Zhan, W.Z.; Gransee, H.M.; Sieck, G.C.; Mantilla, C.B. Diaphragm muscle sarcopenia into very old age in mice. Physiol. Rep. 2020, 8, e14305. [Google Scholar] [CrossRef] [PubMed]
- Dres, M.; Dubé, B.P.; Mayaux, J.; Delemazure, J.; Reuter, D.; Brochard, L.; Similowski, T.; Demoule, A. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am. J. Respir. Crit. Care Med. 2017, 195, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.P.; Dietz, A.R.; Johnson, R.; Shoykhet, M.; Zaidman, C.M. Reliability of bedside ultrasound of limb and diaphragm muscle thickness in critically ill children. Muscle Nerve 2019, 59, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Neto Silva, I.; Duarte, J.A.; Perret, A.; Dousse, N.; Wozniak, H.; Bollen Pinto, B.; Giraud, R.; Bendjelid, K. Diaphragm dysfunction and peripheral muscle wasting in septic shock patients: Exploring their relationship over time using ultrasound technology (the MUSiShock protocol). PLoS ONE 2022, 17, e0266174. [Google Scholar] [CrossRef] [PubMed]
- Carámbula, A.; Pereyra, S.; Barbato, M.; Angulo, M. Combined diaphragm and limb muscle atrophy is associated with increased mortality in mechanically ventilated patients: A pilot study. Arch. Bronconeumol. 2021, 57, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Morisawa, T.; Okamoto, H.; Nakanishi, N.; Matsumoto, N.; Saitoh, M.; Takahashi, T.; Fujiwara, T. Diaphragm dysfunction and ICU-acquired weakness in septic shock patients with or without mechanical ventilation: A pilot prospective observational study. J. Clin. Med. 2023, 12, 5191. [Google Scholar] [CrossRef] [PubMed]
- Maynard-Paquette, A.C.; Poirier, C.; Chartrand-Lefebvre, C.; Dubé, B.P. Ultrasound evaluation of the quadriceps muscle contractile index in patients with stable chronic obstructive pulmonary disease: Relationships with clinical symptoms, disease severity and diaphragm contractility. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Vitor de Carvalho, E.S.; da Silva Santos, G.; Rocha de Siqueira, G.; Branco Pinto Duarte, A.L.; Tavares Dantas, A. Ultrasound assessment of diaphragm and quadriceps muscles and its relationship with handgrip and respiratory muscle strength in patients with systemic sclerosis: A cross-sectional study. Clin. Rheumatol. 2024, 43, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; He, S.; Lu, H.; Liang, G.; Ben, X.; Zhong, W.; Zhang, M.; Wang, H. Prediction of loss of muscle mass in sarcopenia using ultrasonic diaphragm excursion. Contrast Media Mol. Imaging 2021, 2021, 4754705. [Google Scholar] [CrossRef]
- Deniz, O.; Coteli, S.; Karatoprak, N.B.; Pence, M.C.; Varan, H.D.; Kizilarslanoglu, M.C.; Oktar, S.O.; Goker, B. Diaphragmatic muscle thickness in older people with and without sarcopenia. Aging Clin. Exp. Res. 2021, 33, 573–580. [Google Scholar] [CrossRef]
- Lee, Y.; Son, S.; Kim, D.K.; Park, M.W. Association of diaphragm thickness and respiratory muscle strength with indices of sarcopenia. Ann. Rehabil. Med. 2023, 47, 307–314. [Google Scholar] [CrossRef]
- Van Doorn, J.L.M.; Wijntjes, J.; Saris, C.G.J.; Ottenheijm, C.A.C.; van Alfen, N.; Doorduin, J. Association of diaphragm thickness and echogenicity with age, sex, and body mass index in healthy subjects. Muscle Nerve 2022, 66, 197–202. [Google Scholar] [CrossRef]
- Kocjan, J.; Gzik-Zroska, B.; Nowakowska, K.; Burkacki, M.; Suchoń, S.; Michnik, R.; Czyźewski, D.; Adamek, M. Impact of diaphragm function parameters on balance maintenance. PLoS ONE 2018, 13, e0208697. [Google Scholar] [CrossRef] [PubMed]
- Monti, E.; Tagliaferri, S.; Zampieri, S.; Sarto, F.; Sirago, G.; Franchi, M.V.; Ticinesi, A.; Longobucco, Y.; Adorni, E.; Lauretani, F.; et al. Effects of a 2-year exercise training on neuromuscular system health in older individuals with low muscle function. J. Cachexia Sarcopenia Muscle 2023, 14, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Ticinesi, A.; Gionti, L.; Prati, B.; Nouvenne, A.; Tana, C.; Meschi, T.; Maggio, M. Short-Physical Performance Battery (SPPB) score is associated with falls in older outpatients. Aging Clin. Exp. Res. 2019, 31, 1435–1442. [Google Scholar] [CrossRef]
- Güngör, S.; Doğan, A. Diaphragm thickness by ultrasound in pediatric patients with primary malnutrition. Eur. J. Pediatr. 2023, 182, 3347–3354. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, S.; Okoye, C.; Zotti, S.; Lauretani, F.; Nouvenne, A.; Cerundolo, N.; Bruni, A.A.; Torrini, M.; Finazzi, A.; Mazzarone, T.; et al. Advancing healthcare through thoracic ultrasound research in older patients. Aging Clin. Exp. Res. 2023, 35, 2887–2901. [Google Scholar] [CrossRef]


| Author, Year [Ref] | Parameter | Normative Values in Males | Normative Values in Females | Ethnicity of Participants |
|---|---|---|---|---|
| Ueki, 1995 [39] | Thickness at TLC | 4.5 ± 0.9 mm | - | Asian |
| Thickness at FRC | 1.7 ± 0.2 mm | - | ||
| Thickness at RV | 1.6 ± 0.2 mm | - | ||
| Boussuges, 2009 [27] | Excursion on QB | 18 ± 4 mm | 16 ± 4 mm | Caucasian |
| Excursion on DB | 75 ± 9 mm | 64 ± 10 mm | ||
| Boon, 2013 [42] | Thickness at FRC | 3.8 ± 1.5 mm | 2.7 ± 1 mm | Caucasian |
| Thickening difference on DB | 18 ± 5 mm | 18 ± 5 mm | ||
| Harper, 2013 [41] | Thickness at TV | 3.7 ± 1.4 mm | Caucasian | |
| Thickness at FRC | 3.2 ± 1.4 mm | |||
| Thickening ratio on QB | 1.20 ± 0.15 | |||
| Carrillo-Esper, 2016 [43] | Thickness at FRC | 1.9 ± 0.4 mm | 1.4 ± 0.3 mm | Caucasian |
| Scarlata, 2019 [24] | Excursion on DB | 65 ± 13 mm | 55 ± 14 mm | Caucasian |
| Thickness at TLC | 2.8 ± 0.5 mm | 2.4 ± 0.5 mm | ||
| Thickness at FRC | 1.9 ± 0.4 mm | 1.7 ± 0.4 mm | ||
| Spiesshoefer, 2020 [25] | Excursion on QB | 17 ± 6 mm | 15 ± 5 mm | Caucasian |
| Excursion on DB | 91 ± 19 mm | 75 ± 16 mm | ||
| Thickness at TLC | 6.3 ± 1.7 mm | 4.7 ± 1.7 mm | ||
| Thickness at FRC | 2.2 ± 0.8 mm | 1.8 ± 0.5 mm | ||
| Thickening ratio on DB | 3.03 ± 0.95 | 2.77 ± 0.83 | ||
| Kabil, 2022 [40] | Excursion on QB | 24 ± 5 mm | 22 ± 5 mm | Arab |
| Excursion on DB | 57 ± 13 mm | 52 ± 12 mm | ||
| Excursion on QB (over 65) | 23 ± 4 mm | |||
| Excursion on DB (over 65) | 61 ± 22 mm | |||
| Author, Year [Ref] | Population | Exposure Variable (Ultrasound) | Endpoint Assessed | Main Findings |
|---|---|---|---|---|
| Huang, 2017 [72] | 40 ICU patients aged ≥80 under IV for ≥48 h and meeting the criteria for spontaneous breathing trial | Diaphragm excursion (DD defined as <10.7 mm) | Maintenance of spontaneous breathing for >48 h | Diaphragm excursion ≥ 10.7 mm was predictive of weaning success (AUROC 0.839) |
| Kocyigit, 2021 [73] | 60 patients with COPD and respiratory failure needing NIV support in ED (mean age 70) | Diaphragm thickness (DD defined as thickening fraction <20% during spontaneous breathing) | NIV failure (worsened blood gas analysis, altered mental status, worsening dyspnea, need for IV) | DD predicted NIV failure (sensitivity 84.6%, specificity 91.5%, PPV 73.3%, NPV 95.6%) |
| Er, 2023 [74] | 32 ICU patients aged ≥65 under IV for ≥48 h and meeting the criteria for spontaneous breathing trial | Diaphragm thickness and excursion | Weaning failure (reintubation or mortality within 48 h after extubation) | Diaphragm excursion was the only parameter associated with weaning failure |
| Author, Year [Ref] | Population | Mean Age | Ultrasound Variable of Interest | Main Findings |
|---|---|---|---|---|
| An, 2022 [106] | 55 patients with COPD, either stable or exacerbated | 73 ± 8 | Thickening fraction and excursion on maximum inspiration | Reduced thickening fraction (AUROC 0.745) and reduced excursion (AUROC 0.721) were able to classify exacerbation status |
| Lim, 2019 [107] | 10 patients with non-critical acute exacerbation of COPD | 80 ± 8 | Thickening fraction of the right diaphragm on spontaneous breathing | Thickening fraction improved from the acute phase to improvement of symptoms; no variations in excursion |
| Cammarota, 2019 [108] | 21 patients with acute hypercapnic respiratory failure presenting to ED | 70–86 (range) | Diaphragm thickness and excursion under NIV | The amplitude of diaphragmatic excursion predicted NIV success (arterial blood pH > 7.35), but not thickness or thickening fraction |
| Antenora, 2017 [109] | 41 patients with acute exacerbation of COPD and acidosis | 76 | Change in diaphragm thickness under spontaneous breathing (ΔTdi) | ΔTdi correlated with NIV failure, ICU stay and mortality |
| Author, Year [Ref] | Population | Age | Ultrasound Variable of Interest | Main Findings |
|---|---|---|---|---|
| Yamada, 2016 [157] | 40 patients hospitalized with HFpEF | 76 ± 12 | Diaphragm muscle thickening at end-inspiration (cut-off < 3.9 mm) | Diaphragm dysfunction was associated with inspiratory muscle weakness and shorter 6MWD |
| Miyagi, 2018 [156] | 77 patients hospitalized with heart failure | 72 ± 15 | Diaphragm muscle thickening at end-inspiration (cut-off < 4 mm) | Diaphragm dysfunction was associated with older age, lower vital capacity, reduced grip strength, reduced inspiratory muscle strength and shorter 6MWD |
| Kinugasa, 2018 [158] | 62 patients hospitalized with heart failure | 72 ± 15 | Diaphragm muscle thickening at end-inspiration (cut-off < 4 mm) | Diaphragm dysfunction was more prevalent in patients with dynapenia (reduced muscle strength) or sarcopenia (reduced muscle mass and strength) |
| Spiesshoefer, 2021 [159] | 22 patients with HFrEF (A), 8 patients with HFpEF (B), 19 healthy controls (C) | 61 ± 13 (A) 68 ± 9 (B) 57 ± 10 (C) | Diaphragm thickening ratio on maximal inspiration | Diaphragmatic dysfunction was equally present in subjects with HFrEF and in subjects with HFpEF |
| Andriopoulou, 2022 [160] | 25 HFpEF patients, 25 matched controls | 64 ± 12 | Diaphragm excursion during deep breathing | Diaphragm excursion exhibited a strong positive correlation with VO2 in both cases and controls |
| Scarlata, 2024 [155] | 72 acutely decompensated heart failure patients (P), 100 healthy volunteers (C) | 78 (76–81) (P) 41 (38-44) (C) | Diaphragm thickness on tidal volume and TLC Diaphragm motion during deep breathing | Diaphragm excursion on TLC is reduced in acute heart failure, with an inverse correlation with NYHA class. Diaphragm thickness is increased in comparison with controls |
| Author, Year [Ref] | Population | Diaphragm Ultrasound Parameter of Interest | Method of Sarcopenia Assessment | Main Findings |
|---|---|---|---|---|
| Zeng, 2021 [191] | 64 older (age ≥ 60) patients undergoing outpatient evaluation for lung cancer or nodules | Diaphragm excursion | ASM/height based on BIA with internally validated cut-offs | Diaphragm excursion on forced deep breathing ≤ 5.27 cm was associated with increased odds of sarcopenia (AUROC 0.778) |
| Deniz, 2021 [192] | 30 sarcopenic and 30 non-sarcopenic subjects (age ≥ 65) | Diaphragm thickness | EWGSOP criteria | Diaphragm thickness was reduced at all pulmonary volumes in subjects with sarcopenia and was an independent predictor of the sarcopenic status |
| Lee, 2023 [193] | 45 healthy volunteers aged ≥65 | Diaphragm thickness | ASM/BMI based on BIA measurement | ASM/BMI showed significant positive correlation with diaphragm thickness (r = 0.319). ASM/BMI and diaphragm thickness were predictors of maximal expiratory pressure |
| Setting | Condition | Ultrasound Parameters of Interest | Clinical Significance |
|---|---|---|---|
| Intensive care unit | Respiratory failure | Reduced thickening ratio (in adults) Reduced excursion (in older subjects) | Prediction of IV weaning failure |
| Acute-care wards | Exacerbation of COPD | Reduced excursion and thickening ratio on TLC | Prediction of NIV trial failure and duration of hospital stay |
| Congestive heart failure | Reduced excursion and thickness | Associated with increasing NYHA class and exercise intolerance | |
| Bacterial pneumonia | Reduced excursion and thickening fraction | Prediction of progression to respiratory failure, need for IV and mortality | |
| Viral pneumonia (COVID-19) | Reduced excursion and thickness, increased thickening ratio | Prediction of need for NIV, IV, ICU admission and mortality | |
| Long-term care/outpatient clinics | COPD | Excursion on different pulmonary volumes | Association with spirometric parameters |
| Long COVID syndrome | Reduced maximal excursion | Associated with exhaustion and subjective dyspnea (diagnostic aid) | |
| Amyotrophic lateral sclerosis | Reduced thickening ratio | Marker of disease progression to end-stage respiratory failure | |
| Previous stroke | Reduced excursion | Associated with diaphragm paresis, dysphagia and reduced balance | |
| Physical frailty and sarcopenia | Reduced thickness and thickening ratio, reduced excursion | Marker of respiratory involvement of the sarcopenia syndrome, marker of severity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siniscalchi, C.; Nouvenne, A.; Cerundolo, N.; Meschi, T.; Ticinesi, A.; on behalf of the Parma Post-Graduate Specialization School in Emergency-Urgency Medicine Interest Group on Thoracic Ultrasound. Diaphragm Ultrasound in Different Clinical Scenarios: A Review with a Focus on Older Patients. Geriatrics 2024, 9, 70. https://doi.org/10.3390/geriatrics9030070
Siniscalchi C, Nouvenne A, Cerundolo N, Meschi T, Ticinesi A, on behalf of the Parma Post-Graduate Specialization School in Emergency-Urgency Medicine Interest Group on Thoracic Ultrasound. Diaphragm Ultrasound in Different Clinical Scenarios: A Review with a Focus on Older Patients. Geriatrics. 2024; 9(3):70. https://doi.org/10.3390/geriatrics9030070
Chicago/Turabian StyleSiniscalchi, Carmine, Antonio Nouvenne, Nicoletta Cerundolo, Tiziana Meschi, Andrea Ticinesi, and on behalf of the Parma Post-Graduate Specialization School in Emergency-Urgency Medicine Interest Group on Thoracic Ultrasound. 2024. "Diaphragm Ultrasound in Different Clinical Scenarios: A Review with a Focus on Older Patients" Geriatrics 9, no. 3: 70. https://doi.org/10.3390/geriatrics9030070
APA StyleSiniscalchi, C., Nouvenne, A., Cerundolo, N., Meschi, T., Ticinesi, A., & on behalf of the Parma Post-Graduate Specialization School in Emergency-Urgency Medicine Interest Group on Thoracic Ultrasound. (2024). Diaphragm Ultrasound in Different Clinical Scenarios: A Review with a Focus on Older Patients. Geriatrics, 9(3), 70. https://doi.org/10.3390/geriatrics9030070






