Circadian Rhythm of Distal Skin Temperature in Healthy Older and Young Women and Its Relationship with Sleep–Wake Rhythm and Environmental Factors under Natural Living Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Assessment of General Characteristics
2.4. Recording of Distal Skin Temperature
2.5. Assessment of Sleep–Wake Variables, Physical Activity, and Light Exposure
2.6. Statistical Analyses
3. Results
3.1. General Characteristics and Sleep–Wake Rhythm of Participants
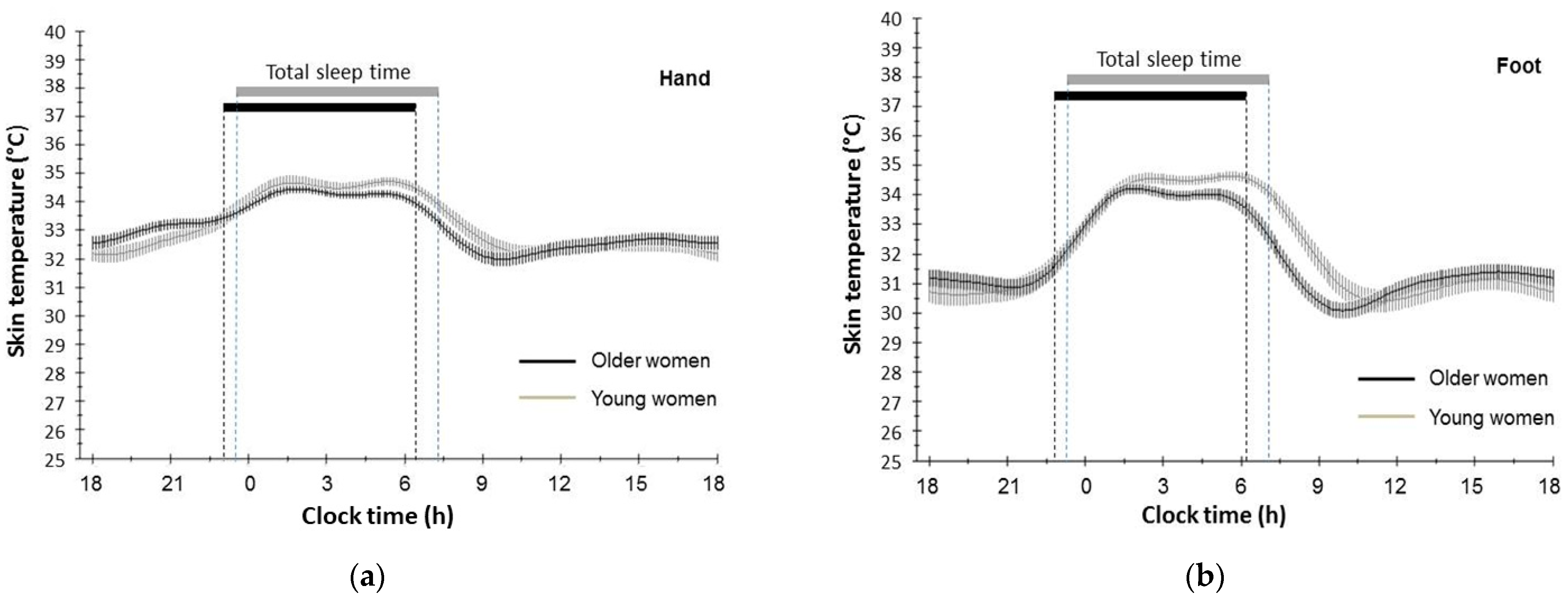
3.2. Circadian Rhythm of Distal Skin Temperature of Hand and Foot in Older and Young Participants
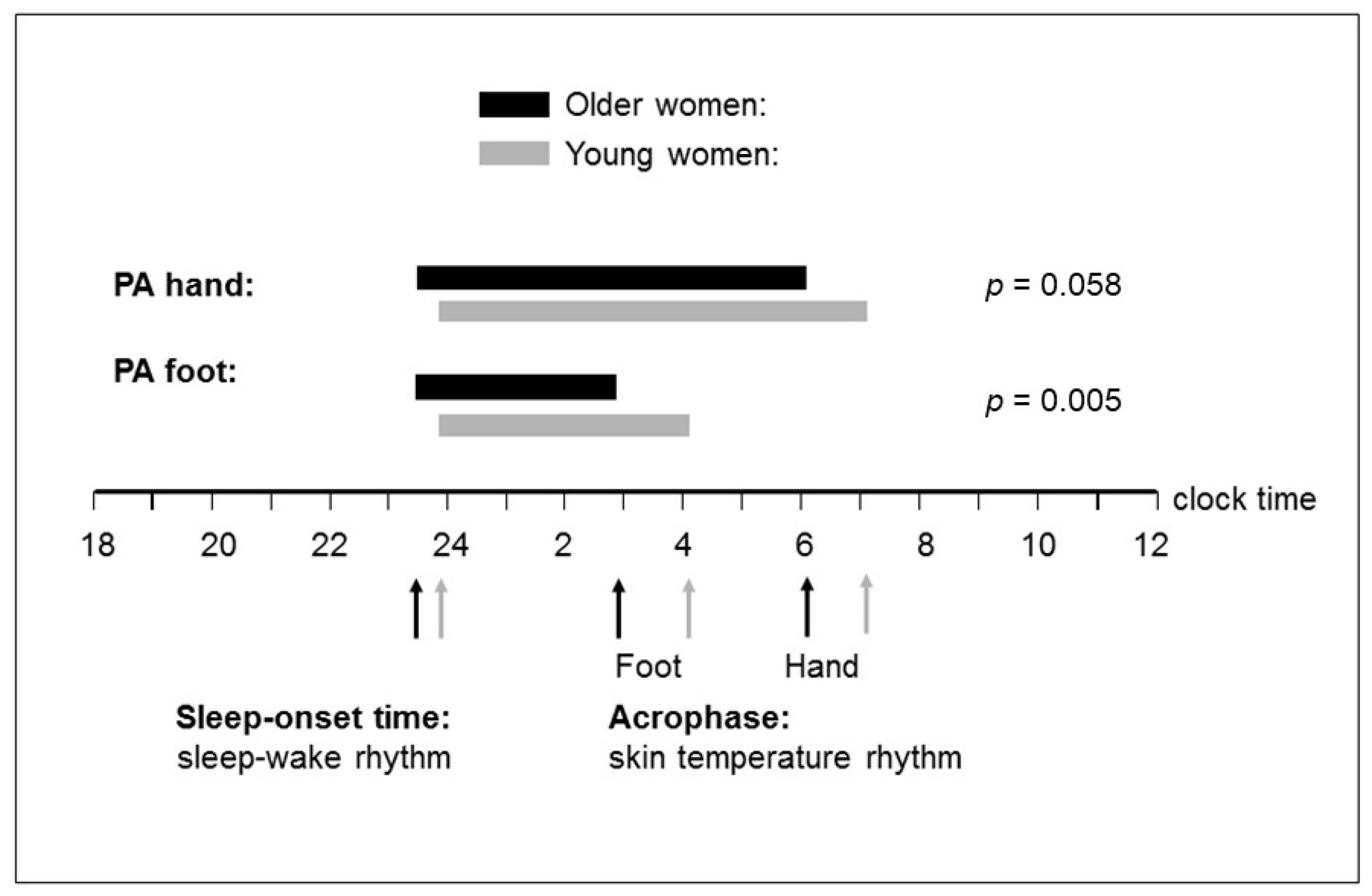
3.3. Phase Relationship (Time Interval) between Distal Skin Temperature Rhythm and Sleep–Wake Rhythm
3.4. Relationship of Distal Skin Temperature Rhythm and Phase Angle with the Environmental Light Exposure and Physical Activity
4. Discussion
4.1. Changes in the Amplitude for Circadian DST Rhythm with Healthy Aging and Environmental Influences
4.2. Changes in the Acrophase for Circadian DST Rhythm with Healthy Aging and Environmental Influences
4.3. Altered Phase Relationship between Circadian DST Rhythm and Sleep–Wake Rhythm with Aging
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gachon, F.; Nagoshi, E.; Brown, S.A.; Ripperger, J.; Schibler, U. The mammalian circadian timing system: From gene expression to physiology. Chromosoma 2004, 113, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.L.; Badia, P. Changes in circadian rhythms and sleep quality with aging: Mechanisms and interventions. Neurosci. Biobehav. Rev. 1995, 19, 553–557. [Google Scholar] [CrossRef]
- Van Gool, W.A.; Mirmiran, M. Aging and circadian rhythms. Prog. Brain Res. 1986, 70, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Weinert, D. Circadian temperature variation and ageing. Ageing Res. Rev. 2010, 9, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, E.D.; Moline, M.L.; Czeisler, C.A.; Zimmerman, J.C. Chronobiology of aging: Temperature, sleep–wake rhythms and entrainment. Neurobiol. Aging 1982, 3, 299–309. [Google Scholar] [CrossRef]
- Buijink, M.R.; Michel, S. A multi-level assessment of the bidirectional relationship between aging and the circadian clock. J. Neurochem. 2021, 157, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, Y.; Zhaon, Z. Impacts of aging on circadian rhythm and related sleep disorders. Biosystems 2024, 236, 105111. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.S.; Murphy, P.J. Relationships between sleep and body temperature in middle-aged and older subjects. J. Am. Geriatr. Soc. 1998, 46, 458–462. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Dumont, M.; Duffy, J.F.; Steinberg, J.D.; Richardson, G.S.; Brown, E.N.; Sánchez, R.; Ríos, C.D.; Ronda, J.M. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet 1992, 340, 933–936. [Google Scholar] [CrossRef]
- Duffy, J.F.; Dijk, D.J.; Klerman, E.B.; Czeisler, C.A. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am. J. Physiol. 1998, 275, R1478–R1487. [Google Scholar] [CrossRef]
- Kim, S.J.; Benloucif, S.; Reid, K.J.; Weintraub, S.; Kennedy, N.; Wolfe, L.F.; Zee, P.C. Phase-shifting response to light in older adults. J. Physiol. 2014, 592, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Nonaka, K.; Nishida, N.; Hayashida, N.; Miyahara, Y.; Kotorii, T.; Matsuoka, K. Comparison of body temperature rhythms between healthy elderly and healthy young adults. Jpn. J. Psychiatry Neurol. 1991, 45, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Monk, T.H.; Kupfer, D.J. Circadian rhythms in healthy aging—Effects downstream from the pacemaker. Chronobiol. Int. 2000, 17, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Renfrew, J.W.; Pettigrew, K.D.; Rapoport, S.I. Motor activity and sleep duration as a function of age in healthy men. Physiol. Behav. 1987, 41, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Haus, E. Alterations with aging of the endocrine and neuroendocrine circadian system in humans. Chronobiol. Int. 2000, 17, 369–390. [Google Scholar] [CrossRef]
- Vitiello, M.V.; Smallwood, R.G.; Avery, D.H.; Pascualy, R.A.; Martin, D.C.; Prinz, P.N. Circadian temperature rhythms in young adult and aged man. Neurobiol. Aging 1986, 7, 97–100. [Google Scholar] [CrossRef]
- Raymann, R.J.; Swaab, D.F.; Van Someren, E.J. Skin temperature and sleep-onset latency: Changes with age and insomnia. Physiol. Behav. 2007, 90, 257–266. [Google Scholar] [CrossRef]
- Harding, E.C.; Franks, N.P.; Wisden, W. The temperature dependence of sleep. Front. Neurosci. 2019, 24, 13:336. [Google Scholar] [CrossRef] [PubMed]
- Batinga, H.; Martinez-Nicolas, A.; Zornoza-Moreno, M.; Sánchez-Solis, M.; Larqué, E.; Mondéjar, M.T.; Moreno-Casbas, M.; García, F.J.; Campos, M.; Rol, M.A.; et al. Ontogeny and aging of the distal skin temperature rhythm in humans. Age 2015, 37, 29. [Google Scholar] [CrossRef]
- Kräuchi, K.; Cajochen, C.; Pache, M.; Flammer, J.; Wirz-Justice, A. Thermoregulatory effects of melatonin in relation to sleepiness. Chronobiol. Int. 2006, 23, 475–484. [Google Scholar] [CrossRef]
- Kräuchi, K.; Cajochen, C.; Werth, E.; Wirz-Justice, A. Functional link between distal vasodilation and sleep-onset latency? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R741–R748. [Google Scholar] [CrossRef]
- Cuesta, M.; Boudreau, P.; Cermakian, N.; Boivin, D.B. Skin temperature rhythms in humans respond to changes in the timing of sleep and light. J. Biol. Rhythms 2017, 32, 257–273. [Google Scholar] [CrossRef]
- Sarabia, J.A.; Rol, M.A.; Mendiola, P.; Madrid, J.A. Circadian rhythm of wrist temperature in normal-living subjects. A candidate of new index of the circadian system. Physiol. Behav. 2008, 95, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Kräuchi, K.; Wirz-Justice, A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am. J. Physiol. 1994, 267, R819–R829. [Google Scholar] [CrossRef]
- Martinez-Nicolas, A.; Ortiz-Tudela, E.; Rol, M.A.; Madrid, J.A. Uncovering different masking factors on wrist skin temperature rhythm in free-living subjects. PLoS ONE 2013, 8, e61142. [Google Scholar] [CrossRef]
- Martinez-Nicolas, A.; Guaita, M.; Santamaría, J.; Montserrat, J.M.; Rol, M.Á.; Madrid, J.A. Circadian impairment of distal skin temperature rhythm in patients with sleep-disordered breathing: The effect of CPAP. Sleep 2017, 40, zsx067. [Google Scholar] [CrossRef] [PubMed]
- Harfmann, B.D.; Schroder, E.A.; England, J.H.; Senn, N.J.; Westgate, P.M.; Esser, K.A.; Kern, P.A. Temperature as a crcadian marker in older human subjects: Relationship to metabolic syndrome and diabetes. J. Endocr. Soc. 2017, 1, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Okamoto-Mizuno, K.; Tsuzuki, K. Effects of season on sleep and skin temperature in the elderly. Int. J. Biometeorol. 2010, 54, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.A.; Swaab, D.F. Influence of aging on the seasonal rhythm of the vasopressin-expressing neurons in the human suprachiasmatic nucleus. Neurobiol. Aging 1995, 16, 965–971. [Google Scholar] [CrossRef]
- Swaab, D.F.; Fliers, E.; Partiman, T.S. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985, 342, 37–44. [Google Scholar] [CrossRef]
- Michel, S.; Meijer, J.H. From clock to functional pacemaker. Eur. J. Neurosci. 2020, 51, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Sutin, E.L.; Dement, W.C.; Heller, H.C.; Kilduff, T.S. Light-induced gene expression in the suprachiasmatic nucleus of young and aging rats. Neurobiol. Aging 1993, 14, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Weinert, D. Age-dependent changes of the circadian system. Chronobiol. Int. 2000, 7, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Bromundt, V.; Frey, S.; Cajochen, C. Age-related neuroendocrine and alerting responses to light. Geroscience 2021, 43, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Charman, W.N. Age, lens transmittance, and the possible effects of light on melatonin suppression. Ophthalmic Physiol. Opt. 2003, 23, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.F.; Zeitzer, J.M.; Czeisler, C.A. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol. Aging 2007, 28, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Sletten, T.L.; Revell, V.L.; Middleton, B.; Lederle, K.A.; Skene, D.J. Age-related changes in acute and phase-advancing responses to monochromatic light. J. Biol. Rhythms 2009, 24, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Júnior, P.R.H.; Sardeli, A.V. The effect of aging on body temperature: A systematic review and meta-analysis. Curr. Aging Sci. 2021, 14, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Lok, R.; van Koningsveld, M.J.; Gordijn, M.C.M.; Beersma, D.G.M.; Hut, R.A. Daytime melatonin and light independently affect human alertness and body temperature. J. Pineal Res. 2019, 67, e12583. [Google Scholar] [CrossRef]
- Yoon, I.Y.; Kripke, D.F.; Elliott, J.A.; Youngstedt, S.D.; Rex, K.M.; Hauger, R.L. Age-related changes of circadian rhythms and sleep-wake cycles. J. Am. Geriatr. Soc. 2003, 51, 1085–1091. [Google Scholar] [CrossRef]
- Campbell, S.S.; Gillin, J.C.; Kripke, D.F.; Erikson, P.; Clopto, P. Gender differences in the circadian temperature rhythms of healthy elderly subjects: Relationship to sleep quality. Sleep 1989, 12, 529–536. [Google Scholar] [PubMed]
- Dörhöfer, R.P.; Pirlich, M. Das B.I.A. Kompendium; Data Input GmbH: Darmstadt, Germany, 2007. [Google Scholar]
- Hasselberg, M.J.; McMahon, J.; Parker, K. The validity, reliability, and utility of the iButton for measurement of body temperature circadian rhythms in sleep/wake research. Sleep Med. 2013, 14, 5–11. [Google Scholar] [CrossRef]
- Van Marken Lichtenbelt, W.D.; Daanen, H.A.M.; Wouters, L.; Fronczek, R.; Raymann, R.J.E.M.; Severens, N.M.W.; Van Someren, E.J.W. Evaluation of wireless determination of skin temperature using iButtons. Physiol. Behav. 2006, 88, 489–497. [Google Scholar] [CrossRef]
- Gompper, B.; Bromundt, V.; Orgül, S.; Flammer, J.; Kräuchi, K. Phase relationship between skin temperature and sleep-wake rhythms in women with vascular dysregulation and controls under real-life conditions. Chronobiol. Int. 2010, 27, 1778–1796. [Google Scholar] [CrossRef]
- Murphy, S.L. Review of physical activity measurement using accelerometers in older adults: Considerations for research design and conduct. Prev. Med. 2009, 48, 108–114. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef]
- Lockley, S.W.; Skene, D.J.; Arendt, J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J. Sleep Res. 1999, 8, 175–183. [Google Scholar] [CrossRef]
- Van de Wouw, E.; Evenhuis, H.M.; Echteld, M.A. Comparison of two types of Actiwatch with polysomnography in older adults with intellectual disability: A pilot study. J. Intellect. Dev. Disabil. 2013, 38, 265–273. [Google Scholar] [CrossRef]
- Lichstein, K.L.; Stone, K.C.; Donaldson, J.; Nau, S.D.; Soeffing, J.P.; Murray, D.; Lester, K.W.; Aguillard, R.N. Actigraphy validation with insomnia. Sleep 2006, 29, 232–239. [Google Scholar] [CrossRef]
- Hoffmann, R.M.; Müller, T.; Hajak, G.; Cassel, W. Abend-Morgenprotokolle in Schlafforschung und Schlafmedizin—Ein Standardinstrument für den deutschsprachigen Raum [Evening and morning protocols in sleep research and sleep medicine—A standard instrument for German-speaking regions]. Somnologie 1997, 1, 103–109. [Google Scholar] [CrossRef]
- Martin, J.L.; Hakim, A.D. Wrist actigraphy. Chest 2011, 139, 1514–1527. [Google Scholar] [CrossRef]
- Zuther, P.; Gorbey, S.; Lemmer, B. Chronos-Fit. Version 1.06. 2009. Available online: https://www.researchgate.net/publication/265600087_Chronos-Fit_revised_Version_106 (accessed on 4 April 2024).
- Mattes, A.; Witte, K.; Hohmann, W.; Lemmer, B. PHARMFIT—A nonlinear fitting program for pharmacology. Chronobiol. Int. 1991, 8, 460–476. [Google Scholar] [CrossRef]
- Sletten, T.L.; Vincenzi, S.; Redman, J.R.; Lockley, S.W.; Rajaratnam, S.M. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front. Neurol. 2010, 1, 137. [Google Scholar] [CrossRef]
- Berardesca, E.; Farinelli, N.; Rabbiosi, G.; Maibach, H.I. Skin bioengineering in the noninvasive assessment of cutaneous aging. Dermatologica 1991, 182, 1–6. [Google Scholar] [CrossRef]
- Van Someren, E.J.W.; Raymann, R.J.E.M.; Scherder, E.J.A.; Daanen, H.A.M.; Swaab, D.F. Circadian and age-related modulation of thermoreception and temperature regulation: Mechanisms and functional implications. Ageing Res. Rev. 2002, 1, 721–778. [Google Scholar] [CrossRef]
- Lok, R.; Woelders, T.; van Koningsveld, M.J.; Oberman, K.; Fuhler, S.G.; Beersma, D.G.M.; Hut, R.A. Bright light decreases peripheral skin temperature in healthy men: A forced desynchrony study under dim and bright light (II). J. Biol. Rhythms. 2022, 37, 417–428. [Google Scholar] [CrossRef]
- Klerman, E.B.; Duffy, J.F.; Dijk, D.J.; Czeisler, C.A. Circadian phase resetting in older people by ocular bright light exposure. J. Investig. Med. 2001, 49, 30–40. [Google Scholar] [CrossRef]
- Benloucif, S.; Green, K.; L’Hermite-Balériaux, M.; Weintraub, S.; Wolfe, L.F.; Zee, P.C. Responsiveness of the aging circadian clock to light. Neurobiol. Aging 2006, 27, 1870–1879. [Google Scholar] [CrossRef]
- Yamazaki, S.; Straume, M.; Tei, H.; Sakaki, Y.; Menaker, M.; Block, G.D. Effects of aging on central and peripheral mammalian clocks. Proc. Natl Acad. Sci. USA 2002, 99, 10801–10806. [Google Scholar] [CrossRef]
- Feinsilver, S.H. Normal and abnormal sleep in the elderly. Clin. Geriatr. Med. 2021, 37, 377–386. [Google Scholar] [CrossRef]
- Hofman, M.A.; Fliers, E.; Goudsmit, E.; Swaab, D.F. Morphometric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: Sex differences and age- dependent changes. J. Anat. 1988, 160, 127–143. [Google Scholar] [PubMed Central]
- Kripke, D.F.; Youngstedt, S.D.; Elliott, J.A.; Tuunainen, A.; Rex, K.M.; Hauger, R.L.; Marler, M.R. Circadian phase in adults of contrasting ages. Chronobiol. Int. 2005, 22, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Kuchel, G.A. Heterogeneity of aging: Individual risk factors, mechanisms, patient priorities, and outcomes. J. Am. Geriatr. Soc. 2021, 69, 610–612. [Google Scholar] [CrossRef]
- Spitler, K.M.; Davies, B.S.J. Aging and plasma triglyceride metabolism. J. Lipid Res. 2020, 61, 1161–1167. [Google Scholar] [CrossRef]
- Shmulevich, R.; Krizhanovsky, V. Cell Senescence, DNA Damage, and Metabolism. Antioxid. Redox Signal. 2021, 34, 324–334. [Google Scholar] [CrossRef]
- Serin, Y.; Acar Tek, N. Effect of Circadian Rhythm on Metabolic Processes and the Regulation of Energy Balance. Ann. Nutr. Metab. 2019, 74, 322–330. [Google Scholar] [CrossRef]
- Rahman, S.A.; Gathungu, R.M.; Marur, V.R.; St Hilaire, M.A.; Scheuermaier, K.; Belenky, M.; Struble, J.S.; Czeisler, C.A.; Lockley, S.W.; Klerman, E.B.; et al. Age-related changes in circadian regulation of the human plasma lipidome. Commun. Biol. 2023, 6, 756. [Google Scholar] [CrossRef]
- Buijink, M.R.; van Weeghel, M.; Harms, A.; Murli, D.S.; Meijer, J.H.; Hankemeier, T.; Michel, S.; Kervezee, L. Loss of temporal coherence in the circadian metabolome across multiple tissues during ageing in mice. Eur. J. Neurosci. 2024, 60, 3843–3857. [Google Scholar] [CrossRef]
- Joyce, D.S.; Zele, A.J.; Feigl, B.; Adhikari, P. The accuracy of artificial and natural light measurements by actigraphs. J. Sleep Res. 2020, 29, e12963. [Google Scholar] [CrossRef]

| Character | Older Women (n = 35) | Young Women (n = 30) | Group Comparison | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t a | p | |
| Anthropometric characters | ||||||
| Age (yrs) | 68.94 | 4.94 | 25.63 | 3.64 | 39.64 | <0.001 |
| Weight (kg) | 67.09 | 6.52 | 65.46 | 8.83 | 0.85 | 0.397 |
| Height (m) | 1.64 | 0.05 | 1.69 | 0.05 | −4.50 | <0.001 |
| BMI (kg/m2) | 24.99 | 2.69 | 22.76 | 2.64 | 3.36 | 0.001 |
| Waist circumference (cm) | 84.47 | 8.32 | 72.98 | 6.55 | 6.11 | <0.001 |
| Hip circumference (cm) | 102.23 | 4.86 | 101.18 | 7.26 | 0.67 | 0.505 |
| Waist–hip index | 0.83 | 0.06 | 0.72 | 0.04 | 7.93 | <0.001 |
| Body composition b | ||||||
| Fat mass (kg) | 23.40 | 6.37 | 20.96 | 6.37 | 1.53 | 0.132 |
| Lean body mass (kg) | 44.17 | 2.68 | 44.46 | 3.73 | −0.36 | 0.718 |
| Body cell mass (kg) | 20.89 | 1.90 | 22.77 | 2.15 | −3.71 | <0.001 |
| Basal metabolic rate (kcal/24 h) | 1276 | 59.33 | 1335 | 66.94 | −3.73 | <0.001 |
| Physical activity c,d | ||||||
| Total activity (counts), day | 255,201 | 93,444 | 264,357 | 65,500 | −0.45 | 0.654 |
| Total activity (counts), night | 21,470 | 53,269 | 14,003 | 5881 | 0.76 | 0.448 |
| Light exposure c,d | ||||||
| Average light intensity (Lux), day | 1019 | 1124 | 744 | 736 | 1.19 | 0.241 |
| Average light intensity (Lux), night | 15 | 70 | 12 | 42 | 0.19 | 0.851 |
| Sleep–wake timing c,f | ||||||
| Awakening time (hh:mm) e | 06:35 | 45.09 | 07:35 | 37.28 | −5.73 | <0.001 |
| Bedtime (hh:mm) e | 22:56 | 35.63 | 23:26 | 37.92 | −3.34 | 0.001 |
| Sleep-onset time (hh:mm) e | 23:22 | 43.11 | 23:43 | 38.57 | −2.05 | 0.045 |
| Sleep onset latency (min) | 26.61 | 20.00 | 17.05 | 13.77 | 2.27 | 0.027 |
| Total sleep time (h) | 6.95 | 0.97 | 7.74 | 0.81 | −3.49 | 0.001 |
| WASO (min) | 60.00 | 27.30 | 50.61 | 33.14 | 1.25 | 0.215 |
| Sleep efficiency (%) | 87.75 | 7.61 | 95.46 | 2.37 | −4.81 | <0.001 |
| Skin Temperature | Older Women (n = 35) | Young Women (n = 30) | ANOVA for Repeated Measures | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Within Subject b | Within Subject b | Between Subjects c | ||||||||
| Older Women | Young Women | |||||||||
| Mean | SEM | Mean | SEM | F | p | F | p | F | p | |
| Hand (wrist) | ||||||||||
| Mesor (°C) | 33.11 | 0.11 | 33.21 | 0.10 | 2.26 | 0.073 | 0.52 | 0.730 | 0.04 | 0.844 |
| Amplitude (°C) | 1.16 | 0.10 | 1.44 | 0.09 | 0.68 | 0.664 | 0.64 | 0.701 | 4.17 | 0.046 |
| Acrophase (hh:mm) a | 05:55 | 19.79 | 07:01 | 12.68 | 1.41 | 0.241 | 1.44 | 0.204 | 8.79 | 0.004 |
| % Rhythm | 68.84 | 1.57 | 67.42 | 1.97 | - | - | - | - | - | - |
| Foot (heel) | ||||||||||
| Mesor (°C) | 31.87 | 0.10 | 32.12 | 0.15 | 0.65 | 0.630 | 0.64 | 0.652 | 0.21 | 0.151 |
| Amplitude (°C) | 1.80 | 0.09 | 2.20 | 0.15 | 0.39 | 0.845 | 2.02 | 0.066 | 0.54 | 0.023 |
| Acrophase (hh:mm) a | 02:48 | 15.95 | 04:01 | 11.10 | 1.81 | 0.127 | 1.81 | 0.099 | 13.06 | 0.001 |
| % Rhythm | 84.56 | 1.17 | 82.78 | 1.18 | - | - | - | - | - | - |
| Character | Older Women (n = 35) | Young Women (n = 30) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amplitude | Acrophase | Phase Angle a | Amplitude | Acrophase | Phase Angle a | |||||||
| r | p | r | p | r | p | r | p | r | p | r | p | |
| Light exposure, Average light intensity (Lux) b,c | ||||||||||||
| Hand, day | −0.42 | 0.023 | 0.30 | 0.106 | 0.33 | 0.072 | −0.02 | 0.903 | −0.10 | 0.608 | −0.05 | 0.806 |
| Foot, day | −0.45 | 0.007 | 0.00 | 0.999 | 0.07 | 0.678 | 0.03 | 0.867 | 0.04 | 0.850 | 0.12 | 0.523 |
| Hand, night | −0.09 | 0.647 | 0.17 | 0.372 | 0.15 | 0.424 | −0.04 | 0.838 | −0.11 | 0.583 | −0.20 | 0.299 |
| Foot, night | −0.10 | 0.572 | −0.06 | 0.753 | −0.06 | 0.719 | −0.01 | 0.964 | −0.10 | 0.593 | −0.23 | 0.217 |
| Physical activity, Total activity (counts) b,c | ||||||||||||
| Hand, day | −0.11 | 0.549 | −0.33 | 0.080 | −0.30 | 0.112 | −0.21 | 0.276 | −0.25 | 0.198 | −0.37 | 0.053 |
| Foot, day | −0.06 | 0.717 | −0.29 | 0.091 | −0.30 | 0.085 | −0.24 | 0.204 | −0.08 | 0.681 | −0.24 | 0.207 |
| Hand, night | −0.01 | 0.950 | −0.15 | 0.428 | −0.09 | 0.605 | −0.12 | 0.558 | −0.16 | 0.415 | −0.10 | 0.606 |
| Foot, night | −0.08 | 0.661 | −0.01 | 0.954 | 0.03 | 0.878 | −0.19 | 0.305 | 0.002 | 0.991 | 0.07 | 0.733 |
| Awakening time (hh:mm) b,d | ||||||||||||
| Hand | −0.05 | 0.802 | −0.03 | 0.880 | - | - | −0.27 | 0.181 | 0.42 | 0.031 | - | - |
| Foot | 0.06 | 0.728 | 0.31 | 0.074 | - | - | 0.24 | 0.213 | 0.56 | 0.002 | - | - |
| Sleep-onset time (hh:mm) b,d | ||||||||||||
| Hand | 0.002 | 0.991 | −0.11 | 0.548 | - | - | −0.31 | 0.106 | 0.37 | 0.050 | - | - |
| Foot | 0.01 | 0.975 | 0.33 | 0.057 | - | - | −0.03 | 0.888 | 0.59 | 0.001 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dittmar, M.; Stark, T.; Wedell, S. Circadian Rhythm of Distal Skin Temperature in Healthy Older and Young Women and Its Relationship with Sleep–Wake Rhythm and Environmental Factors under Natural Living Conditions. Geriatrics 2024, 9, 102. https://doi.org/10.3390/geriatrics9040102
Dittmar M, Stark T, Wedell S. Circadian Rhythm of Distal Skin Temperature in Healthy Older and Young Women and Its Relationship with Sleep–Wake Rhythm and Environmental Factors under Natural Living Conditions. Geriatrics. 2024; 9(4):102. https://doi.org/10.3390/geriatrics9040102
Chicago/Turabian StyleDittmar, Manuela, Tina Stark, and Stefanie Wedell. 2024. "Circadian Rhythm of Distal Skin Temperature in Healthy Older and Young Women and Its Relationship with Sleep–Wake Rhythm and Environmental Factors under Natural Living Conditions" Geriatrics 9, no. 4: 102. https://doi.org/10.3390/geriatrics9040102






