Physical Function Trajectory among High-Functioning Long-Term Care Facility Residents: Utilizing Japanese National Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Population
2.2. Data Collection
2.3. Outcome
2.4. Exposure
2.5. Statistical Analysis
3. Results
3.1. Participant Selection and Follow-Up
3.2. Participant Characteristics
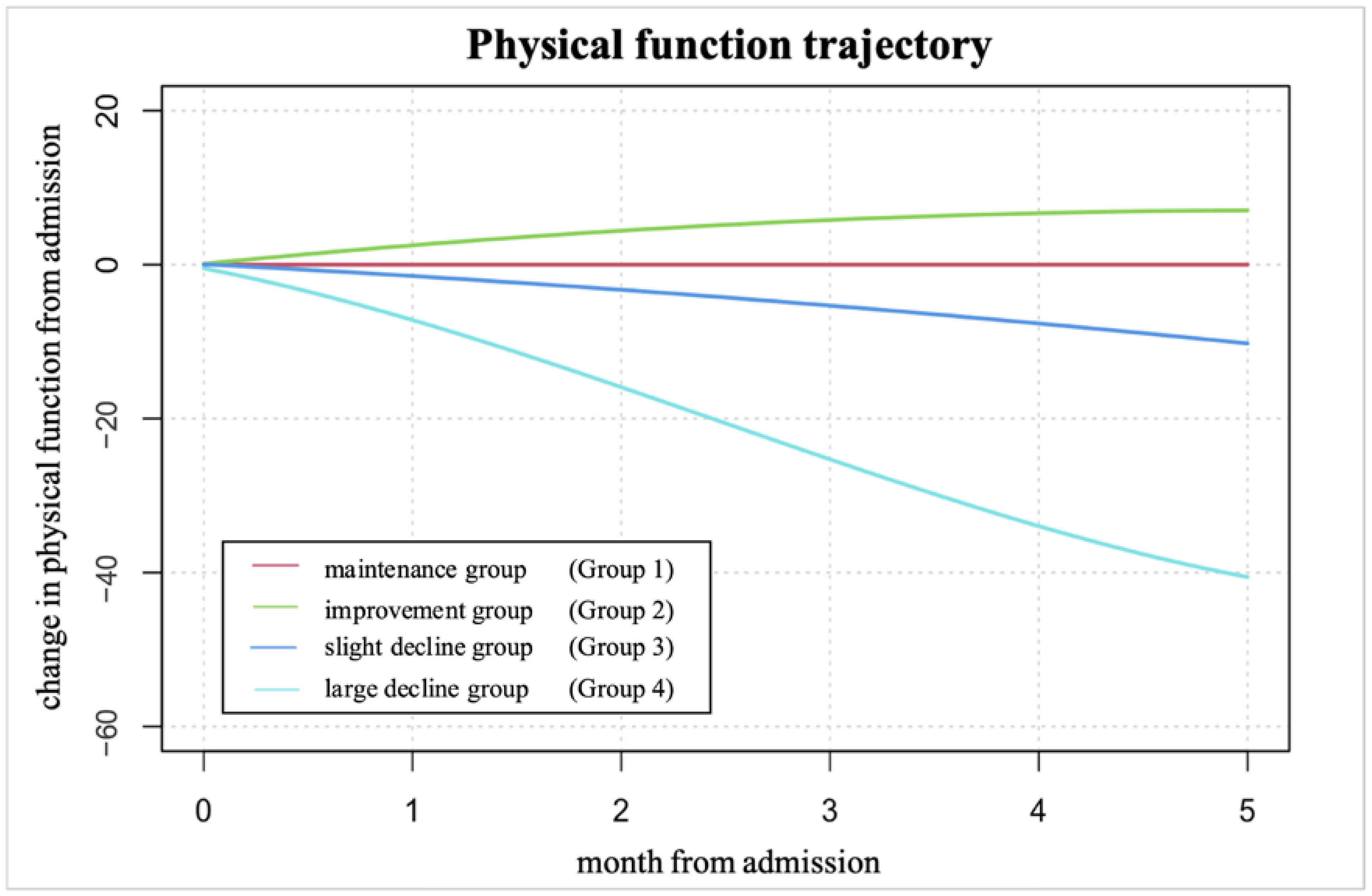
3.3. Trajectory
3.4. Characteristics of Residents for Each Identified PFT
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 7 September 2024).
- Ministry of Health, Labour and Welfare. Recent Developments in the Long-Term Care Insurance System. Available online: https://www.mhlw.go.jp/content/12300000/000917423.pdf (accessed on 7 September 2024).
- Ward, K.T.; Reuben, D.B. Comprehensive Geriatric Assessment. UpToDate 2016, 4, 13–18. [Google Scholar]
- Amankwaa, I.; Nelson, K.; Rook, H.; Hales, C. Association between body mass index, multi-morbidity and activities of daily living among New Zealand nursing home older adults: A retrospective analysis of nationwide InterRAI Data. BMC Geriatr. 2022, 22, 62. [Google Scholar] [CrossRef]
- Jindai, K.; Nielson, C.M.; Vorderstrasse, B.A.; Quiñones, A.R. Multimorbidity and functional limitations among adults 65 or older, NHANES 2005-2012. Prev. Chronic Dis. 2016, 13, E151. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.-P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.M.E.E.G.; Mahanani, W.R.; et al. The world report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-F.; Lin, P.-L. Frail phenotype and mortality prediction: A systematic review and meta-analysis of prospective cohort studies. Int. J. Nurs. Stud. 2015, 52, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwée, D.; Habbig, A.-K.; Scafoglieri, A.; Jansen, B.; Bautmans, I. Gerontopole Brussels study group frailty and the prediction of negative health outcomes: A meta-analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1–1163.e17. [Google Scholar] [CrossRef]
- Banaszak-Holl, J.; Liang, J.; Quiñones, A.; Cigolle, C.; Lee, I.-C.; Verbrugge, L.M. Trajectories of functional change among long stayers in nursing homes: Does baseline impairment matter? J. Aging Health 2011, 23, 862–882. [Google Scholar] [CrossRef]
- Charles, A.; Detilleux, J.; Buckinx, F.; Reginster, J.-Y.; Gruslin, B.; Bruyère, O. Physical performance trajectories and mortality among nursing home residents: Results of the SENIOR cohort. Age Ageing 2020, 49, 800–806. [Google Scholar] [CrossRef]
- Kuo, H.-T.; Lin, K.-C.; Lan, C.-F.; Li, I.-C. Activities of daily living Trajectories among institutionalised older adults: A prospective study. J. Clin. Nurs. 2017, 26, 4756–4767. [Google Scholar] [CrossRef]
- Yuan, Y.; Lapane, K.L.; Tjia, J.; Baek, J.; Liu, S.-H.; Ulbricht, C.M. Trajectories of physical frailty and cognitive impairment in older adults in United States nursing homes. BMC Geriatr. 2022, 22, 339. [Google Scholar] [CrossRef]
- Noguchi-Watanabe, M.; Ishikawa, T.; Ikuta, K.; Aishima, M.; Nonaka, S.; Takahashi, K.; Anzai, T.; Fukui, S. Physical function decline predictors in nursing home residents using new national quality indicators. Geriatr. Gerontol. Int. 2023, 24, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Nitta, J.; Sasaki, H.; Watanabe, T.; Sakamoto, T.; Komoto, S.; Arai, H. Japan’s long-term care issues: Construction and adoption of the LIFE database for establishing evidence-based care practice. J. Am. Med. Dir. Assoc. 2022, 23, 1433–1434. [Google Scholar] [CrossRef] [PubMed]
- Saito, J.; Kondo, N.; Saito, M.; Takagi, D.; Tani, Y.; Haseda, M.; Tabuchi, T.; Kondo, K. Exploring 2.5-Year Trajectories of Functional Decline in Older Adults by Applying a Growth Mixture Model and Frequency of Outings as a Predictor: A 2010–2013 JAGES Longitudinal Study. J. Epidemiol. 2019, 29, 65–72. [Google Scholar]
- United Nations. World Population Ageing 2015; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Statista. Demographics of Japan—Statistics & Facts. Available online: https://www.statista.com/topics/4675/demographics-in-japan/#topicOverview (accessed on 10 February 2024).
- Ministry of Health, Labour and Welfare. Number of Care Workers Needed Based on the Eighth Long-Term Care Insurance Plan. Available online: https://www.mhlw.go.jp/stf/houdou/0000207323_00005.html (accessed on 2 January 2024).
- Aishima, M.; Ishikawa, T.; Ikuta, K.; Noguchi-Watanabe, M.; Nonaka, S.; Takahashi, K.; Anzai, T.; Fukui, S. Unplanned hospital visits and poor oral health with undernutrition in nursing home residents. J. Am. Med. Dir. Assoc. 2023, 24, 1855–1860.e1. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Kinoshita, S.; Isowa, T.; Sakai, K.; Tohara, H.; Momosaki, R. Impact of motivation for eating habits, appetite and food satisfaction, and food consciousness on food intake and weight loss in older nursing home patients. Ann. Geriatr. Med. Res. 2024, 28, 110–115. [Google Scholar] [CrossRef]
- Bernabeu-Wittel, M.; Díez-Manglano, J.; Nieto-Martín, D.; Ramírez-Duque, N.; Ollero-Baturone, M.; En representación de los investigadores del Proyecto PROFUND; Investigadores del Proyecto PROFUND. Simplification of the Barthel Scale for Screening for Frailty and Severe Dependency in Polypathological Patients. Rev. Clin. Esp. 2019, 219, 433–439. [Google Scholar] [CrossRef]
- Nakanishi, M.; Hattori, K.; Nakashima, T.; Sawamura, K. Health care and personal care needs among residents in nursing homes, group homes, and congregate housing in Japan: Why does transition occur, and where can the frail elderly establish a permanent residence? J. Am. Med. Dir. Assoc. 2014, 15, 76.e1–76.e6. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the Sensitivity of the Barthel Index for Stroke Rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Moreno-Martin, P.; Jerez-Roig, J.; Rierola-Fochs, S.; Oliveira, V.R.; Farrés-Godayol, P.; Bezerra de Souza, D.L.; Giné-Garriga, M.; Booth, J.; Skelton, D.A.; Minobes-Molina, E. Incidence and predictive factors of functional decline in older people living in nursing homes: A systematic review. J. Am. Med. Dir. Assoc. 2022, 23, 1815–1825.e9. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. Long-Term Care Insurance in Japan. Survey of Institutions and Establishments for Long-Term Care. Available online: https://www.mhlw.go.jp/english/database/db-hss/dl/siel-2010-04.pdf (accessed on 1 September 2024).
- Tago, M.; Katsuki, N.E.; Yaita, S.; Nakatani, E.; Yamashita, S.; Oda, Y.; Yamashita, S.-I. High inter-rater reliability of Japanese bedriddenness ranks and cognitive function scores: A hospital-based prospective observational study. BMC Geriatr. 2021, 21, 168. [Google Scholar] [CrossRef]
- Baumgarten, M.; Becker, R.; Gauthier, S. Validity and reliability of the Dementia Behavior Disturbance Scale. J. Am. Geriatr. Soc. 1990, 38, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, T.; Iijima, S.; Eto, F.; Ishizuka, A.; Orimo, H. Reliability and validity of a Japanese version of the Dementia Behavior Disturbance Scale. Nihon Ronen Igakkai Zasshi 1993, 30, 835–840. [Google Scholar] [PubMed]
- Ito, K.; Ogisawa, F.; Furuta, K.; Awata, S.; Toba, K. Development of a five-item short-form version of the Dementia Behavior Disturbance Scale. Geriatr. Gerontol. Int. 2021, 21, 870–871. [Google Scholar] [CrossRef] [PubMed]
- Toba, K.; Nakai, R.; Akishita, M.; Iijima, S.; Nishinaga, M.; Mizoguchi, T.; Yamada, S.; Yumita, K.; Ouchi, Y. Vitality Index as a useful tool to assess elderly with dementia. Geriatr. Gerontol. Int. 2002, 2, 23–29. [Google Scholar] [CrossRef]
- Nagin, D.S.; Odgers, C.L. Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 2010, 6, 109–138. [Google Scholar] [CrossRef]
- Saito, J.; Murayama, H.; Ueno, T.; Saito, M.; Haseda, M.; Saito, T.; Kondo, K.; Kondo, N. Functional disability trajectories at the end of life among Japanese older adults: Findings from the Japan Gerontological Evaluation Study (JAGES). Age Ageing 2022, 51, afac260. [Google Scholar] [CrossRef]
- van de Schoot, R.; Sijbrandij, M.; Winter, S.D.; Depaoli, S.; Vermunt, J.K. The GRoLTS-Checklist: Guidelines for reporting on latent trajectory studies. Struct. Equ. Model. 2017, 24, 451–467. [Google Scholar] [CrossRef]
- Beer, N.; Riffat, A.; Volkmer, B.; Wyatt, D.; Lambe, K.; Sheehan, K.J. Patient perspectives of recovery after hip fracture: A systematic review and qualitative synthesis. Disabil. Rehabil. 2022, 44, 6194–6209. [Google Scholar] [CrossRef]
- Jeong, E.-H.; Park, J.-H. The relationship among leisure activities, depression and quality of life in community-dwelling elderly Koreans. Gerontol. Geriatr. Med. 2020, 6, 2333721420923449. [Google Scholar] [CrossRef]
- de Labra, C.; Guimaraes-Pinheiro, C.; Maseda, A.; Lorenzo, T.; Millán-Calenti, J.C. Effects of physical exercise interventions in frail older adults: A systematic review of randomized controlled trials. BMC Geriatr. 2015, 15, 154. [Google Scholar] [CrossRef]
- Crowley, K. Sleep and sleep disorders in older adults. Neuropsychol. Rev. 2011, 21, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Auerswald, T.; Meyer, J.; von Holdt, K.; Voelcker-Rehage, C. Application of activity trackers among nursing home residents-A pilot and feasibility study on physical activity behavior, usage behavior, acceptance, usability and motivational impact. Int. J. Environ. Res. Public Health 2020, 17, 6683. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Yin, H.; Jia, Y.; Zhao, J.; Meng, X.; Chen, L.; Liu, P. Nonpharmacological interventions to improve sleep in nursing home residents: A systematic review. Geriatr. Nurs. 2019, 40, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.-P.; Claßen, K.; Wahl, H.-W.; Hauer, K. Effects of interventions on physical activity in nursing home residents. Eur. J. Ageing 2015, 12, 261–271. [Google Scholar] [CrossRef]
- Ishii, S.; Weintraub, N.; Mervis, J.R. Apathy: A common psychiatric syndrome in the elderly. J. Am. Med. Dir. Assoc. 2009, 10, 381–393. [Google Scholar] [CrossRef]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef]
- Yoshida, M.; Suzuki, R.; Kikutani, T. Nutrition and oral status in elderly people. Jpn. Dent. Sci. Rev. 2014, 50, 9–14. [Google Scholar] [CrossRef]
- Saijo, M.; Takeshita, A.; Matsumoto, M.; Fukai, T.; Irie, K.; Kita, K.; Ono, Y.; Maruyama, Y.; Yasui, T. Relationship between degree of independence in daily activities and denture wearing status of residents of special nursing homes for elderly persons. Conn. Dent. Stud. J. 2021, 71, 147–152. [Google Scholar]
- Wöstmann, B.; Michel, K.; Brinkert, B.; Melchheier-Weskott, A.; Rehmann, P.; Balkenhol, M. Influence of denture improvement on the nutritional status and quality of life of geriatric patients. J. Dent. 2008, 36, 816–821. [Google Scholar] [CrossRef]

| N | 718 |
| Age (mean (SD)) | 85.69 (7.01) |
| Female (%) | 463 (64.5) |
| Low BMI * (%) | 179 (24.9) |
| Low cognitive function ‡ (%) | 492 (68.5) |
| History of aspiration pneumonia (%) | 7 (1.0) |
| Diagnosis of dementia (%) | 268 (37.3) |
| Physical function requiring assistance | |
| Feeding (%) | 19 (2.6) |
| Chair/bed transfer (%) | 77 (10.7) |
| Personal hygiene (%) | 125 (17.4) |
| Toilet (%) | 124 (17.3) |
| Self-bathing (%) | 578 (80.5) |
| Ambulation (%) | 298 (41.5) |
| Stair climbing (%) | 580 (80.8) |
| Dressing (%) | 198 (27.6) |
| Bowel control (%) | 223 (31.1) |
| Bladder control (%) | 126 (17.5) |
| Eating preference (soft food) (%) | 76 (10.6) |
| Using dentures (%) | 289 (40.3) |
| Choking easily (%) | 36 (5.0) |
| One item of the Vitality Index | |
| Low motivation (%) | 300 (41.8) |
| Five items of the DBD-13 | |
| Lack of interest in daily activities (%) | 196 (27.3) |
| Waking at night (%) | 121 (16.9) |
| Making an accusation (%) | 98 (13.6) |
| Walking around (%) | 159 (22.1) |
| Repeating the same action (%) | 122 (17.0) |
| Group 1 | Group 2 | Group 3 | Group 4 | p-Value | |
|---|---|---|---|---|---|
| Maintenance Group | Improvement Group | Slight Decline Group | Large Decline Group | ||
| N | 474 | 68 | 119 | 57 | |
| Barthel index at admission | 82.83 (10.56) | 76.76 (9.13) | 82.06 (10.56) | 82.63 (11.22) | <0.001 |
| Age (mean (SD)) | 85.48 (7.37) | 86.03 (7.06) | 85.95 (6.22) | 86.49 (5.27) | 0.688 |
| Female (%) | 305 (64.3) | 44 (64.7) | 80 (67.2) | 34 (59.6) | 0.807 |
| Low BMI * (%) | 113 (23.8) | 11 (16.2) | 31 (26.1) | 24 (42.1) | 0.007 |
| Low cognitive function ‡ (%) | 320 (67.5) | 46 (67.6) | 83 (69.7) | 43 (75.4) | 0.660 |
| Physical function requiring assistance | |||||
| Feeding (%) | 7 (1.5) | 4 (5.9) | 5 (4.2) | 3 (5.3) | 0.048 |
| Chair/bed transfer (%) | 46 (9.7) | 13 (19.1) | 10 (8.4) | 8 (14.0) | 0.077 |
| Personal hygiene (%) | 76 (16.0) | 15 (22.1) | 26 (21.8) | 8 (14.0) | 0.292 |
| Toilet (%) | 75 (15.8) | 18 (26.5) | 22 (18.5) | 9 (15.8) | 0.177 |
| Self-bathing (%) | 380 (80.2) | 62 (91.2) | 93 (78.2) | 43 (75.4) | 0.097 |
| Ambulation (%) | 190 (40.1) | 42 (61.8) | 47 (39.5) | 19 (33.3) | 0.003 |
| Stair climbing (%) | 384 (81.0) | 61 (89.7) | 91 (76.5) | 44 (77.2) | 0.145 |
| Dressing (%) | 120 (25.3) | 28 (41.2) | 36 (30.3) | 14 (24.6) | 0.042 |
| Bowel control (%) | 137 (28.9) | 29 (42.6) | 43 (36.1) | 14 (24.6) | 0.049 |
| Bladder control (%) | 76 (16.0) | 14 (20.6) | 23 (19.3) | 13 (22.8) | 0.469 |
| History of aspiration pneumonia (%) | 4 (0.8) | 1 (1.5) | 1 (0.8) | 1 (1.8) | 0.888 |
| Diagnosis of dementia (%) | 174 (36.7) | 23 (33.8) | 43 (36.1) | 28 (49.1) | 0.273 |
| Eating preference (soft food) (%) | 44 (9.3) | 9 (13.2) | 16 (13.4) | 7 (12.3) | 0.465 |
| Using dentures (%) | 201 (42.4) | 26 (38.2) | 45 (37.8) | 17 (29.8) | 0.273 |
| Choking easily (%) | 20 (4.2) | 3 (4.4) | 7 (5.9) | 6 (10.5) | 0.212 |
| One item of the Vitality Index | |||||
| Low motivation (%) | 186 (39.2) | 28 (41.2) | 57 (47.9) | 29 (50.9) | 0.169 |
| Five items of the DBD-13 | |||||
| Not showing interest (%) | 134 (28.3) | 13 (19.1) | 30 (25.2) | 19 (33.3) | 0.281 |
| Awaking at midnight (%) | 67 (14.1) | 9 (13.2) | 29 (24.4) | 16 (28.1) | 0.005 |
| Making an accusation (%) | 59 (12.4) | 9 (13.2) | 19 (16.0) | 11 (19.3) | 0.444 |
| Walking around (%) | 99 (20.9) | 14 (20.6) | 29 (24.4) | 17 (29.8) | 0.420 |
| Repeating an action (%) | 77 (16.2) | 8 (11.8) | 23 (19.3) | 14 (24.6) | 0.233 |
| Improvement Group (G2) | Slight Decline Group (G3) | Large Decline Group (G4) | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (years) | 1.01 (0.97–1.05) | 0.781 | 1.01 (0.98–1.04) | 0.487 | 1.04 (0.99–1.09) | 0.095 |
| Female | 1.13 (0.63–2.02) | 0.693 | 1.19 (0.75–1.89) | 0.461 | 0.60 (0.32–1.14) | 0.117 |
| Low BMI ‡ | 0.54 (0.26–1.12) | 0.097 | 0.94 (0.58–1.53) | 0.802 | 2.42 (1.29–4.55) | 0.006 |
| Diagnosis of dementia | 0.91 (0.48–1.73) | 0.768 | 0.82 (0.50–1.34) | 0.422 | 1.63 (0.80–3.32) | 0.180 |
| Eating preference (soft food) | 1.12 (0.43–2.90) | 0.815 | 1.46 (0.72–2.95) | 0.293 | 1.00 (0.34–2.95) | 0.997 |
| Using dentures | 0.65 (0.36–1.15) | 0.139 | 0.74 (0.47–1.16) | 0.185 | 0.49 (0.26–0.95) | 0.035 |
| Choking easily | 0.71 (0.16–3.04) | 0.641 | 1.26 (0.46–3.45) | 0.653 | 2.25 (0.65–7.74) | 0.199 |
| One item of the Vitality Index | ||||||
| Low motivation | 1.42 (0.79–2.57) | 0.246 | 1.58 (1.01–2.47) | 0.044 | 1.43 (0.76–2.69) | 0.267 |
| Five items of the DBD-13 | ||||||
| Lack of interest in daily activities | 0.45 (0.21–0.97) | 0.042 | 0.65 (0.38–1.11) | 0.113 | 0.81 (0.40–1.66) | 0.563 |
| Awaking at midnight | 0.81 (0.33–2.03) | 0.658 | 2.21 (1.19–4.10) | 0.012 | 2.09 (0.89–4.91) | 0.089 |
| Making an accusation | 1.15 (0.45–2.91) | 0.769 | 1.27 (0.65–2.45) | 0.483 | 1.00 (0.40–2.49) | 0.999 |
| Walking around | 1.47 (0.59–3.67) | 0.412 | 0.80 (0.38–1.71) | 0.565 | 1.28 (0.47–3.52) | 0.630 |
| Repeating the same action | 0.66 (0.23–1.84) | 0.423 | 1.09 (0.50–2.40) | 0.830 | 1.04 (0.36–2.99) | 0.943 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikuta, K.; Noguchi-Watanabe, M.; Aishima, M.; Anzai, T.; Takahashi, K.; Fukui, S. Physical Function Trajectory among High-Functioning Long-Term Care Facility Residents: Utilizing Japanese National Data. Geriatrics 2024, 9, 123. https://doi.org/10.3390/geriatrics9050123
Ikuta K, Noguchi-Watanabe M, Aishima M, Anzai T, Takahashi K, Fukui S. Physical Function Trajectory among High-Functioning Long-Term Care Facility Residents: Utilizing Japanese National Data. Geriatrics. 2024; 9(5):123. https://doi.org/10.3390/geriatrics9050123
Chicago/Turabian StyleIkuta, Kasumi, Maiko Noguchi-Watanabe, Miya Aishima, Tatsuhiko Anzai, Kunihiko Takahashi, and Sakiko Fukui. 2024. "Physical Function Trajectory among High-Functioning Long-Term Care Facility Residents: Utilizing Japanese National Data" Geriatrics 9, no. 5: 123. https://doi.org/10.3390/geriatrics9050123
APA StyleIkuta, K., Noguchi-Watanabe, M., Aishima, M., Anzai, T., Takahashi, K., & Fukui, S. (2024). Physical Function Trajectory among High-Functioning Long-Term Care Facility Residents: Utilizing Japanese National Data. Geriatrics, 9(5), 123. https://doi.org/10.3390/geriatrics9050123







