Optimal Cardiac Resynchronization Therapy with Conduction System Pacing Guided by Electro-Anatomical Mapping: A Case Report
Abstract
:1. Introduction
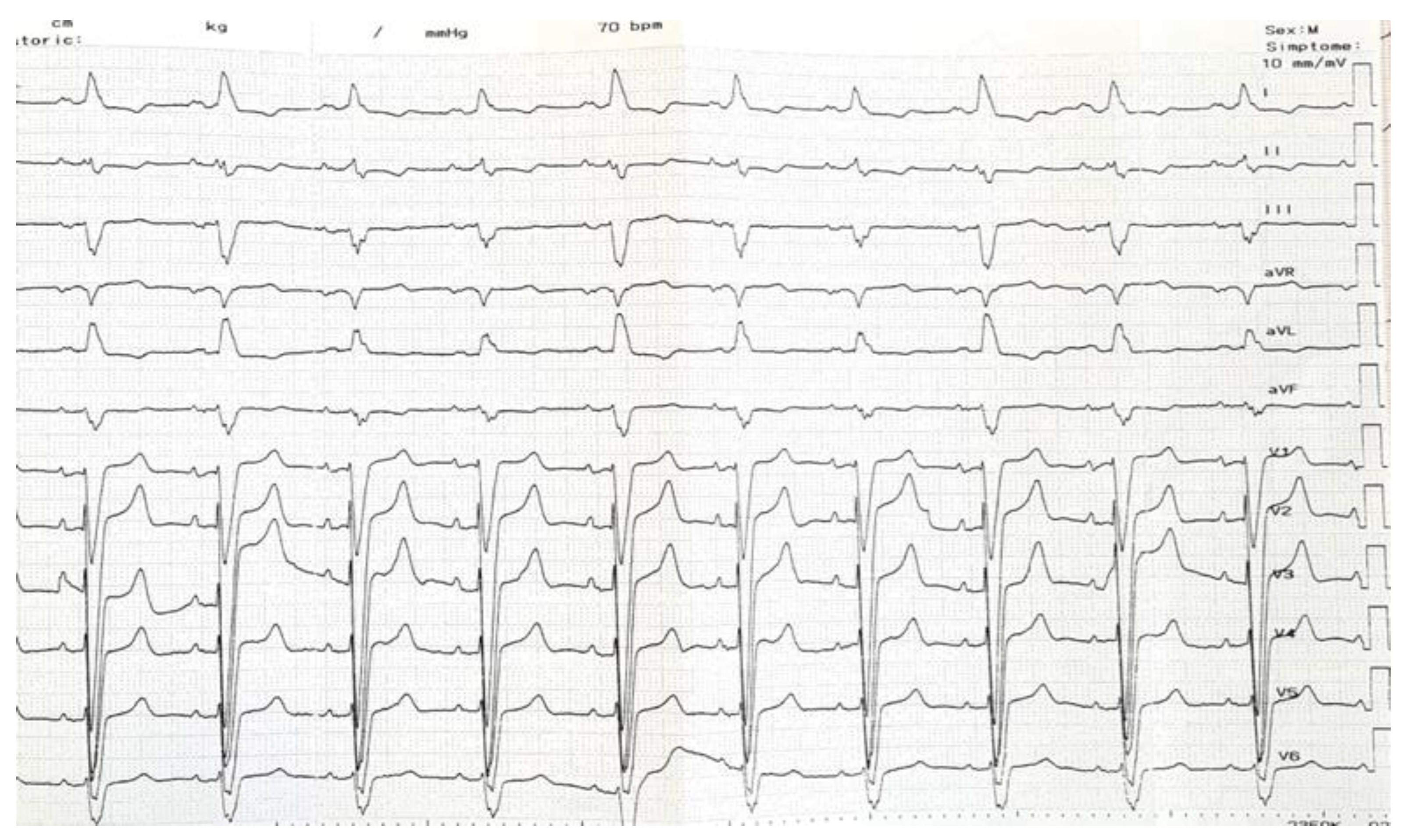
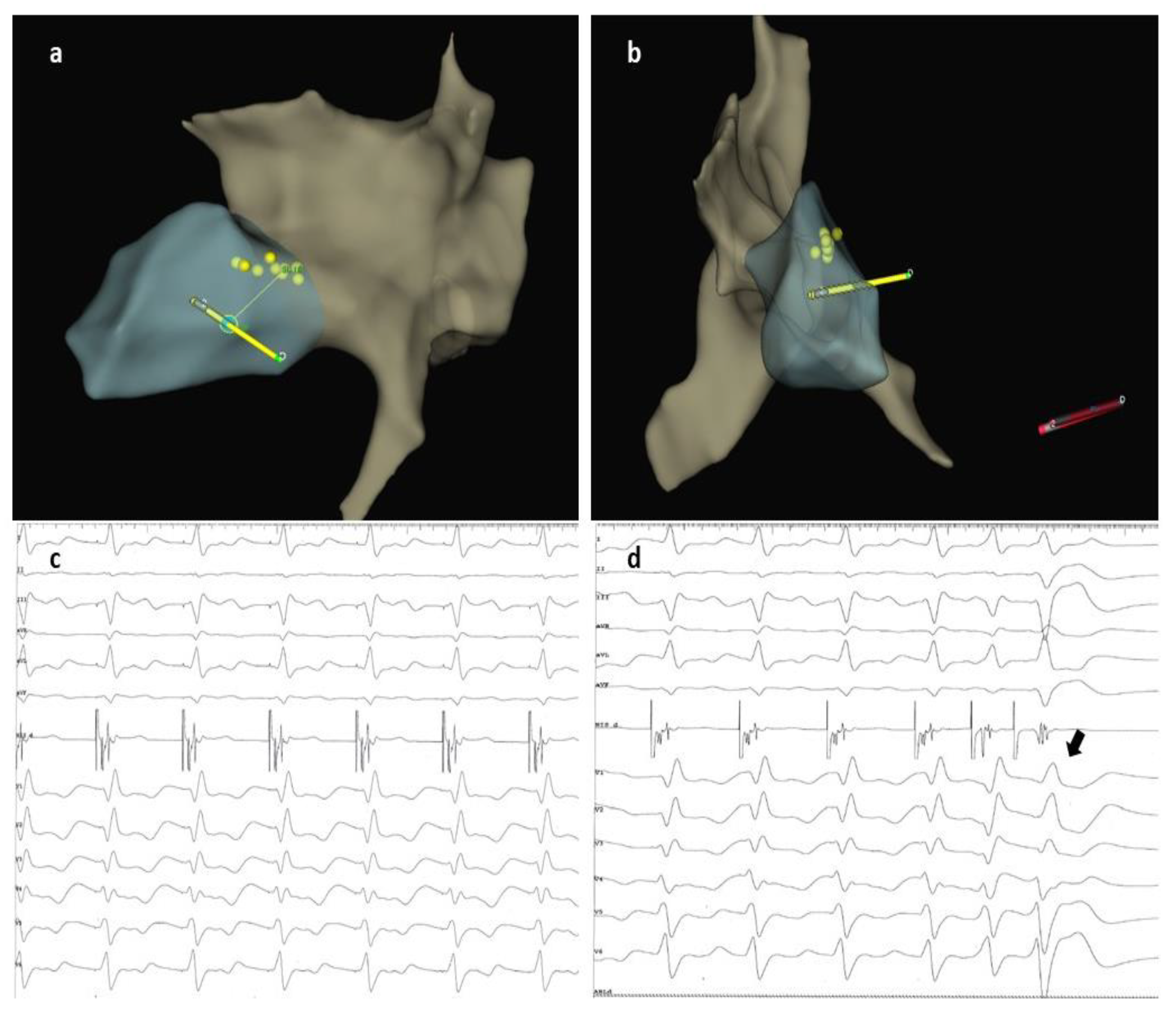
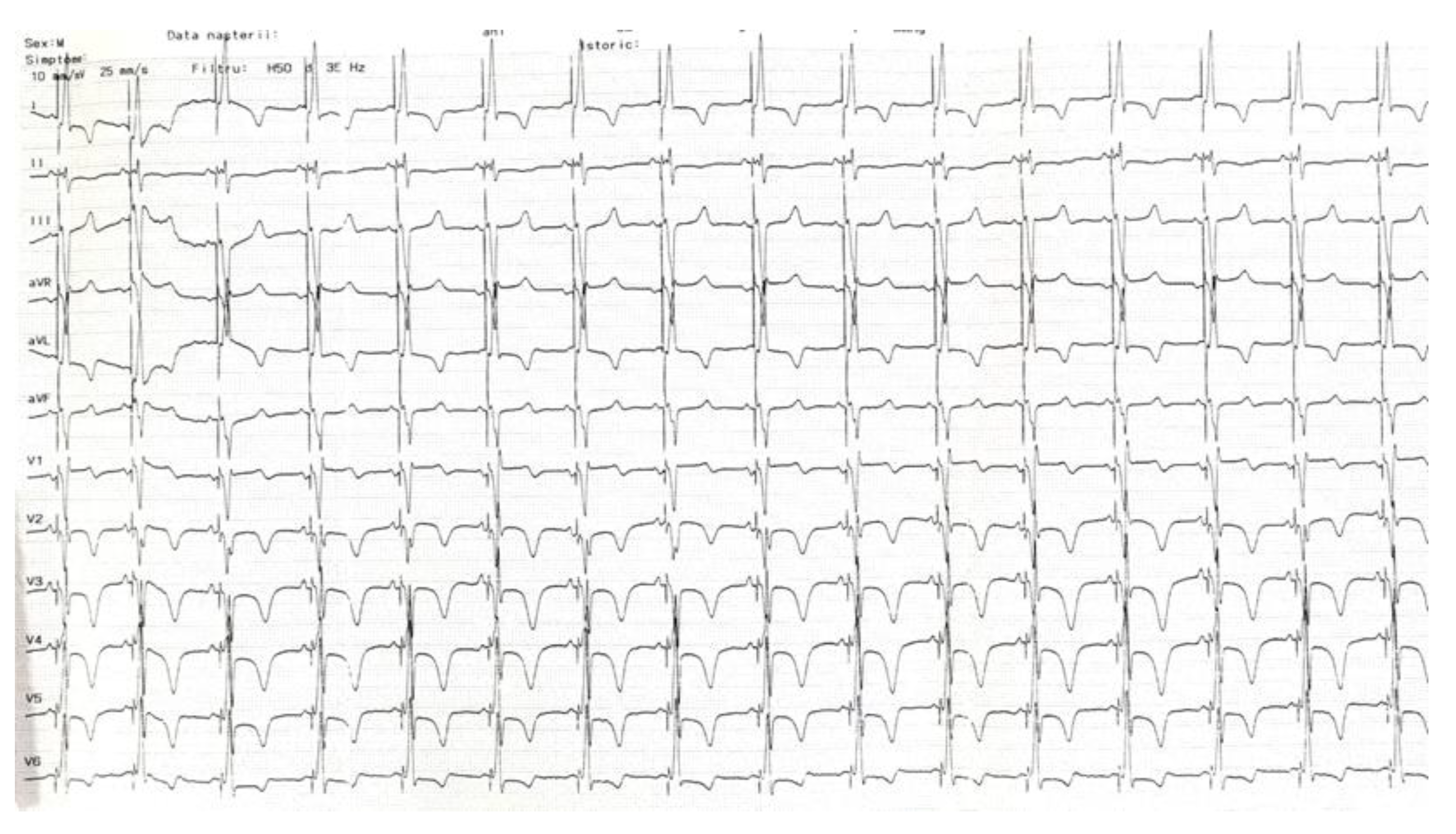
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Senes, J.; Mascia, G.; Bottoni, N.; Oddone, D.; Donateo, P.; Grimaldi, T.; Minneci, C.; Bertolozzi, I.; Brignole, M.; Puggioni, E.; et al. Is His-optimized superior to conventional cardiac resynchronization therapy in improving heart failure? Results from a propensity-matched study. Pacing Clin. Electrophysiol. 2021, 44, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, S.E.; Ellenbogen, K.A.; Vijayaraman, P.; Chelu, M.G. Clinical Outcomes of Conduction System Pacing versus Biventricular Pacing for Cardiac Resynchronization Therapy: A Systematic Review and Meta-Analysis. J. Cardiovasc. Electrophysiol. 2023, 34, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Sharma, P.S.; Cano, Ó.; Ponnusamy, S.S.; Herweg, B.; Zanon, F.; Jastrzębski, M.; Zou, J.; Chelu, M.G.; Vernooy, K.; et al. Comparison of Left Bundle Branch Area Pacing and Biventricular Pacing in Candidates for Resynchronization Therapy. J. Am. Coll. Cardiol. 2023, 82, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Pestrea, C.; Cicala, E.; Ivascu, M.; Gherghina, A.; Pintilie, I.; Orțan, F.; Pop, D. The Impact of Cardiac Chamber Volumes on Permanent His Bundle Pacing Procedural Outcomes—a Single Center Experience. J. Clin. Med. 2022, 11, 7076. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, G.A.; Cherian, T.; Shatz, D.Y.; Beaser, A.D.; Aziz, Z.; Ozcan, C.; Broman, M.T.; Nayak, H.M.; Tung, R. Intracardiac Delineation of Septal Conduction in Left Bundle-Branch Block Patterns. Circulation 2019, 139, 1876–1888. [Google Scholar] [CrossRef] [PubMed]
- Burri, H.; Jastrzebski, M.; Cano, Ó.; Čurila, K.; de Pooter, J.; Huang, W.; Israel, C.; Joza, J.; Romero, J.; Vernooy, K.; et al. EHRA Clinical Consensus Statement on Conduction System Pacing Implantation: Endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). Europace 2023, 25, 1208–1236. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębski, M.; Kiełbasa, G.; Cano, O.; Curila, K.; Heckman, L.; De Pooter, J.; Chovanec, M.; Rademakers, L.; Huybrechts, W.; Grieco, D.; et al. Left Bundle Branch Area Pacing Outcomes: The Multicentre European MELOS Study. Eur. Heart J. 2022, 43, 4161–4173. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Panikkath, R.; Mascarenhas, V.; Bauch, T.D. Left Bundle Branch Pacing Utilizing Three Dimensional Mapping. J. Cardiovasc. Electrophysiol. 2019, 30, 3050–3056. [Google Scholar] [CrossRef] [PubMed]
- Marcantoni, L.; Centioni, M.; Pastore, G.; Aneris, F.; Baracca, E.; Zanon, F. Conduction System Pacing in Difficult Cardiac Anatomies: Systematic Approach with the 3D Electroanatomic Mapping Guide. Indian Pacing Electrophysiol. J. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Gebauer, R.; Ebert, M.; Moscoso Ludueña, C.; Scheller, D.; Lucas, J.; König, S.; Paetsch, I.; Hindricks, G.; Doering, M. Electroanatomical Mapping–Guided Left Bundle Branch Area Pacing in Patients with Structural Heart Disease and Advanced Conduction Abnormalities. Europace 2022, 25, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, S.; Pieragnoli, P.; Haugaa, K.H.; Potpara, T.S.; Rasero, L.; Ramacciati, N.; Ricciardi, G.; Solimene, F.; Mascia, G.; Mascioli, G.; et al. The influence of age on the psychological profile of patients with cardiac implantable electronic devices: Results from the Italian population in a multicenter study conducted by the European Heart Rhythm Association. Aging Clin. Exp. Res. 2019, 31, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Duncker, D.; Friedel, K.; König, T.; Schreyer, H.; Lüsebrink, U.; Duncker, M.; Oswald, H.; Klein, G.; Gardiwal, A. Cardiac resynchronization therapy improves psycho-cognitive performance in patients with heart failure. Europace. 2015, 17, 1415–1421. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pestrea, C.; Enache, R.; Cicala, E.; Vatasescu, R. Optimal Cardiac Resynchronization Therapy with Conduction System Pacing Guided by Electro-Anatomical Mapping: A Case Report. J. Cardiovasc. Dev. Dis. 2023, 10, 456. https://doi.org/10.3390/jcdd10110456
Pestrea C, Enache R, Cicala E, Vatasescu R. Optimal Cardiac Resynchronization Therapy with Conduction System Pacing Guided by Electro-Anatomical Mapping: A Case Report. Journal of Cardiovascular Development and Disease. 2023; 10(11):456. https://doi.org/10.3390/jcdd10110456
Chicago/Turabian StylePestrea, Catalin, Roxana Enache, Ecaterina Cicala, and Radu Vatasescu. 2023. "Optimal Cardiac Resynchronization Therapy with Conduction System Pacing Guided by Electro-Anatomical Mapping: A Case Report" Journal of Cardiovascular Development and Disease 10, no. 11: 456. https://doi.org/10.3390/jcdd10110456
APA StylePestrea, C., Enache, R., Cicala, E., & Vatasescu, R. (2023). Optimal Cardiac Resynchronization Therapy with Conduction System Pacing Guided by Electro-Anatomical Mapping: A Case Report. Journal of Cardiovascular Development and Disease, 10(11), 456. https://doi.org/10.3390/jcdd10110456





