Risk of Cardiac Implantable Electronic Device Infection after Early versus Delayed Lead Repositioning
Abstract
1. Introduction
2. Methods
2.1. Patient Population
2.2. Study Endpoints
2.3. Statistical Analysis
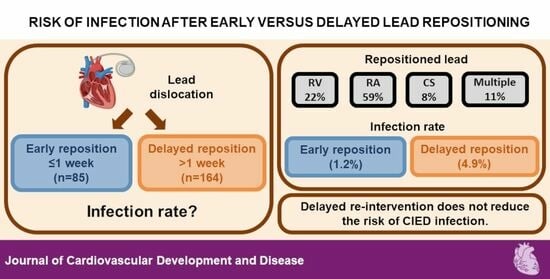
3. Results
3.1. Patients Characteristics
3.2. Study Endpoints
4. Discussion
4.1. Main Findings
4.2. Known Risk Factors of CIED Infection
4.3. Reasons Supporting Delayed or Early Reintervention
4.4. Treatment Options for Early Wound Infection
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 932–987. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Baumbach, A.; Böhm, M.; Burri, H.; Čelutkiene, J.; Chioncel, O.; Cleland, J.G.F.; Coats, A.J.S.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; De Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; De Chillou, C.; et al. 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, M.G.; Kennergren, C.; Butter, C.; Deharo, J.C.; Kutarski, A.; Rinaldi, C.A.; Romano, S.L.; Maggioni, A.P.; Andarala, M.; Auricchio, A.; et al. The European Lead Extraction ConTRolled (ELECTRa) Study: A European Heart Rhythm Association (EHRA) Registry of Transvenous Lead Extraction Outcomes. Eur. Heart J. 2017, 38, 2995–3005. [Google Scholar] [CrossRef]
- Monsefi, N.; Waraich, H.S.; Vamos, M.; Erath, J.; Sirat, S.; Moritz, A.; Hohnloser, S.H. Efficacy and Safety of Transvenous Lead Extraction in 108 Consecutive Patients: A Single-Centre Experience. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 704–708. [Google Scholar] [CrossRef]
- Zsigmond, E.-J.; Miklos, M.; Vida, A.; Benak, A.; Makai, A.; Schvartz, N.; Klausz, G.; Hegedus, Z.; Bogats, G.; Saghy, L. Reimplantation and Long-Term Mortality after Transvenous Lead Extraction in a High-Risk, Single-Center Cohort. J. Interv. Card. Electrophysiol. 2021, 66, 847–855. [Google Scholar] [CrossRef]
- Blomström-Lundqvist, C.; Traykov, V.; Erba, P.A.; Burri, H.; Nielsen, J.C.; Bongiorni, M.G.; Poole, J.; Boriani, G.; Costa, R.; Deharo, J.-C.; et al. European Heart Rhythm Association (EHRA) International Consensus Document on How to Prevent, Diagnose, and Treat Cardiac Implantable Electronic Device Infections-Endorsed of Clinical Microbiology and Infectious Diseases (ESCMID) in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace 2020, 22, 515–549. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA Expert Consensus Statement on Lead Extraction: Recommendations on Definitions, Endpoints, Research Trial Design, and Data Collection Requirements for Clinical Scientific Studies and Registries: Endorsed by APHRS/HRS/LAHRS. Europace 2018, 20, 1217. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.C.; Ellenbogen, K.A.; et al. 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef] [PubMed]
- Traykov, V.; Blomström-Lundqvist, C. Antibiotic-Eluting Envelopes for the Prevention of Cardiac Implantable Electronic Device Infections: Rationale, Efficacy, and Cost-Effectiveness. Front. Cardiovasc. Med. 2022, 9, 855233. [Google Scholar] [CrossRef] [PubMed]
- Frausing, M.H.J.P.; Kronborg, M.B.; Johansen, J.B.; Nielsen, J.C. Avoiding Implant Complications in Cardiac Implantable Electronic Devices: What Works? Europace 2021, 23, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Vamos, M.; Erath, J.W.; Benz, A.P.; Duray, G.Z. Editorial: Developments in Cardiac Implantable Electronic Device Therapy: How Can We Improve Clinical Implementation? Front. Cardiovasc. Med. 2023, 10, 1177882. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Zou, T.; Dong, M.; Liu, J.; Cui, W.; Tong, J.; Shi, H.; Chen, H.; Chong, J.; Lyu, Y.; et al. Risk Stratifying and Prognostic Analysis of Subclinical Cardiac Implantable Electronic Devices Infection: Insight from Traditional Bacterial Culture. J. Am. Heart Assoc. 2021, 10, e022260. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, Y.; Sakamoto, S.; Kawase, Y.; Oomori, H.; Watanabe, Y.; Kurita, J.; Maruyama, Y.; Sasaki, T.; Ishii, Y. Temporal and Microbiological Analysis of Cardiac Implantable Electrical Device Infections—A Retrospective Study. Circ. Rep. 2021, 3, 488–496. [Google Scholar] [CrossRef]
- Döring, M.; Richter, S.; Hindricks, G. Übersichtsarbeit: Diagnostik Und Therapie von Infektionen Kardialer Elektronischer Implantate. Dtsch. Arztebl. Int. 2018, 115, 445–452. [Google Scholar] [CrossRef]
- Polyzos, K.A.; Konstantelias, A.A.; Falagas, M.E. Risk Factors for Cardiac Implantable Electronic Device Infection: A Systematic Review and Meta-Analysis. Europace 2015, 17, 767–777. [Google Scholar] [CrossRef]
- Garweg, C.; Vandenberk, B.; Jentjens, S.; Foulon, S.; Hermans, P.; Poels, P.; Haemers, P.; Ector, J.; Willems, R. Bacteraemia after Leadless Pacemaker Implantation. J. Cardiovasc. Electrophysiol. 2020, 31, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.Z.; Blomström-Lundqvist, C.; Bloom, H.; Cooper, C.; Ellis, C.; Goette, A.; Greenspon, A.J.; Love, C.J.; Johansen, J.B.; Philippon, F.; et al. Use of Healthcare Claims to Validate the Prevention of Arrhythmia Device Infection Trial Cardiac Implantable Electronic Device Infection Risk Score. Europace 2021, 23, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.R.; Uslan, D.Z.; Khan, A.H.; Friedman, P.A.; Hayes, D.L.; Wilson, W.R.; Steckelberg, J.M.; Stoner, S.M.; Baddour, L.M. Risk Factor Analysis of Permanent Pacemaker Infection. Clin. Infect. Dis. 2007, 45, 166–173. [Google Scholar] [CrossRef]
- Ghani, A.; Delnoy, P.P.H.M.; Ramdat Misier, A.R.; Smit, J.J.J.; Adiyaman, A.; Ottervanger, J.P.; Elvan, A. Incidence of Lead Dislodgement, Malfunction and Perforation during the First Year Following Device Implantation. Neth. Heart J. 2014, 22, 286–291. [Google Scholar] [CrossRef]
- Prutkin, J.M.; Reynolds, M.R.; Bao, H.; Curtis, J.P.; Al-Khatib, S.M.; Aggarwal, S.; Uslan, D.Z. Rates of and Factors Associated with Infection in 200 909 Medicare Implantable Cardioverter-Defibrillator Implants Results from the National Cardiovascular Data Registry. Circulation 2014, 130, 1037–1043. [Google Scholar] [CrossRef]
- Lin, Y.S.; Hung, S.P.; Chen, P.R.; Yang, C.H.; Wo, H.T.; Chang, P.C.; Wang, C.C.; Chou, C.C.; Wen, M.S.; Chung, C.M.; et al. Risk Factors Influencing Complications of Cardiac Implantable Electronic Device Implantation: Infection, Pneumothorax and Heart Perforation: A Nationwide Population-Based Cohort Study. Medicine 2014, 93, e213. [Google Scholar] [CrossRef]
- Lekkerkerker, J.C.; Van Nieuwkoop, C.; Trines, S.A.; Van der Bom, J.G.; Bernards, A.; Van de Velde, E.T.; Bootsma, M.; Zeppenfeld, K.; Jukema, J.W.; Borleffs, J.W.; et al. Risk Factors and Time Delay Associated with Cardiac Device Infections: Leiden Device Registry. Heart 2009, 95, 715–720. [Google Scholar] [CrossRef]
- Ann, H.W.; Ahn, J.Y.; Jeon, Y.D.; Jung, I.Y.; Jeong, S.J.; Joung, B.; Lee, M.H.; Ku, N.S.; Han, S.H.; Kim, J.M.; et al. Incidence of and Risk Factors for Infectious Complications in Patients with Cardiac Device Implantation. Int. J. Infect. Dis. 2015, 36, e9–e14. [Google Scholar] [CrossRef]
- Qin, D.; Filippaios, A.; Murphy, J.; Berg, M.; Lampert, R.; Schloss, E.J.; Noone, M.; Mela, T. Short- and Long-Term Risk of Lead Dislodgement Events: Real-World Experience from Product Surveillance Registry. Circ. Arrhythmia Electrophysiol. 2022, 15, 530–538. [Google Scholar] [CrossRef]
- Pakarinen, S.; Oikarinen, L.; Toivonen, L. Short-Term Implantation-Related Complications of Cardiac Rhythm Management Device Therapy: A Retrospective Single-Centre 1-Year Survey. Europace 2010, 12, 103–108. [Google Scholar] [CrossRef]
- Cheng, A.; Wang, Y.; Curtis, J.P.; Varosy, P.D. Acute Lead Dislodgements and In-Hospital Mortality in Patients Enrolled in the National Cardiovascular Data Registry Implantable Cardioverter Defibrillator Registry. J. Am. Coll. Cardiol. 2010, 56, 1651–1656. [Google Scholar] [CrossRef]
- Pelosi, F. Reducing CIED Lead Dislodgements: Faithful Alignment to Small Things. Pacing Clin. Electrophysiol. 2019, 42, 63–64. [Google Scholar] [CrossRef]
- Afzal, M.R.; Horner, S.; Matre, N.B.; Blake, P.; Dunham, K.; Pinkhas, D.; Okabe, T.; Tyler, J.; Houmsse, M.; Kalbfleisch, S.J.; et al. Comprehensive Strategy to Reduce the Incidence of Lead Dislodgement for Cardiac Implantable Electronic Devices. Pacing Clin. Electrophysiol. 2019, 42, 58–62. [Google Scholar] [CrossRef]
- Klug, D.; Balde, M.; Pavin, D.; Hidden-Lucet, F.; Clementy, J.; Sadoul, N.; Rey, J.L.; Lande, G.; Lazarus, A.; Victor, J.; et al. Risk Factors Related to Infections of Implanted Pacemakers and Cardioverter-Defibrillators: Results of a Large Prospective Study. Circulation 2007, 116, 1349–1355. [Google Scholar] [CrossRef]
- Esposito, M.; Kennergren, C.; Holmström, N.; Nilsson, S.; Eckerdal, J.; Thomsen, P. Morphologic and Immunohistochemical Observations of Tissues Surrounding Retrieved Transvenous Pacemaker Leads. J. Biomed. Mater. Res. 2002, 63, 548–558. [Google Scholar] [CrossRef]
- Ernst, M.; Sághy, L.; Hohnloser, S.H.; Vámos, M. Avoiding ICD Lead Revision in a Patient with Chronically Low R-Wave Amplitudes. Cardiol. Hung. 2020, 50, 199–201. [Google Scholar] [CrossRef]
- Ruetz, L.L.; Koehler, J.L.; Brown, M.L.; Jackson, T.E.; Belk, P.; Swerdlow, C.D. Sinus Rhythm R-Wave Amplitude as a Predictor of Ventricular Fibrillation Undersensing in Patients with Implantable Cardioverter-Defibrillator. Heart Rhythm 2015, 12, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Nair, G.M.; Nair, V.; Healey, J.S.; Morillo, C.A. Automatic Implantable Cardioverter Defibrillator Lead Dislodgement Resulting in Sudden Cardiac Death: A Case Report. Can. J. Cardiol. 2014, 30, 1460.e7–1460.e9. [Google Scholar] [CrossRef] [PubMed]


| Total | Early Revision (≤1 Week) | Delayed Revision (>1 Week) | p-Value | |
|---|---|---|---|---|
| Number of patients | 249 | 85 | 164 | |
| Age in years (median (IQR)) | 72.0 (15.4) | 76.0 (68.9–81.8) | 69.4 (62.3–77.9) | 0.001 |
| Male sex | 134 (54%) | 50 (59%) | 84 (51%) | 0.254 |
| Type of device | ||||
| VVI PM | 48 (19%) | 24 (28%) | 24 (15%) | 0.021 |
| VVI ICD | 26 (10%) | 8 (9%) | 18 (11%) | |
| DDD PM | 105 (42%) | 40 (47%) | 65 (40%) | |
| DDD ICD | 16 (6%) | 4 (5%) | 12 (7%) | |
| CRT-P | 13 (5%) | 1 (1%) | 12 (7%) | |
| CRT-D | 41 (17%) | 9 (11%) | 32 (20%) | |
| Number of leads | 0.006 | |||
| (mean ± SD) | 1.9 ± 0.7 | 1.7 ± 0.7 | 2.0 ± 0.7 | |
| (median (IQR)) | 2.0 (1) | 2.0 (1–2) | 2.0 (1–3) | |
| Diabetes | 65 (26%) | 22 (26%) | 43 (26%) | 0.954 |
| Heart failure | 76 (31%) | 21 (25%) | 55 (34%) | 0.151 |
| Fever prior to implant | 2 (0.8%) | 0 (0%) | 2 (1.2%) | 0.307 |
| Anticoagulation | 89 (36%) | 29 (34%) | 60 (37%) | 0.700 |
| NOAC full dose | 19 (8%) | 4 (5%) | 15 (9%) | 0.468 |
| NOAC reduced dose | 6 (2%) | 2 (2%) | 4 (2%) | |
| VKA | 53 (21%) | 17 (20%) | 36 (22%) | |
| LMWH | 11 (4%) | 6 (7%) | 5 (3%) | |
| Platelet inhibition | 118 (47%) | 43 (50%) | 75 (46%) | 0.467 |
| Prolonged antibiotic therapy | 17 (7%) | 4 (5%) | 13 (8%) | 0.339 |
| Corticosteroids | 2 (0.8%) | 1 (1%) | 1 (0.6%) | 0.635 |
| Temporary pacemaker | 28 (11%) | 13 (15%) | 15 (9%) | 0.145 |
| Creatinine in umol/L (median (IQR)) a | 93.5 (40.5) | 97.2 (81–137.6) | 89.0 (71–110.5) | 0.023 |
| White blood cell (WBC) count in/L) (median (IQR)) | ||||
| At baseline b | 7.7 (3.2) | 8.2 (6.6–10.4) | 7.6 (6.4–9.2) | 0.455 |
| At revision c | 7.6 (2.8) | 7.8 (6.9–10.4) | 7.5 (6.3–8.9) | 0.142 |
| C-reactive protein (CRP) in mg/dL (median (IQR)) | ||||
| At baseline d | 1.3 (6.3) | 2.6 (0.4–12.5) | 0.82 (0.2–4.1) | 0.023 |
| At revision e | 1.8 (9.0) | 2.9 (0.9–14.4) | 1.2 (0.6–7.8) | 0.160 |
| Total | Early Revision (≤1 Week) | Delayed Revision (>1 Week) | OR, 95% CI, p-Value | Adjusted OR, 95% CI, p-Value | |
|---|---|---|---|---|---|
| Number of patients | 249 | 85 | 164 | ||
| Infection | 9 (3.6%) | 1 (1.2%) | 8 (4.9%) | 0.232 0.029–1.888 p = 0.172 | 0.264 0.032–2.179 p = 0.216 |
| Explantation/extraction due to infection | 7 (2.8%) | 0 (0%) | 7 (4.3%) | 0.128 0.01–2.273 p = 0.161 | N/A |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Early revision | 0.232 | 0.029–1.888 | 0.172 |
| Number of leads | 0.790 | 0.310–2.013 | 0.621 |
| Diabetes | 1.435 | 0.348–5.913 | 0.617 |
| Heart failure | 1.144 | 0.278–4.699 | 0.852 |
| Fever prior implant | 29.875 | 1.711–521.624 | 0.020 |
| Corticosteroid | N/A | ||
| Anticoagulation | 1.459 | 0.382–5.578 | 0.581 |
| Platelet inhibition | 0.543 | 0.133–2.224 | 0.396 |
| Temporary pacemaker | 0.986 | 0.119–8.191 | 0.990 |
| Adjusted OR | 95% CI | p-Value | |
|---|---|---|---|
| Step 1 | |||
| Early revision | 0.264 | 0.032–2.179 | 0.216 |
| Fever prior implant | 22.143 | 1.251–391.868 | 0.035 |
| Step 2 | |||
| Fever prior implant | 29.875 | 1.711–521.624 | 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schvartz, N.; Haidary, A.; Wakili, R.; Hecker, F.; Kupusovic, J.; Zsigmond, E.-J.; Miklos, M.; Saghy, L.; Szili-Torok, T.; Erath, J.W.; et al. Risk of Cardiac Implantable Electronic Device Infection after Early versus Delayed Lead Repositioning. J. Cardiovasc. Dev. Dis. 2024, 11, 117. https://doi.org/10.3390/jcdd11040117
Schvartz N, Haidary A, Wakili R, Hecker F, Kupusovic J, Zsigmond E-J, Miklos M, Saghy L, Szili-Torok T, Erath JW, et al. Risk of Cardiac Implantable Electronic Device Infection after Early versus Delayed Lead Repositioning. Journal of Cardiovascular Development and Disease. 2024; 11(4):117. https://doi.org/10.3390/jcdd11040117
Chicago/Turabian StyleSchvartz, Noemi, Arian Haidary, Reza Wakili, Florian Hecker, Jana Kupusovic, Elod-Janos Zsigmond, Marton Miklos, Laszlo Saghy, Tamas Szili-Torok, Julia W. Erath, and et al. 2024. "Risk of Cardiac Implantable Electronic Device Infection after Early versus Delayed Lead Repositioning" Journal of Cardiovascular Development and Disease 11, no. 4: 117. https://doi.org/10.3390/jcdd11040117
APA StyleSchvartz, N., Haidary, A., Wakili, R., Hecker, F., Kupusovic, J., Zsigmond, E.-J., Miklos, M., Saghy, L., Szili-Torok, T., Erath, J. W., & Vamos, M. (2024). Risk of Cardiac Implantable Electronic Device Infection after Early versus Delayed Lead Repositioning. Journal of Cardiovascular Development and Disease, 11(4), 117. https://doi.org/10.3390/jcdd11040117