Left Ventricular Global Longitudinal Strain as a Parameter of Mild Myocardial Dysfunction in Athletes after COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography
2.3. Statistical Analysis
3. Results
3.1. Cohort Characteristics
3.2. Echocardiographic Parameters
3.3. Initial Symptoms and Correlation Analysis with GLS and GRS
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhmerov, A.; Marban, E. COVID-19 and the Heart. Circ. Res. 2020, 126, 1443–1455. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Li, H.; Liu, M.; Can, B.; Cheng, Z.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Krzywański, J.; Mikulski, T.; Krysztofiak, H.; Pokrywka, A.; Młyńczak, M.; Małek, A.; Kwiatkowska, D.; Kuchar, E. Elite athletes with COVID-19—Predictors of the course of disease. J. Sci. Med. Sport 2022, 25, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From COVID-19 Infection. JAMA Cardiol. 2021, 6, 116–118. [Google Scholar] [CrossRef]
- Schumacher, Y.O.; Tabben, M.; Hassoun, K.; Al Marwani, A.; Al Hussein, I.; Coyle, P.; Abbassi, A.K.; Ballan, H.T.; Al-Kuwari, A.; Chamari, K.; et al. Resuming professional football (soccer) during the COVID-19 pandemic in a country with high infection rates: A prospective cohort study. Br. J. Sport. Med. 2021, 55, 1092–1098. [Google Scholar] [CrossRef]
- Clark, D.E.; Parikh, A.; Dendy, J.M.; Diamond, A.B.; George-Durrett, K.; Fish, F.A.; Fitch, W.; Hughes, S.G.; Soslow, J.H. COVID-19 Myocardial Pathology Evaluation in Athletes With Cardiac Magnetic Resonance (COMPETE CMR). Circ. J. 2021, 143, 609–612. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Moulson, N.; Petek, B.J.; Drezner, J.A.; Harmon, K.G.; Kliethermes, S.A.; Patel, M.R.; Baggish, A.L. SARS-CoV-2 Cardiac Involvement in Young Competitive Athletes. Circulation 2021, 144, 256–266. [Google Scholar] [CrossRef]
- Martinez, M.W.; Tucker, A.M.; Bloom, O.J.; Green, G.; DiFiori, J.P.; Solomon, G.; Phelan, D.; Kim, J.H.; Meeuwisse, W.; Sills, A.K.; et al. Prevalence of Inflammatory Heart Disease Among Professional Athletes with Prior COVID-19 Infection Who Received Systematic Return-to-Play Cardiac Screening. JAMA Cardiol. 2021, 6, 745–752. [Google Scholar] [CrossRef]
- Steinacker, J.M.; Schellenberg, J.; Bloch, W.; Deibert, P.; Friedmann-Bette, B.; Grim, C.; Halle, M.; Hirschmüller, A.; Hollander, K.; Kerling, A.; et al. Recommendations for Return-to-Sport after COVID-19: Expert Consensus. Dtsch. Z. Sportmed. 2022, 73, 127–136. [Google Scholar]
- Wilson, M.G.; Hull, J.H.; Rogers, J.; Pollock, N.; Dodd, M.; Haines, J.; Harris, S.; Loosemore, M.; Malhotra, A.; Pieles, G.; et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: A practical guide for sport and exercise medicine physicians. Br. J. Sport. Med. 2020, 54, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Halle, M.; Bloch, W.; Niess, A.M.; Predel, H.G.; Reinsberger, C.; Scharhag, J.; Steinacker, J.; Wolfarth, B.; Scherr, J.; Niebauer, J. Exercise and sports after COVID-19-Guidance from a clinical perspective. Transl. Sport. Med. 2021, 4, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-de-Las-Penas, C.; Florencio, L.L.; Gomez-Mayordomo, V.; Cuadrado, M.L.; Palacios-Cena, D.; Raveendran, A.V. Proposed integrative model for post-COVID symptoms. Diabetes Metab. Syndr. 2021, 15, 102159. [Google Scholar] [CrossRef]
- Iqbal, F.M.; Lam, K.; Sounderajah, V.; Clarke, J.M.; Ashrafian, H.; Darzi, A. Characteristics and predictors of acute and chronic post-COVID syndrome: A systematic review and meta-analysis. EClinicalMedicine 2021, 36, 100899. [Google Scholar] [CrossRef]
- Peter, R.S.; Nieters, A.; Kräusslich, H.-G.; Brockmann, S.O.; Göpel, S.; Kindle, G.; Merle, U.; Steinacker, J.M.; Rothenbacher, D.; Kern, W.V.; et al. Post-acute sequelae of COVID-19 six to 12 months after infection: Population based study. BMJ 2022, 379, e071050. [Google Scholar] [CrossRef]
- Hull, J.H.; Wootten, M.; Moghal, M.; Heron, N.; Martin, R.; Walsted, E.S.; Biswas, A.; Loosemore, M.; Elliott, N.; Ranson, C. Clinical patterns, recovery time and prolonged impact of COVID-19 illness in international athletes: The UK experience. Br. J. Sport. Med. 2022, 56, 4–11. [Google Scholar] [CrossRef]
- Petek, B.J.; Moulson, N.; Baggish, A.L.; Kliethermes, S.A.; Patel, M.R.; Churchill, T.W.; Harmon, K.G.; Drezner, J.A.; Investigators, O. Prevalence and clinical implications of persistent or exertional cardiopulmonary symptoms following SARS-CoV-2 infection in 3597 collegiate athletes: A study from the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA). Br. J. Sport. Med. 2022, 56, 913–918. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Szabo, L.; Juhasz, V.; Dohy, Z.; Fogarasi, C.; Kovacs, A.; Lakatos, B.K.; Kiss, O.; Sydo, N.; Csulak, E.; Suhai, F.I.; et al. Is cardiac involvement prevalent in highly trained athletes after SARS-CoV-2 infection? A cardiac magnetic resonance study using sex-matched and age-matched controls. Br. J. Sport. Med. 2022, 56, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Croft, L.B.; Krishnamoorthy, P.; Ro, R.; Anastasius, M.; Zhao, W.; Buckley, S.; Goldman, M.; Argulian, E.; Sharma, S.K.; Kini, A.; et al. Abnormal left ventricular global longitudinal strain by speckle tracking echocardiography in COVID-19 patients. Future Cardiol. 2021, 17, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Stobe, S.; Richter, S.; Seige, M.; Stehr, S.; Laufs, U.; Hagendorff, A. Echocardiographic characteristics of patients with SARS-CoV-2 infection. Clin. Res. Cardiol. 2020, 109, 1549–1566. [Google Scholar] [CrossRef]
- Li, R.; Wang, H.; Ma, F.; Cui, G.L.; Peng, L.Y.; Li, C.Z.; Zeng, H.S.; Marian, A.J.; Wang, D.W. Widespread myocardial dysfunction in COVID-19 patients detected by myocardial strain imaging using 2-D speckle-tracking echocardiography. Acta Pharmacol. Sin. 2021, 42, 1567–1574. [Google Scholar] [CrossRef]
- Minhas, A.S.; Gilotra, N.A.; Goerlich, E.; Metkus, T.; Garibaldi, B.T.; Sharma, G.; Bavaro, N.; Phillip, S.; Michos, E.D.; Hays, A.G. Myocardial Work Efficiency, A Novel Measure of Myocardial Dysfunction, Is Reduced in COVID-19 Patients and Associated with In-Hospital Mortality. Front. Cardiovasc. Med. 2021, 8, 667721. [Google Scholar] [CrossRef] [PubMed]
- Baum, P.; Do, L.; Deterding, L.; Lier, J.; Kunis, I.; Saur, D.; Classen, J.; Wirtz, H.; Laufs, U. Cardiac function in relation to functional status and fatigue in patients with post-COVID syndrome. Sci. Rep. 2022, 12, 19575. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Kunal, S.; Shah, B.; Garg, S.; Palleda, G.M.; Bansal, A.; Batra, V.; Yusuf, J.; Mukhopadhyay, S.; Kumar, S.; et al. Left ventricular global longitudinal strain in COVID-19 recovered patients. Echocardiography 2021, 38, 1722–1730. [Google Scholar] [CrossRef]
- Kujur, P.P.; Jhala, M.; Bhondve, A.; Lanjewar, C.; Matta, R.; Deshmukh, H. Left ventricular global longitudinal strain imaging in identifying subclinical myocardial dysfunction among COVID-19 survivors. Indian Heart J. 2022, 74, 51–55. [Google Scholar] [CrossRef]
- Oikonomou, E.; Lampsas, S.; Theofilis, P.; Souvaliotis, N.; Papamikroulis, G.A.; Katsarou, O.; Kalogeras, K.; Pantelidis, P.; Papaioannou, T.G.; Tsatsaragkou, A.; et al. Impaired left ventricular deformation and ventricular-arterial coupling in post-COVID-19: Association with autonomic dysregulation. Heart Vessel. 2023, 38, 381–393. [Google Scholar] [CrossRef]
- Lakatos, B.K.; Tokodi, M.; Fabian, A.; Ladanyi, Z.; Vago, H.; Szabo, L.; Sydo, N.; Csulak, E.; Kiss, O.; Babity, M.; et al. Frequent Constriction-Like Echocardiographic Findings in Elite Athletes Following Mild COVID-19: A Propensity Score-Matched Analysis. Front. Cardiovasc. Med. 2021, 8, 760651. [Google Scholar] [CrossRef]
- Fikenzer, S.; Kogel, A.; Pietsch, C.; Lavall, D.; Stobe, S.; Rudolph, U.; Laufs, U.; Hepp, P.; Hagendorff, A. SARS-CoV2 infection: Functional and morphological cardiopulmonary changes in elite handball players. Sci. Rep. 2021, 11, 17798. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.A.; Otto, C.; Stoddard, M.; Waggoner, A.; Zoghbi, W.A. Recommendations for quantification of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2002, 15, 167–184. [Google Scholar] [CrossRef] [PubMed]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Task Force, M.; Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Scharhag, J.; Schneider, G.; Urhausen, A.; Rochette, V.; Kramann, B.; Kindermann, W. Athlete’s heart: Right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J. Am. Coll. Cardiol. 2002, 40, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Abergel, E.; Chatellier, G.; Hagege, A.A.; Oblak, A.; Linhart, A.; Ducardonnet, A.; Menard, J. Serial left ventricular adaptations in world-class professional cyclists: Implications for disease screening and follow-up. J. Am. Coll. Cardiol. 2004, 44, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the Eacvi Norre study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 833–840. [Google Scholar] [CrossRef]
- D’Elia, N.; Caselli, S.; Kosmala, W.; Lancellotti, P.; Morris, D.; Muraru, D.; Takeuchi, M.; van den Bosch, A.; van Grootel, R.W.J.; Villarraga, H.; et al. Normal Global Longitudinal Strain: An Individual Patient Meta-Analysis. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 167–169. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Daraban, A.M.; Unlu, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181.e2. [Google Scholar] [CrossRef]
- Zghal, F.; Bougteb, H.; Reant, P.; Lafitte, S.; Roudaut, R. Assessing global and regional left ventricular myocardial function in elderly patients using the bidimensional strain method. Echocardiography 2011, 28, 978–982. [Google Scholar] [CrossRef]
- Skaarup, K.G.; Lassen, M.C.H.; Johansen, N.D.; Olsen, F.J.; Lind, J.N.; Jorgensen, P.G.; Jensen, G.; Schnohr, P.; Prescott, E.; Sogaard, P.; et al. Age- and sex-based normal values of layer-specific longitudinal and circumferential strain by speckle tracking echocardiography: The Copenhagen City Heart Study. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 629–640. [Google Scholar] [CrossRef]
- Galderisi, M.; Esposito, R.; Schiano-Lomoriello, V.; Santoro, A.; Ippolito, R.; Schiattarella, P.; Strazzullo, P.; de Simone, G. Correlates of global area strain in native hypertensive patients: A three-dimensional speckle-tracking echocardiography study. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Saghir, M.; Areces, M.; Makan, M. Strain rate imaging differentiates hypertensive cardiac hypertrophy from physiologic cardiac hypertrophy (athlete’s heart). J. Am. Soc. Echocardiogr. 2007, 20, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Majstorovic, A.; Pencic, B.; Ivanovic, B.; Neskovic, A.; Badano, L.; Stanisavljevic, D.; Scepanovic, R.; Stevanovic, P.; Celic, V. The impact of high-normal blood pressure on left ventricular mechanics: A three-dimensional and speckle tracking echocardiography study. Int. J. Cardiovasc. Imaging 2014, 30, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, P.; Sjoblom, P.; Nilsson, M.; Wijkman, M.; Engvall, M.; Lanne, T.; Nystrom, F.H.; Ostgren, C.J.; Engvall, J. Overweight and obesity impair left ventricular systolic function as measured by left ventricular ejection fraction and global longitudinal strain. Cardiovasc. Diabetol. 2018, 17, 113. [Google Scholar] [CrossRef]
- Arenas, I.A.; Podesta, C.A.; Issa, O.; Lin, J.; Brenes, J.C. Myocardial longitudinal strain, fitness, and heart failure risk factors in young adults. Echocardiography 2020, 37, 404–411. [Google Scholar] [CrossRef]
- Siurana, J.M.; Ventura, P.S.; Yeste, D.; Riaza-Martin, L.; Arciniegas, L.; Clemente, M.; Torres, M.; Amigo, N.; Giralt, G.; Roses-Noguer, F.; et al. Myocardial Geometry and Dysfunction in Morbidly Obese Adolescents (BMI 35–40 kg/m(2)). Am. J. Cardiol. 2021, 157, 128–134. [Google Scholar] [CrossRef]
- D’Andrea, A.; Bossone, E.; Radmilovic, J.; Caso, P.; Calabro, R.; Russo, M.G.; Galderisi, M. The role of new echocardiographic techniques in athlete’s heart. F1000Research 2015, 4, 289. [Google Scholar] [CrossRef]
- Galderisi, M.; Lomoriello, V.S.; Santoro, A.; Esposito, R.; Olibet, M.; Raia, R.; Di Minno, M.N.; Guerra, G.; Mele, D.; Lombardi, G. Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: A speckle-tracking echocardiography study. J. Am. Soc. Echocardiogr. 2010, 23, 1190–1198. [Google Scholar] [CrossRef]
- Caruso, M.R.; Garg, L.; Martinez, M.W. Cardiac Imaging in the Athlete: Shrinking the “Gray Zone”. Curr. Treat. Options Cardiovasc. Med. 2020, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Jaroszewski, D.E.; Velazco, C.S.; Pulivarthi, V.; Arsanjani, R.; Obermeyer, R.J. Cardiopulmonary Function in Thoracic Wall Deformities: What Do We Really Know? Eur. J. Pediatr. Surg. 2018, 28, 327–346. [Google Scholar] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Granato, A.; Lombardo, M.; Anza, C.; Ambrosio, G. Reduced Myocardial Strain Parameters in Subjects with Pectus Excavatum: Impaired Myocardial Function or Methodological Limitations Due to Chest Deformity? Semin. Thorac. Cardiovasc. Surg. 2021, 33, 251–262. [Google Scholar] [CrossRef] [PubMed]
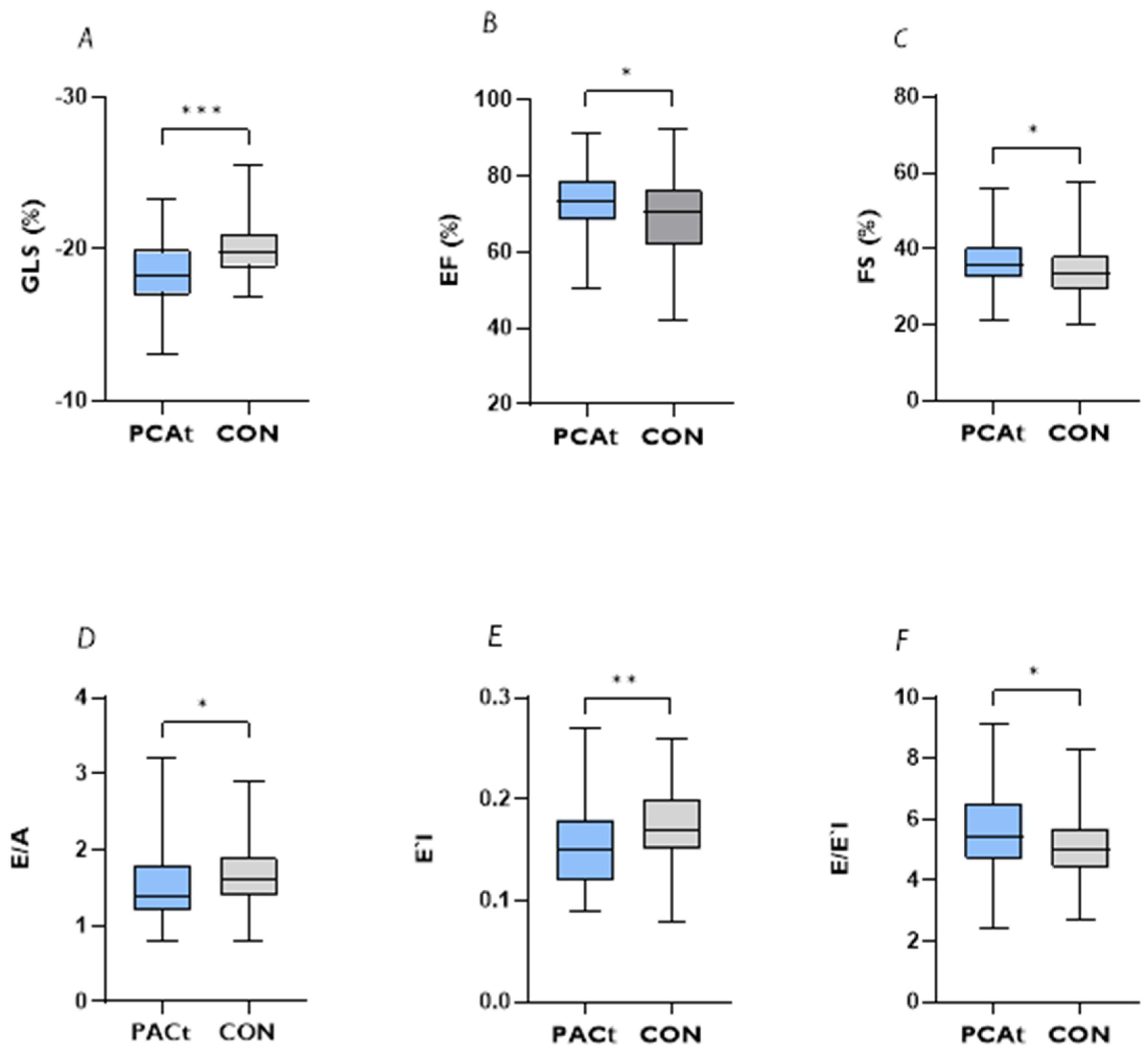
| PCAt | CON | Test Statistic | p-Value | |
|---|---|---|---|---|
| Number | 88 | 52 | ||
| Sex (female/male) | 31/57 (35%/65%) | 20/32 (38%/62%) | φ = 0.04 | 0.741 |
| Age (years), median (IQR) | 29 (22–40) | 22 (19–28) | W = 2655.5 | <0.010 ** |
| Weight (kg), median (IQR) | 73.3 (64.2–84.5) | 72.4 (61.4–80.9) | W = 2735 | 0.212 |
| Height (cm), median (IQR) | 176 (168–189) | 179 (171–184) | W = 3332.5 | 0.4072 |
| BMI (kg/m2), median (IQR) | 22.8 (21.6–25.9) | 22.7 (20.8–24.3) | W = 2522.5 | <0.050 * |
| Systolic blood pressure, mmHg, median (IQR) | 120 (110–125) | 120 (110–130) | W = 2746 | 0.1418 |
| Diastolic blood pressure, mmHg, median (IQR) | 80 (70–80) | 80 (71.25–90) | W = 2559 | 0.033 * |
| HR (bpm), median (IQR) | 62 (55–68) | 62 (56–68) | W = 5709 | 0.6317 |
| Training volume | at least three times per week >20 MET | >three times per week >20 MET |
| PCAt | CON | W | p-Value | |
|---|---|---|---|---|
| EDV (mL), median (IQR) | 123.00 (108.00–146.00) | 141.50 (104.00–169.00) | 4207.5 | 0.246 |
| ESV (mL), median (IQR) | 32.50 (24.18–44.53) | 38.80 (28.62–53.40) | 4488.0 | 0.047 * |
| LV Mass (g), median (IQR) | 149.50 (124.00–184.75) | 172.00 (134.00–202.50) | 4457.5 | 0.037 * |
| LV-EF (%), median (IQR) | 73.25 (67.85–78.57) | 70.35 (61.95–76.00) | 3130.5 | 0.042 * |
| FS (%), median (IQR) | 35.75 (32.32–40.30) | 33.40 (29.35–38.00) | 2949.0 | 0.015 * |
| GLS (%), median (IQR) | −18.27 (−19.72–−17.14) | −19.76 (−20.72–18.99) | 2059.5 | <0.001 *** |
| GRS (%), median (IQR) | 10.42 (5.60–15.91) | 11.12 (6.11–19.45) | 4020.5 | 0.349 |
| Stroke Volume (ml), median (IQR) | 89.10 (77.60–110.00) | 92.30 (71.85–115.00) | 3788.5 | 0.855 |
| E/A, median (IQR) | 1.40 (1.20–1.80) | 1.60 (1.40–1.90) | 4416.0 | 0.020 * |
| E′l, median (IQR) | 0.15 (0.12–0.18) | 0.16 (0.15–0.20) | 4057.0 | 0.009 ** |
| E′m, median (IQR) | 0.11 (0.09–0.13) | 0.12 (0.10–0.13) | 3129.5 | 0.414 |
| E/E′l, median (IQR) | 5.45 (4.70–6.60) | 5.00 (4.40–5.70) | 2629.0 | 0.024 * |
| E/E′m, median (IQR) | 7.30 (6.30–9.15) | 7.20 (6.50–8.10) | 3585.5 | 0.618 |
| Vmax A, median (IQR) | 0.55 (0.46–0.64) | 0.52 (0.46–0.58) | 3078.0 | 0.076 |
| Vmax E, median (IQR) | 0.81 (0.67–0.96) | 0.83 (0.72–0.94) | 3974.0 | 0.332 |
| Dec Time, median (IQR) | 164.00 (129.00–198.00) | 183.50 (144.25–218.50) | 2415.0 | 0.168 |
| Symptoms | Present | Not Present |
|---|---|---|
| fever | 26 (46%) | 30 (54%) |
| cough | 31 (55%) | 25 (45%) |
| rhinorrhea | 37 (66%) | 19 (34%) |
| sore throat | 33 (59%) | 23 (41%) |
| resting dyspnea | 14 (25%) | 42 (75%) |
| exertional dyspnea during COVID-19 | 32 (57%) | 24 (43%) |
| exertional dyspnea after COVID-19 | 34 (62%) | 21 (38%) |
| palpitations | 20 (36%) | 36 (64%) |
| chest pain | 20 (36%) | 36 (64%) |
| increased resting heart rate | 25 (45%) | 31 (55%) |
| subjective perceived performance limitation | 37 (66%) | 19 (34%) |
| dizziness | 25 (45%) | 30 (55%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schellenberg, J.; Ahathaller, M.; Matits, L.; Kirsten, J.; Kersten, J.; Steinacker, J.M. Left Ventricular Global Longitudinal Strain as a Parameter of Mild Myocardial Dysfunction in Athletes after COVID-19. J. Cardiovasc. Dev. Dis. 2023, 10, 189. https://doi.org/10.3390/jcdd10050189
Schellenberg J, Ahathaller M, Matits L, Kirsten J, Kersten J, Steinacker JM. Left Ventricular Global Longitudinal Strain as a Parameter of Mild Myocardial Dysfunction in Athletes after COVID-19. Journal of Cardiovascular Development and Disease. 2023; 10(5):189. https://doi.org/10.3390/jcdd10050189
Chicago/Turabian StyleSchellenberg, Jana, Magdalena Ahathaller, Lynn Matits, Johannes Kirsten, Johannes Kersten, and Juergen Michael Steinacker. 2023. "Left Ventricular Global Longitudinal Strain as a Parameter of Mild Myocardial Dysfunction in Athletes after COVID-19" Journal of Cardiovascular Development and Disease 10, no. 5: 189. https://doi.org/10.3390/jcdd10050189
APA StyleSchellenberg, J., Ahathaller, M., Matits, L., Kirsten, J., Kersten, J., & Steinacker, J. M. (2023). Left Ventricular Global Longitudinal Strain as a Parameter of Mild Myocardial Dysfunction in Athletes after COVID-19. Journal of Cardiovascular Development and Disease, 10(5), 189. https://doi.org/10.3390/jcdd10050189








