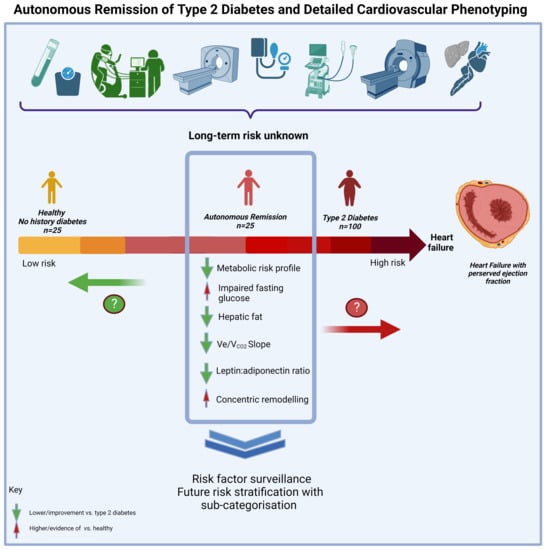
Impact of the Remission of Type 2 Diabetes on Cardiovascular Structure and Function, Exercise Capacity and Risk Profile: A Propensity Matched Analysis
Abstract
:1. Introduction
2. Materials and Methods
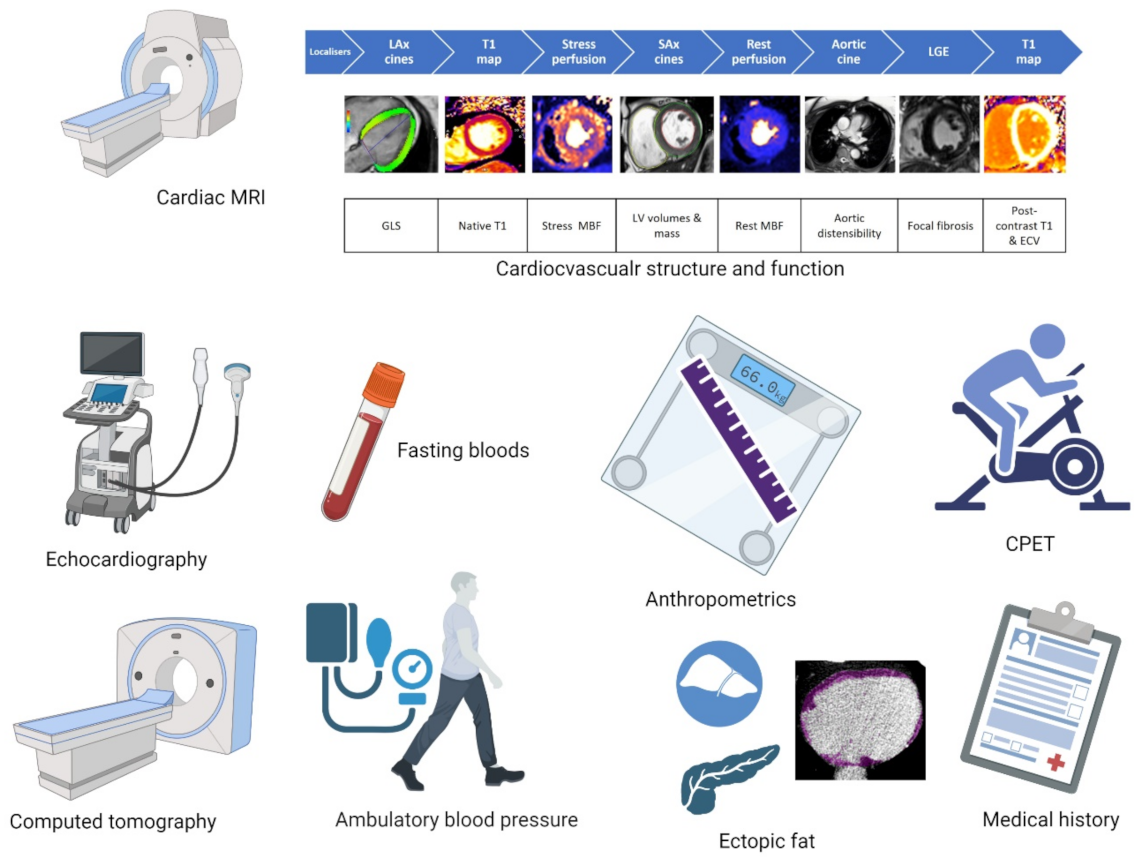
2.1. Assessments
2.2. Statistical Analysis
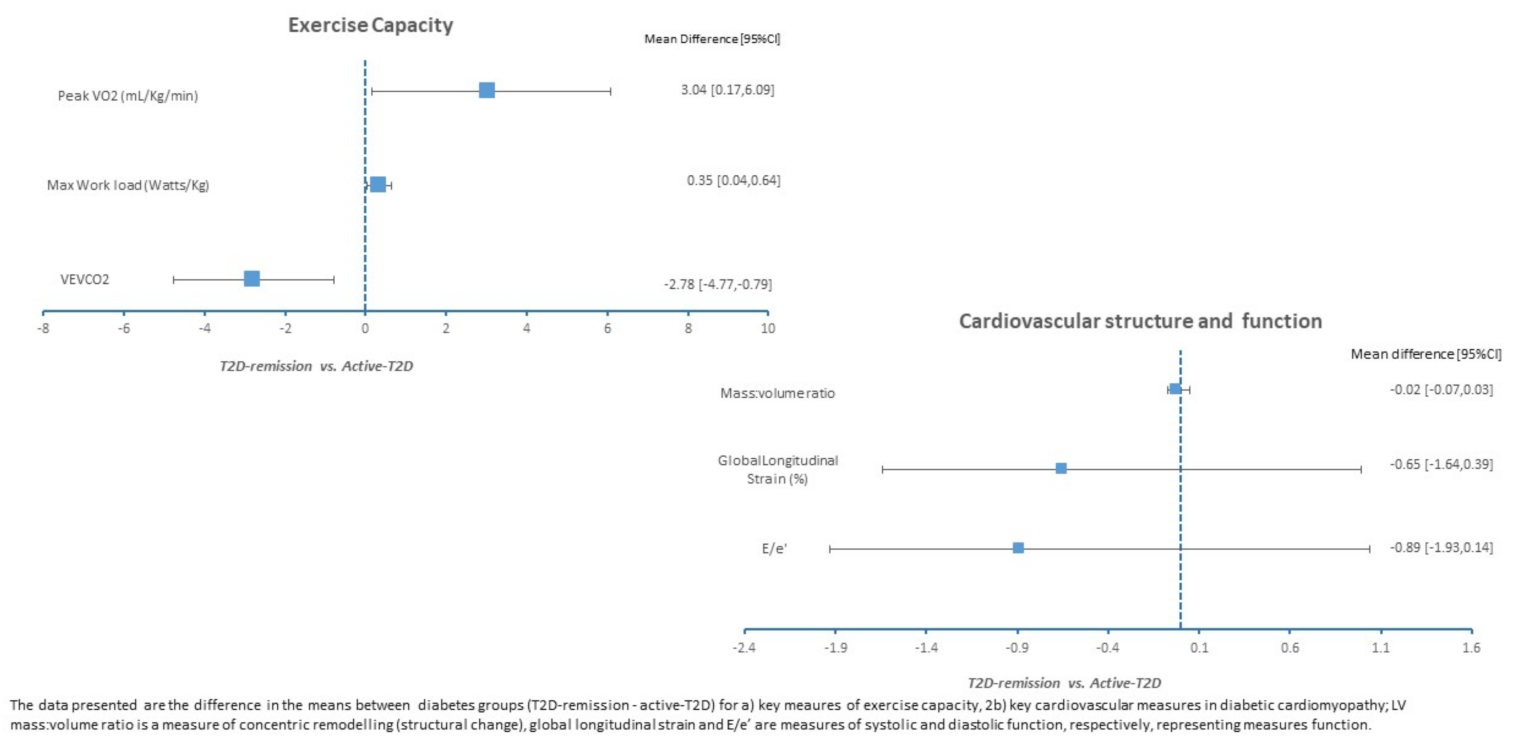
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Borlaug, B. The pathophysiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2014, 11, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Athithan, L.; Gulsin, G.S.; McCann, G.P.; Levelt, E. Diabetic cardiomyopathy: Pathophysiology, theories and evidence to date. World J. Diabetes 2019, 10, 490–510. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. J. Card. Fail. 2021, 27, 387–413. [Google Scholar]
- Seyoum, B.; O Estacio, R.; Berhanu, P.; Schrier, R.W. Exercise capacity is a predictor of cardiovascular events in patients with type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2006, 3, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Bilak, J.M.; Gulsin, G.S.; McCann, G.P. Cardiovascular and systemic determinants of exercise capacity in people with type 2 diabetes mellitus. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018820980235. [Google Scholar] [CrossRef]
- Pandey, A.; Shah, S.J.; Butler, J.; Kellogg, D.L., Jr.; Lewis, G.D.; Forman, D.E.; Mentz, R.J.; Borlaug, B.A.; Simon, M.A.; Chirinos, J.A.; et al. Exercise Intolerance in Older Adults with Heart Failure with Preserved Ejection Fraction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1166–1187. [Google Scholar] [CrossRef] [PubMed]
- Gulsin, G.S.; Swarbrick, D.J.; Athithan, L.; Brady, E.M.; Henson, J.; Baldry, E.; Argyridou, S.; Jaicim, N.B.; Squire, G.; Walters, Y.; et al. Effects of Low-Energy Diet or Exercise on Cardiovascular Function in Working-Age Adults with Type 2 Diabetes: A Prospective, Randomized, Open-Label, Blinded End Point Trial. Diabetes Care 2020, 43, 1300–1310. [Google Scholar] [CrossRef]
- Cuspidi, C.; Rescaldani, M.; Tadic, M.; Sala, C.; Grassi, G. Effects of Bariatric Surgery on Cardiac Structure and Function: A Systematic Review and Meta-Analysis. Am. J. Hypertens. 2014, 27, 146–156. [Google Scholar] [CrossRef]
- Riddle, M.C.; Cefalu, W.T.; Evans, P.H.; Gerstein, H.C.; Nauck, M.A.; Oh, W.K.; Rothberg, A.E.; le Roux, C.W.; Rubino, F.; Schauer, P.; et al. Consensus Report: Definition and Interpretation of Remission in Type 2 Diabetes. Diabetes Care 2021, 44, 2438–2444. [Google Scholar] [CrossRef]
- Dambha-Miller, H.; Day, A.; Strelitz, J.; Irving, G.; Griffin, S. Behaviour change, weight loss and remission of Type 2 diabetes: A community-based prospective cohort study. Diabet. Med. 2020, 37, 681–688. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Triantafyllidi, H.; Birmpa, D.; Benas, D.; Trivilou, P.; Fambri, A.; Iliodromitis, E.K. Cardiopulmonary Exercise Testing: The ABC for the Clinical Cardiologist. Cardiology 2022, 147, 62–71. [Google Scholar] [CrossRef]
- Yeo, J.L.; Gulsin, G.S.; Brady, E.M.; Dattani, A.; Bilak, J.M.; Marsh, A.-M.; Sian, M.; Athithan, L.; Parke, K.S.; Wormleighton, J.; et al. Association of ambulatory blood pressure with coronary microvascular and cardiac dysfunction in asymptomatic type 2 diabetes. Cardiovasc. Diabetol. 2022, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Statistical Criteria for Selecting the Optimal Number of Untreated Subjects Matched to Each Treated Subject When Using Many-to-One Matching on the Propensity Score. Am. J. Epidemiology 2010, 172, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Austin, P.C.; Tu, J.V.; Ko, D.T.; Mazer, C.D.; Kiss, A.; Fremes, S.E. A Review of Propensity-Score Methods and Their Use in Cardiovascular Research. Can. J. Cardiol. 2016, 32, 259–265. [Google Scholar] [CrossRef] [PubMed]
- St. Pierre, T.G.; House, M.J.; Bangma, S.J.; Pang, W.; Bathgate, A.; Gan, E.K.; Ayonrinde, O.T.; Bhathal, P.S.; Clouston, A.; Olynyk, J.K.; et al. Stereological Analysis of Liver Biopsy Histology Sections as a Reference Standard for Validating Non-Invasive Liver Fat Fraction Measurements by MRI. PLoS ONE. 2016, 11, e0160789. [Google Scholar] [CrossRef]
- Moore, B.; Brubaker, P.H.; Stewart, K.P.; Kitzman, D.W. VE/VCO2 Slope in Older Heart Failure Patients with Normal Versus Reduced Ejection Fraction Compared with Age-Matched Healthy Controls. J. Card. Fail. 2007, 13, 259–262. [Google Scholar] [CrossRef]
- Arnold, J.; Kanagala, P.; Budgeon, C.; Jerosch-Herold, M.; Singh, A.; Khan, J.; Gulsin, G.; Squire, I.; Ng, L.; McCann, G. Prevalence of microvascular dysfunction and association with clinical outcomes in heart failure with preserved ejection fraction. Eur. Heart J. 2020, 41, ehaa946-0253. [Google Scholar] [CrossRef]
- Taylor, R. Calorie restriction for long-term remission of type 2 diabetes. Clin. Med. 2019, 19, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Koepp, K.E.; Obokata, M.; Reddy, Y.N.; Olson, T.P.; Borlaug, B.A. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure with Preserved Ejection Fraction. JACC: Heart Fail. 2020, 8, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Patel, K.V.; Vaduganathan, M.; Sarma, S.; Haykowsky, M.J.; Berry, J.D.; Lavie, C.J. Physical Activity, Fitness, and Obesity in Heart Failure with Preserved Ejection Fraction. JACC: Heart Fail. 2018, 6, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Myers, J.; Aslam, S.S.; Varughese, E.B.; Peberdy, M.A. Peak VO2 and VE/VCO2 slope in patients with heart failure: A prognostic comparison. Am. Heart J. 2004, 147, 354–360. [Google Scholar] [CrossRef]
- Alcendor, R.R.; Gao, S.; Zhai, P.; Zablocki, D.; Holle, E.; Yu, X.; Tian, B.; Wagner, T.; Vatner, S.F.; Sadoshima, J. Sirt1 Regulates Aging and Resistance to Oxidative Stress in the Heart. Circ. Res. 2007, 100, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Corbi, G.; Polito, M.V.; Ciccarelli, M.; Manzo, V.; Torsiello, M.; De Bellis, E.; D’auria, F.; Vitulano, G.; Piscione, F.; et al. Sirt1 Activity in PBMCs as a Biomarker of Different Heart Failure Phenotypes. Biomolecules 2020, 10, 1590. [Google Scholar] [CrossRef]
- Ministrini, S.; Puspitasari, Y.M.; Beer, G.; Liberale, L.; Montecucco, F.; Camici, G.G. Sirtuin 1 in Endothelial Dysfunction and Cardiovascular Aging. Front. Physiol. 2021, 12, 1589. [Google Scholar] [CrossRef]
- Sellitto, C.; Corbi, G.; Stefanelli, B.; Manzo, V.; Trucillo, M.; Charlier, B.; Mensitieri, F.; Izzo, V.; Lucariello, A.; Perna, A.; et al. Antioxidant Supplementation Hinders the Role of Exercise Training as a Natural Activator of SIRT1. Nutrients 2022, 14, 2092. [Google Scholar] [CrossRef] [PubMed]
- Gulsin, G.S.; Henson, J.; Brady, E.M.; Sargeant, J.A.; Wilmot, E.G.; Athithan, L.; Htike, Z.Z.; Marsh, A.-M.; Biglands, J.D.; Kellman, P.; et al. Cardiovascular Determinants of Aerobic Exercise Capacity in Adults with Type 2 Diabetes. Diabetes Care 2020, 43, 2248–2256. [Google Scholar] [CrossRef]
- Bilak, J.M.; Alam, U.; A Miller, C.; McCann, G.P.; Arnold, J.R.; Kanagala, P. Microvascular Dysfunction in Heart Failure with Preserved Ejection Fraction: Pathophysiology, Assessment, Prevalence and Prognosis. Card. Fail. Rev. 2022, 8, e24. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. The emerging challenge in diabetes: The “metabolic memory”. Vasc. Pharmacol. 2012, 57, 133–138. [Google Scholar] [CrossRef]
- Berezin, A. Metabolic memory phenomenon in diabetes mellitus: Achieving and perspectives. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S176–S183. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Song, Q.; Hu, C.; Da, X.; Yu, Y.; He, Z.; Xu, C.; Chen, Q.; Wang, Q.K. Endothelial cell metabolic memory causes cardiovascular dysfunction in diabetes. Cardiovasc. Res. 2021, 118, 196–211. [Google Scholar] [CrossRef] [PubMed]
- MacKay, D.; Chan, C.; Dasgupta, K.; Dominy, C.; Gagner, M.; Jin, S.; Kim, J.; Little, J.P.; MacDonald, B.; McInnes, N.; et al. Remission of Type 2 Diabetes. Can. J. Diabetes 2022, 46, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, Å.; Bouchard, C.; Carlsson, B.; Karason, K.; Lönroth, H.; et al. Association of Bariatric Surgery with Long-term Remission of Type 2 Diabetes and With Microvascular and Macrovascular Complications. JAMA 2014, 311, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
| T2D Remission (n = 25) | Active T2D (n = 100) | Controls (n = 25) | |
|---|---|---|---|
| Demographic, Anthropometric and Glycaemic Characteristics | |||
| Age (years) | 62.1 ± 8.9 | 62.1 ± 7.0 | 59.4 ±7.3 |
| Male (%) | 15 (60) | 63 (63) | 13 (52) |
| White Ethnicity (%) | 20 (80) | 80 (80) | 13 (52) |
| Smoking, Never (%) | 14 (56) | 53 (53) | 17 (68) |
| Height (cm) | 169.5 ± 9.8 | 170.49 ± 9.01 | 170.4 ± 9.2 |
| Weight (kg) | 78.3 ± 17.3 | 93.2 ± 19.1 | 79.4 ± 15.2 |
| BMI (kg/m2) | 27.1 ± 4.7 | 32.1 ± 6.4 | 27.3 ± 3.9 |
| Weight–height ratio (kg/cm) | 0.46 ± 0.09 | 0.55 ± 0.11 | 0.46 ± 0.07 |
| Waist circumference (cm) | 99.1 ± 13.1 | 109.9 ± 11.1 | 97.0 ± 11.8 |
| Waist–hip ratio | 0.93 ± 0.07 | 0.97 ± 0.06 | 0.91 ± 0.07 |
| Years since T2D diagnosis | 5.7 ±4.4 | 5.6 ± 4.2 | - |
| Remission (years) | 4.2 ± 2.5 | - | - |
| Medication | |||
| Number of antihypertensives $ | |||
| 0 | 17 (68) | 49 (49) | 23 (92) |
| 1 | 3 (12) | 25 (25) | 2 (8) |
| ≥2 | 5 (20) | 26 (26) | 0 (0) |
| Statin (%) | 9 (36) | 65 (65) | 6 (24) |
| Dietary supplement (%) | 6 (24) | 28 (28) | 12 (48) |
| 24 h ambulatory blood pressure and heart rate | |||
| Systolic (mmHg) | 127.3 ± 11.2 | 127.6 ± 12.7 | 118.7 ± 11.3 † |
| Diastolic (mmHg) | 76.7 ± 8.3 | 75.2 ± 7.1 | 73.0 ± 6.6 |
| Heart rate (bpm) | 73.0 ± 10.8 | 75.9 ± 10.3 | 68.1 ± 6.9 |
| Biochemistry | |||
| eGFR (mL/min/1.72 m2) | 80 (70, 89) | 78 (74, 84) | 79 (73, 83) |
| NT-proBNP (pg/mL) | 62 (4, 111) | 59 (41.5, 93.0) | 48 (39, 99) |
| Hs-Trop I (ng/L) | 5.80 (3.20, 7.60) | 3.35 (2.70, 5.30) | 4.20 (3.05, 4.65) |
| Leptin (ng/mL) | 6.30 (4.15, 12.60) | 17.40 (12.14, 29.43) * | 9.52 (4.53, 21.77) |
| Adiponectin (g/mL) | 9.39 (8.02, 13.21) | 8.02 (6.96, 9.74) | 10.42 (8.11, 11.90) |
| Leptin:Adiponectin Ratio (LAR) | 0.75 (0.37, 1.50) | 2.24 (1.43, 4.10) * | 0.89 (0.46, 2.07) |
| HbA1C (%) | 5.84 ± 0.44 | 7.39 ± 1.09 | 5.49 ± 0.32 |
| HbA1c (mmol/mol) | 40.24 ± 4.95 | 57.24 ± 12.00 | 36.46 ± 3.49 |
| Fasting glucose (mmol/L) | 6.15 ± 0.82 | 8.30 ± 2.05 | 5.21 ± 0.56 |
| IFG pre-diabetes range (5.6–6.9 mmol/l, (n (%)) | 15 (60) | 17 (17) * | 7 (28) † |
| IFG T2D range (> 7.0 mmol/l, (n (%)) | 5 (22) | 75 (75) * | 0 (0) † |
| Insulin μIU/mL | 12.99 (11.56, 16.39) | 18.56 (11.21, 26.70) | 6.43 (4.89, 10.50) |
| HOMA-IR | 3.38 (2.88, 4.61) | 6.84 (4.20, 11.11) | 1.64 (1.08, 2.50) |
| Cholesterol (mmol/L) | 4.90 ± 1.33 | 4.43 ± 1.01 | 5.40 ± 1.08 |
| HDL cholesterol (mmol/L) | 1.40 (1.20, 1.90) | 1.20 (1.00, 1.50) * | 1.65 (1.23, 2.08) |
| LDL cholesterol (mmol/L) | 2.62 ± 1.02 | 2.32 ± 0.81 | 3.18 ± 0.97 |
| Triglycerides (mmol/L) | 1.45 (0.96, 1.74) | 1.69 (1.3, 2.2) * | 1.18 (0.98, 1.45) |
| T2D Remission (n = 25) | Active T2D (n = 100) | Controls (n = 25) | |
|---|---|---|---|
| Echocardiography | |||
| E/A ratio | 0.94 ± 0.31 | 0.91 ± 0.21 | 1.04 ± 0.19 |
| E/e’ | 8.1 ± 2.2 | 9.0 ± 2.0 | 8.7 ± 1.5 |
| Magnetic resonance imaging | |||
| Hepatic fat (%) | 3.20 (2.20, 7.33) | 9.40 (4.80, 15.20) * | 2.70 (1.90, 3.70) |
| Pancreatic fat (%) | 3.80 (3.15, 6.45) | 6.20 (3.60, 9.30) | 3.30 (1.80, 5.90) |
| LV mass/height (g/m) | 0.66 ± 0.13 | 0.71 ± 0.16 | 0.67 ± 0.14 |
| LVEDV/height (ml/m) | 0.77 ± 0.14 | 0.80 ± 0.19 | 0.84 ± 0.14 |
| LV mass: volume | 0.88 ± 0.10 | 0.90 ± 0.13 | 0.80 ± 0.10 † |
| LV EF (%) | 66.1 ± 7.3 | 66.7± 6.8 | 67.5 ± 3.9 |
| LV GCS (%) | 18.8 ± 2.4 | 19.1 ± 2.3 | 19.5 ± 1.8 |
| LV GLS (%) | 15.8 ± 2.3 | 16.4 ± 2.2 | 17.4 ± 1.9 |
| ECV (%) | 27.0 ± 2.7 | 27.0 ± 2.5 | 26.0 ± 1.7 |
| LGE infarction (n (%)) | 10 (40) | 28 (28) | 14 (56) |
| Myocardial perfusion reserve | 2.84 ± 0.75 | 2.88 ± 0.80 | 3.13 ± 0.87 |
| Cardiac computed tomography | |||
| Calcium score $ <1 | 13 (56.5) | 30 (30.6) | 13 (52.0) |
| Calcium score $ 1–100 | 4 (17.4) | 37 (37.7) | 4 (16.0) |
| Calcium score $ >100 | 6 (26.1) | 31 (31.6) | 8 (32.0) |
| Epicardial adipose volume (mL) | 111.36 ± 67.38 | 144.22 ± 54.84 | 89.41 ± 40.48 |
| Cardiopulmonary exercise testing | |||
| Max. workload (watts) | 127.68 ± 54.59 | 124.73 ± 46.68 | 152.28 ± 53.46 |
| Max. workload/weight (watts/Kg) | 1.65 ± 0.69 | 1.30 ± 0.54 | 1.91 ± 0.55 |
| Peak VO2 (L/min) | 1.72 ± 0.60 | 1.74 ± 0.51 | 1.89 ± 0.55 |
| Peak VO2 (mL/kg/min) | 22.20 ± 7.05 | 19.16 ± 4.90 | 23.94 ± 5.55 |
| VE/VCO2 slope | 27.74 ± 3.95 | 30.52 ± 5.46 * | 27.09 ± 4.57 |
| Max. exercise heart rate (bpm) | 155.8 ± 18.6 | 150.8 ± 18.2 | 157.4 ± 15.0 |
| Respiratory exchange ratio peak | 1.06 ± 0.06 | 1.06 ± 0.08 | 1.10 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilak, J.M.; Yeo, J.L.; Gulsin, G.S.; Marsh, A.-M.; Sian, M.; Dattani, A.; Ayton, S.L.; Parke, K.S.; Bain, M.; Pang, W.; et al. Impact of the Remission of Type 2 Diabetes on Cardiovascular Structure and Function, Exercise Capacity and Risk Profile: A Propensity Matched Analysis. J. Cardiovasc. Dev. Dis. 2023, 10, 191. https://doi.org/10.3390/jcdd10050191
Bilak JM, Yeo JL, Gulsin GS, Marsh A-M, Sian M, Dattani A, Ayton SL, Parke KS, Bain M, Pang W, et al. Impact of the Remission of Type 2 Diabetes on Cardiovascular Structure and Function, Exercise Capacity and Risk Profile: A Propensity Matched Analysis. Journal of Cardiovascular Development and Disease. 2023; 10(5):191. https://doi.org/10.3390/jcdd10050191
Chicago/Turabian StyleBilak, Joanna M., Jian L. Yeo, Gaurav S. Gulsin, Anna-Marie Marsh, Manjit Sian, Abhishek Dattani, Sarah L. Ayton, Kelly S. Parke, Moira Bain, Wenjie Pang, and et al. 2023. "Impact of the Remission of Type 2 Diabetes on Cardiovascular Structure and Function, Exercise Capacity and Risk Profile: A Propensity Matched Analysis" Journal of Cardiovascular Development and Disease 10, no. 5: 191. https://doi.org/10.3390/jcdd10050191
APA StyleBilak, J. M., Yeo, J. L., Gulsin, G. S., Marsh, A.-M., Sian, M., Dattani, A., Ayton, S. L., Parke, K. S., Bain, M., Pang, W., Boulos, S., Pierre, T. G. S., Davies, M. J., Yates, T., McCann, G. P., & Brady, E. M. (2023). Impact of the Remission of Type 2 Diabetes on Cardiovascular Structure and Function, Exercise Capacity and Risk Profile: A Propensity Matched Analysis. Journal of Cardiovascular Development and Disease, 10(5), 191. https://doi.org/10.3390/jcdd10050191