Differences in Long-Term Heart Rate Variability between Subjects with and without Metabolic Syndrome: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Inclusion/Exclusion Criteria
2.3. Quality Assessment
2.4. Data Extraction
3. Results
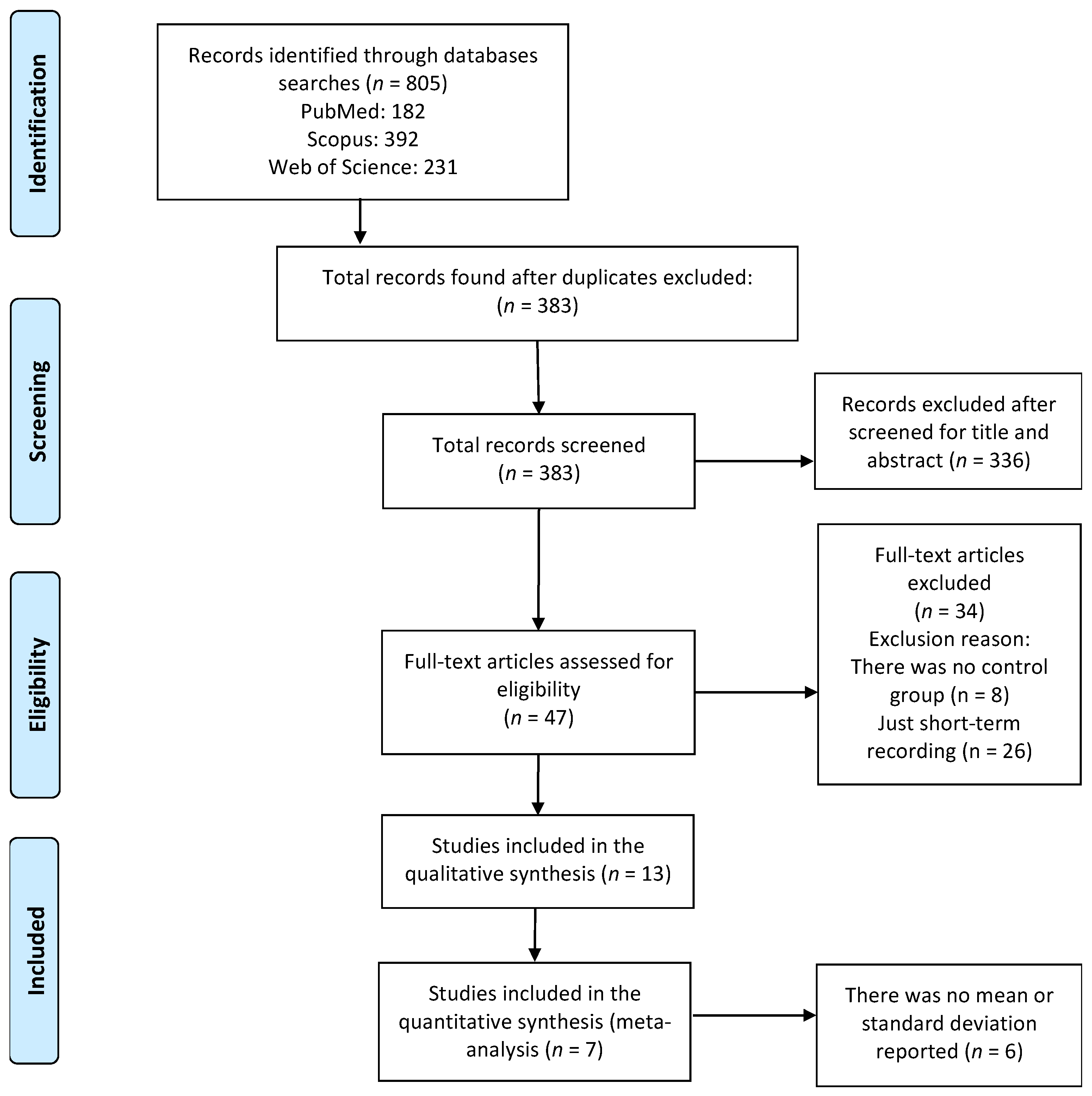
3.1. Identification of Studies
3.2. Quality Assessment
3.3. Study and Patient Characteristics
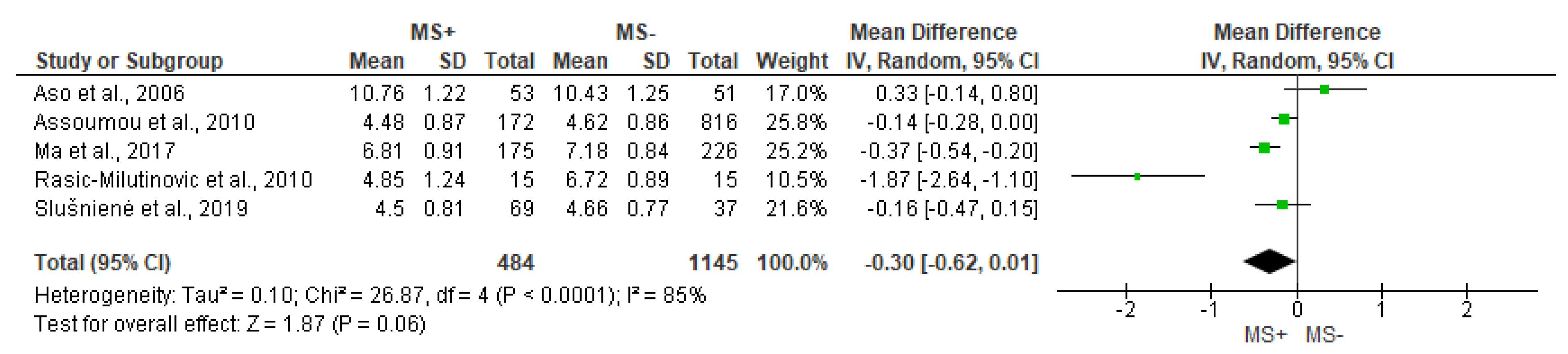
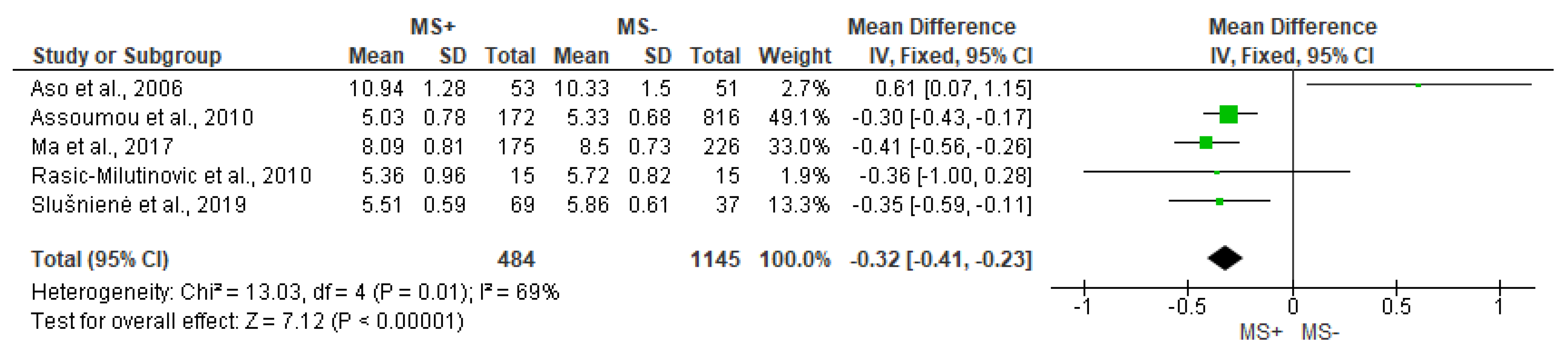
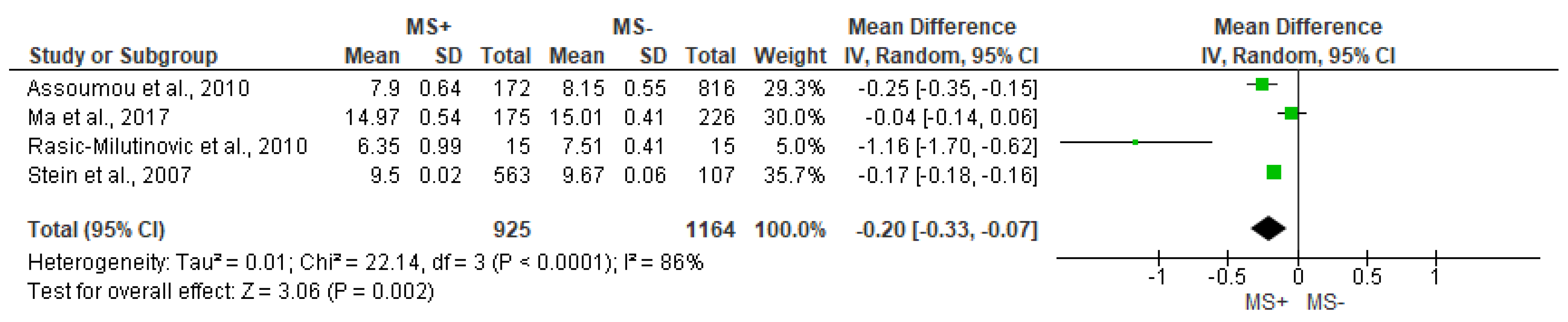
3.4. Time Domain Analysis Outcomes
3.5. Frequency Domain Analysis Outcomes
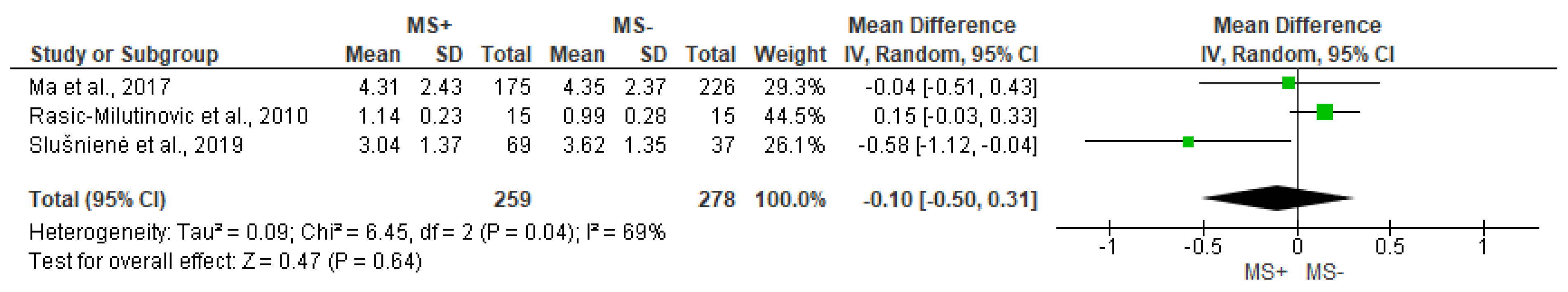
3.6. Non-Linear Analysis Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameter | Description |
|---|---|
| SDNN | Standard deviation of the R-R interval series (overall variability). |
| SDANN | Standard deviation of the average R-R intervals for each 5 min segment of a 24 h HRV recording |
| SDNNi | Mean of the standard deviations of all the R-R intervals for each 5 min segment of a 24 h HRV recording |
| rMSSD | The root mean square of differences of successive R-R intervals. |
| pNN50 | Successive R-R intervals that differ by more than 50 ms (expressed in percentage) |
| pNN20 | Successive R-R intervals that differ by more than 20 ms (expressed in percentage) |
| R-R interval | Mean of successive R-R intervals. |
| Parameter | Description |
|---|---|
| LF, HF, VLF, ULF | Ranges of the spectral components of the HRV |
| LF: low frequency (0.04–0.15 Hz) | |
| HF: high frequency (0.15–0.4 Hz) VLF: very low frequency (0.0033–0.04 Hz) ULF: ultra-low frequency (<0.0033 Hz) | |
| LF/HF | Ratio between LF and HF bands. |
| TP | Total power and includes all the bands together (<0.4 Hz). |
| Parameter | Description |
|---|---|
| SD1 | Standard deviation of the perpendicular point along the line of identity of the Poincaré plot. It represents the instantaneous beat-to-beat short-term variability. |
| SD2 | Standard deviation of the perpendicular point along the line of identity of the Poincaré plot. It represents the instantaneous beat-to-beat long-term variability. |
| α1 | Short terms fluctuations (4–12 beats) of detrended fluctuation analysis. The slopes of a log-log plot (correlation measure as a function of segment length). |
| Multiscale entropy | Measures the complexity of R-R time series, based on the analysis of sample entropy |
References
- Reaven, G.M. Role of insulin resistance in human disease. (Banting Lecture 1988). Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C.; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Sperling, L.S.; Mechanick, J.I.; Neeland, I.J.; Herrick, C.J.; Després, J.P.; Ndumele, C.E.; Vijayaraghavan, K.; Handelsman, Y.; Puckrein, G.A.; Araneta, M.R.G.; et al. The CardioMetabolic Health Alliance Working toward a New Care Model for the Metabolic Syndrome. J. Am. Coll. Cardiol. 2015, 66, 1050–1067. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, J.-H.; Shin, A.-R.; Song, K.-B.; Amano, A.; Choi, Y.-H. Association of Adiposity with Periodontitis and Metabolic Syndrome: From the Third National Health and Nutrition Examination Survey of United States. Int. J. Environ. Res. Public Health 2023, 20, 2533. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Yu, T.Y.; Lee, M.K. Autonomic dysfunction, diabetes and metabolic syndrome. J. Diabetes Investig. 2021, 12, 2108–2111. [Google Scholar] [CrossRef]
- Iellamo, F.; Perrone, M.A.; Cimini, A.; Caminiti, G.; Chiaravalloti, A.; Parisi, A.; Schillaci, O. Complementary Role of Combined Indirect and Direct Cardiac Sympathetic (Hyper)Activity Assessment in Patients with Heart Failure by Spectral Analysis of Heart Rate Variability and Nuclear Imaging: Possible Application in the Evaluation of Exercise Traini. J. Cardiovasc. Dev. Dis. 2022, 9, 181. [Google Scholar] [CrossRef]
- Endukuru, C.K.; Gaur, G.S.; Yerrabelli, D.; Sahoo, J.; Vairappan, B. Impaired baroreflex sensitivity and cardiac autonomic functions are associated with cardiovascular disease risk factors among patients with metabolic syndrome in a tertiary care teaching hospital of South-India. Diabetes Metab. Syndr. Res. Rev. 2020, 14, 2043–2051. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Z.-H.; Zeng, F.; Zhou, L. Associations between the severity of metabolic syndrome and cardiovascular autonomic function in a Chinese population. J. Endocrinol. Investig. 2013, 36, 993–999. [Google Scholar]
- Azulay, N.; Olsen, R.B.; Nielsen, C.S.; Stubhaug, A.; Jenssen, T.G.; Schirmer, H.; Frigessi, A.; Rosseland, L.A.; Tronstad, C. Reduced heart rate variability is related to the number of metabolic syndrome components and manifest diabetes in the sixth Tromsø study 2007–2008. Sci. Rep. 2022, 12, 11998. [Google Scholar] [CrossRef]
- Ma, Y.; Tseng, P.-H.P.-H.; Ahn, A.; Wu, M.-S.M.-S.; Ho, Y.-L.; Chen, M.-F.M.-F.; Peng, C.-K. Cardiac Autonomic Alteration and Metabolic Syndrome: An Ambulatory ECG-based Study in A General Population. Sci. Rep. 2017, 7, 44363. [Google Scholar] [CrossRef] [PubMed]
- Ksela, J.; Rupert, L.; Djordjevic, A.; Antonic, M.; Avbelj, V.; Jug, B. Altered Heart Rate Turbulence and Variability Parameters Predict 1-Year Mortality in Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Dev. Dis. 2022, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Camm, A.J.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Shaffer, F.; Meehan, Z.M.; Zerr, C.L. A Critical Review of Ultra-Short-Term Heart Rate Variability Norms Research. Front. Neurosci. 2020, 14, 594880. [Google Scholar] [CrossRef]
- McCraty, R.; Shaffer, F. Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob. Adv. Health Med. 2015, 4, 46–61. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2009, 8, 336–341. [Google Scholar] [CrossRef]
- Law, M.; Stewart, D.; Letts, L.; Pollock, N.; Bosch, J.; Westmorland, M.; Critical Review form–Quantitative Studies. McMaster University Occupational Therapy Evidence-Based Practice Research Group, Hamilton, ON, Canada. 1998. Available online: www.fhs.mcmaster.ca/rehab/ebp/pdf/qualguidelines.pdf (accessed on 1 January 2023).
- Faber, I.R.; Bustin, P.M.; Oosterveld, F.G.; Elferink-Gemser, M.T.; Nijhuis-Van der Sanden, M.W. Assessing personal talent determinants in young racquet sport players: A systematic review. J. Sport Sci. 2016, 34, 395–410. [Google Scholar] [CrossRef]
- Shuster, J.J. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Stein, P.K.; Barzilay, J.I.; Domitrovich, P.P.; Chaves, P.M.; Gottdiener, J.S.; Heckbert, S.R.; Kronmal, R.A. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome. Cardiovasc. Health Study Diabet. Med. 2007, 24, 855–863. [Google Scholar]
- Gehi, A.K.; Lampert, R.; Veledar, E.; Lee, F.; Goldberg, J.; Jones, L.; Murrah, R.N.N.; Ashraf, A.; Vaccarino, V. A Twin Study of Metabolic Syndrome and Autonomic Tone. J. Cardiovasc. Electrophysiol. 2009, 20, 422–428. [Google Scholar] [CrossRef]
- Wulsin, L.R.; Horn, P.S.; Perry, J.L.; Massaro, J.M.; D’Agostino Sr, R.B.; D’Agostino, R.B. The Contribution of Autonomic Imbalance to the Development of Metabolic Syndrome. Psychosom. Med. 2016, 78, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Poliwczak, A.R.; Tylinska, M.; Broncel, M. Effect of short-term testosterone replacement therapy on heart rate variability in men with hypoandrogen-metabolic syndrome. Pol. Arch. Med. Wewn. Arch. Intern. Med. 2013, 123, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Maciorowska, M.; Krzesinski, P.; Wierzbowski, R.; Gielerak, G. Heart Rate Variability in Patients with Hypertension: The Effect of Metabolic Syndrome and Antihypertensive Treatment. Cardiovasc. Ther. 2020, 2020, 8563135. [Google Scholar] [CrossRef] [PubMed]
- Assoumou, H.G.N.; Pichot, V.; Barthelemy, J.C.; Dauphinot, V.; Celle, S.; Gosse, P.; Kossovsky, M.; Gaspoz, J.M.; Roche, F. Metabolic Syndrome and Short-Term and Long-Term Heart Rate Variability in Elderly Free of Clinical Cardiovascular Disease: The PROOF Study. Rejuvenation Res. 2010, 13, 653–663. [Google Scholar] [CrossRef]
- Rasic-Milutinovic, Z.R.; Milicevic, D.R.; Milovanovic, B.D.; Perunicic-Pekovic, G.B.; Pencic, B.D. Do components of metabolic syndrome contribute to cardiac autonomic neuropathy in non-diabetic patients? Saudi Med. J. 2010, 31, 650–657. [Google Scholar]
- Jarczok, M.N.; Li, J.; Mauss, D.; Fischer, J.E.; Thayer, J.F. Heart Rate Variability is Associated with Glycemic Status After Controlling for Components of the Metabolic Syndrome. Int. J. Cardiol. 2013, 167, 855–861. [Google Scholar] [CrossRef]
- Balcioglu, A.S.; Akinci, S.; Cicek, D.; Eldem, H.O.; Coner, A.; Bal, U.A.; Müderrisoğlu, H. Which is responsible for cardiac autonomic dysfunction in non-diabetic patients with metabolic syndrome: Prediabetes or the syndrome itself? Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S13–S20. [Google Scholar] [CrossRef]
- Slušnienė, A.; Laucevičius, A.; Navickas, P.; Ryliškytė, L.; Stankus, V.; Stankus, A.; Navickas, R.; Laucevičienė, I.; Kasiulevičius, V. Daily heart rate variability indices in subjects with and without metabolic syndrome before and after the elimination of the influence of day-time physical activity. Medicina 2019, 55, 700. [Google Scholar] [CrossRef]
- Aso, Y.; Wakabayashi, S.; Nakano, T.; Yamamoto, R.; Takebayashi, K.; Inukai, T. High serum high-sensitivity C-reactive protein concentrations are associated with relative cardiac sympathetic overactivity during the early morning period in type 2 diabetic patients with metabolic syndrome. Metabolism 2006, 55, 1014–1021. [Google Scholar] [CrossRef]
- Yoo, H.J.; Hwang, S.Y.; Choi, K.M.; Baik, S.H.; Lee, E.M.; Kim, E.J.; Rha, S.-W.; Park, C.G.; Oh, D.J.; Seo, H.S. Clinical implication of body size phenotype on heart rate variability. Metabolism 2016, 65, 1589–1596. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef] [PubMed]
- DeGiorgio, C.M.; Miller, P.; Meymandi, S.; Chin, A.; Epps, J.; Gordon, S.; Gornbein, J.; Harper, R.M. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: The SUDEP-7 Inventory. Epilepsy Behav. 2010, 19, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, A.B.; Siedlik, J.A.; Wecht, J.M.; Deckert, J.A.; Nguyen, N.D.; Weir, J.P. Reminder: RMSSD and SD1 are identical heart rate variability metrics. Muscle Nerve 2017, 56, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Jarczok, M.N.; Weimer, K.; Braun, C.; Williams, D.P.; Thayer, J.F.; Gündel, H.O.; Balint, E.M. Heart rate variability in the prediction of mortality: A systematic review and meta-analysis of healthy and patient populations. Neurosci. Biobehav. Rev. 2022, 143, 104907. [Google Scholar] [CrossRef]
- Berntson, G.G.; Cacioppo, J.T.; Grossman, P. Whither vagal tone. Biol. Psychol. 2007, 74, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Rosenberg, A.A.; Weiser-Bitoun, I.; Billman, G.E.; Yaniv, Y. Signatures of the autonomic nervous system and the heart’s pacemaker cells in canine electrocardiograms and their applications to humans. Sci. Rep. 2020, 10, 9971. [Google Scholar] [CrossRef]
- Yaniv, Y.; Lakatta, E.G.; Maltsev, V.A. From two competing oscillators to one coupled-clock pacemaker cell system. Front. Physiol. 2015, 6, 28. [Google Scholar] [CrossRef]
- Tsuji, H.; Venditti, F.J.; Manders, E.S.; Evans, J.C.; Larson, M.G.; Feldman, C.L.; Levy, D. Reduced heart rate variability and mortality risk in an elderly cohort: The Framingham heart study. Circulation 1994, 90, 878–883. [Google Scholar] [CrossRef]
- Hadase, M.; Azuma, A.; Zen, K.; Asada, S.; Kawasaki, T.; Kamitani, T.; Kawasaki, S.; Sugihara, H.; Matsubara, H. Very Low Frequency Power of Heart Rate Variability is a Powerful Predictor of Clinical Prognosis in Patients with Congestive Heart Failure. Circ. J. 2004, 68, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Müller-Werdan, U.; Hoffmann, T.; Francis, D.P.; Piepoli, M.F.; Rauchhaus, M.; Prondzinsky, R.; Loppnow, H.; Buerke, M.; Hoyer, D.; et al. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit. Care Med. 2005, 33, 1994–2002. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.M.; Freedland, K.E.; Stein, P.K.; Miller, G.E.; Steinmeyer, B.; Rich, M.W.; Duntley, S.P. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J. Psychosom. Res. 2007, 62, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Lampert, R.; Bremner, J.D.; Su, S.; Miller, A.; Lee, F.; Cheema, F.; Goldberg, J.; Vaccarino, V. Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am. Heart J. 2008, 156, 759.e1–759.e7. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Shoaibinobarian, N.; Noormohammadi, M.; Fakhr Mousavi, A.; Savar Rakhsh, A.; Salari, A.; Ghorbani, Z. Inflammatory Markers and Atherogenic Coefficient: Early Markers of Metabolic Syndrome. Int. J. Endocrinol. Metab. 2022, 20, e127445. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-77952241896&doi=10.1016%2Fj.ijcard.2009.09.543&partnerID=40&md5=8ab0272b19df198c0839289f4b7b377b (accessed on 1 January 2023). [CrossRef]
- Eller, N.H. Total power and high frequency components of heart rate variability and risk factors for atherosclerosis. Auton. Neurosci. Basic Clin. 2007, 131, 123–130. [Google Scholar] [CrossRef]
- Meszaros, M.; Bikov, A. Obstructive Sleep Apnoea and Lipid Metabolism: The Summary of Evidence and Future Perspectives in the Pathophysiology of OSA-Associated Dyslipidaemia. Biomedicines 2022, 10, 2754. [Google Scholar] [CrossRef]
- Campo, A.; Frühbeck, G.; Zulueta, J.J.; Iriarte, J.; Seijo, L.M.; Alcaide, A.B.; Galdiz, J.B.; Salvador, J. Hyperleptinaemia, respiratory drive and hypercapnic response in obese patients. Eur. Respir. J. 2007, 30, 223–231. Available online: http://erj.ersjournals.com/cgi/doi/10.1183/09031936.00115006 (accessed on 1 January 2023). [CrossRef]



| Database | Search Equation |
|---|---|
| PubMed | ((“heart rate variability” [Title/Abstract] OR “autonomic control” [Title/Abstract] OR “HRV” [Title/Abstract] OR “cardiac autonomic control” [Title/Abstract] OR “cardiac autonomic function” [Title/Abstract] OR “cardiac autonomic modulation” [Title/Abstract]) AND (“metabolic syndrome” [Title/Abstract])) |
| Web of Science | (“heart rate variability” OR “autonomic control” OR “HRV” OR “cardiac autonomic control” OR “cardiac autonomic function” OR “cardiac autonomic modulation”) AND (“metabolic syndrome”) |
| Scopus | (TITLE-ABS-KEY (“metabolic syndrome”) AND TITLE-ABS-KEY (“heart rate variability”) O TITLE-ABS-KEY (“autonomic control”) OR TITLE-ABS-KEY (“HRV”) OR TITLE-ABS-KEY (“cardiac autonomic control”) OR TITLE-ABS-KEY (“cardiac autonomic function”) OR TITLE-ABS-KEY (“cardiac autonomic modulation”)) |
| Reference | Methodological Evaluation (%) | n | Age (Years) | Sex (F/M) | MS Definition | Analyzed Variables | ||
|---|---|---|---|---|---|---|---|---|
| Time | Frequency | Non-Linear | ||||||
| Aso et al., 2006 [31] | 94% | 104 | 43–70 | Both (48/52) | NCEP ATP III | No | HF, LF, LF/HF | No |
| Stein et al., 2007 [21] | 75% | 899 | 67–76 | Both (56/44) | NCEP ATP III | SDNN, SDANN, SDNNi, rMSSD, pNN50 | HF, LF, TP, VLF, ULF | DFA-1 |
| Gehi et al., 2009 [22] | 88% | 288 | 50–57 | Men (0/100) | AHA y NHLBI | No | HF, LF, TP, VLF, ULF | No |
| Assoumou et al., 2010 [26] | 94% | 1010 | 64–66 | Both (60/40) | NCEP-ATP III | No | TP, HF, LF, VLF, ULF, LF/HF | No |
| Rasic-Milutinovic et al., 2010 [27] | 88% | 47 | 50–60 | Both (60/40) | NCEP ATP III | SDNN, rMSSD | HF, LF, LF/HF, TP, VLF | No |
| Poliwczak et al., 2013 [24] | 94% | 80 | 50–55 | Men (0/100) | IDF | SDNN, SDANN, SDNNi, rMSSD, pNN50 | HF, LF, LF/HF, TP, VLF, ULF | No |
| Jarczok et al., 2013 [28] | 88% | 2441 | 18–67 | Both (24/76) | IC | SDNN, rMSSD | HF, LF, LF/HF | No |
| Wulsin et al., 2016 [23] | 94% | 1143 | 40–57 | Both (57/43) | IC | SDNN, rMSSD | No | No |
| Yoo et al., 2016 [32] | 94% | 1200 | 50–60 | Both (60/40) | NCEP ATP III | SDNN, SDANN, rMSSD | No | No |
| Balcioglu et al., 2016 [29] | 94% | 150 | 48–74 | Both (65/35) | NCEP ATP III | SDNN, SDANN, SDNNi, rMSSD, pNN50 | No | No |
| Ma et al., 2017 [11] | 94% | 401 | 46–64 | Both (41/59) | NCEP ATP III | SDNN, rMSSD, pNN50, pNN20 | HF, LF, LF/HF, TP, VLF | SD1, SD2, SD1/SD2, Multiscale entropy |
| Slušnienė et al., 2019 [30] | 81% | 106 | 50–55 | Both (49/51) | NCEP ATP III | SDNN, SDANN, SDNNi, rMSSD, pNN50 | HF, LF, LF/HF, VLF | No |
| MacIorowska et al., 2020 [25] | 69% | 118 | 34–58 | Both (32/86) | IDF | SDNN, rMSSD, pNN50 | HF, LF, LF/HF, TP | No |
| Reference | SDNN | SDANN | SDNNi | rMSSD | pNN50 | pNN20 | R-R |
|---|---|---|---|---|---|---|---|
| Stein et al., 2007 [21] | ↓ | ↓ | = | ||||
| Rasic-Milutinovic et al., 2010 [27] | = | = | |||||
| Poliwczak et al., 2013 [24] M | ↓ | ↓ | ↓ | ↓ | ↓ | ||
| Wulsin et al., 2016 [23] | ↓ | ↓ | |||||
| Yoo et al., 2016 [32] | ↓ | ↓ | ↓ | ||||
| Balcioglu et al., 2016 [29] | ↓ | ↓ | ↓ | ↓ | ↓ | = | |
| Ma et al., 2017 [11] | ↓ | = | = | ↓ | = | ||
| Slušnienė et al., 2019 [30] | = | = | ↓ | = | = | = | |
| MacIorowska et al., 2020 [25] | ↓ | ↓ | ↓ |
| Reference | HF | LF | LF/HF | TP | VLF | ULF |
|---|---|---|---|---|---|---|
| Aso et al., 2006 [31] | = | ↑ | ↑ | |||
| Stein et al., 2007 [21] | ↓ | = | ↓ | |||
| Gehi et al., 2009 [22] | ↓ | ↓ | ↓ | ↓ | ↓ | |
| Assoumou et al., 2010 [26] | = | ↓ | ↓ | ↓ | ↓ | ↓ |
| Rasic-Milutinovic et al., 2010 [27] | ↓ | ↓ | ↓ | ↓ | ↓ | |
| Poliwczak et al., 2013 [24] M | ↓ | ↓ | = | ↓ | ↓ | ↓ |
| Ma et al., 2017 [11] | ↓ | ↓ | = | = | ↓ | |
| Slušnienė et al., 2019 [30] | = | ↓ | ↓ | = | ||
| Maciorowska et al., 2020 [25] | = | = | ↑ | = |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Guzmán, J.E.; Mollà-Casanova, S.; Arias-Mutis, Ó.J.; Bizy, A.; Calvo, C.; Alberola, A.; Chorro, F.J.; Zarzoso, M. Differences in Long-Term Heart Rate Variability between Subjects with and without Metabolic Syndrome: A Systematic Review and Meta-Analysis. J. Cardiovasc. Dev. Dis. 2023, 10, 203. https://doi.org/10.3390/jcdd10050203
Ortiz-Guzmán JE, Mollà-Casanova S, Arias-Mutis ÓJ, Bizy A, Calvo C, Alberola A, Chorro FJ, Zarzoso M. Differences in Long-Term Heart Rate Variability between Subjects with and without Metabolic Syndrome: A Systematic Review and Meta-Analysis. Journal of Cardiovascular Development and Disease. 2023; 10(5):203. https://doi.org/10.3390/jcdd10050203
Chicago/Turabian StyleOrtiz-Guzmán, Johan E., Sara Mollà-Casanova, Óscar J. Arias-Mutis, Alexandra Bizy, Conrado Calvo, Antonio Alberola, Francisco J. Chorro, and Manuel Zarzoso. 2023. "Differences in Long-Term Heart Rate Variability between Subjects with and without Metabolic Syndrome: A Systematic Review and Meta-Analysis" Journal of Cardiovascular Development and Disease 10, no. 5: 203. https://doi.org/10.3390/jcdd10050203
APA StyleOrtiz-Guzmán, J. E., Mollà-Casanova, S., Arias-Mutis, Ó. J., Bizy, A., Calvo, C., Alberola, A., Chorro, F. J., & Zarzoso, M. (2023). Differences in Long-Term Heart Rate Variability between Subjects with and without Metabolic Syndrome: A Systematic Review and Meta-Analysis. Journal of Cardiovascular Development and Disease, 10(5), 203. https://doi.org/10.3390/jcdd10050203








