Assessment and Therapeutic Modulation of Heart Rate Variability: Potential Implications in Patients with COVID-19
Abstract
:1. Introduction
2. Search Strategy
3. Heart Rate Variability
3.1. Physiological Principles
3.2. Clinical Assessment
3.2.1. Time Domain Analysis
- SDNN (milliseconds): Standard deviation of the NN intervals. As the name implies, this is the square root of variance. Since the variance increases with longer recording times, this value is highly dependent of the duration of measurement, which means that SDNN can only be used to compare HRV if both recordings lasted the same amount of time. The most commonly used recording settings are 5 min resting ECG and 24 h Holter ECG.
- SDANN (milliseconds): The recording is divided in 5 min segments, and the average of NN intervals for each segment is calculated. SDANN is the standard deviation of those averages.
- SDNN index (milliseconds): The recording is divided in 5 min segments, and for each segment, the standard deviation of all NN intervals is calculated. SDNN index is the mean of those standard deviations.
- RMSSD (milliseconds): Root mean square of successive differences. Here, each NN interval is subtracted from its neighbor, and the result is squared to yield only positive values. A mean of those values is calculated, and the RMSSD is the square root of this mean.
- NN50 (no units, natural number): the number of subsequent NN pairs whose difference is greater than 50 milliseconds.
- pNN50 (%): The value of NN50 divided by the total number of NN intervals.
- Geometric methods: The series of NN intervals are plotted as a geometric pattern, and the variability is analyzed using mathematical formulae based on the graphical or geometric traits.
3.2.2. Frequency Domain Analysis
3.3. HRV as Biomarker of Cardiovascular Health
3.4. Baroreflex Sensitivity
4. COVID-19 and Cardiac Damage
5. Neurocardiac Autonomic Dysfunction in COVID-19
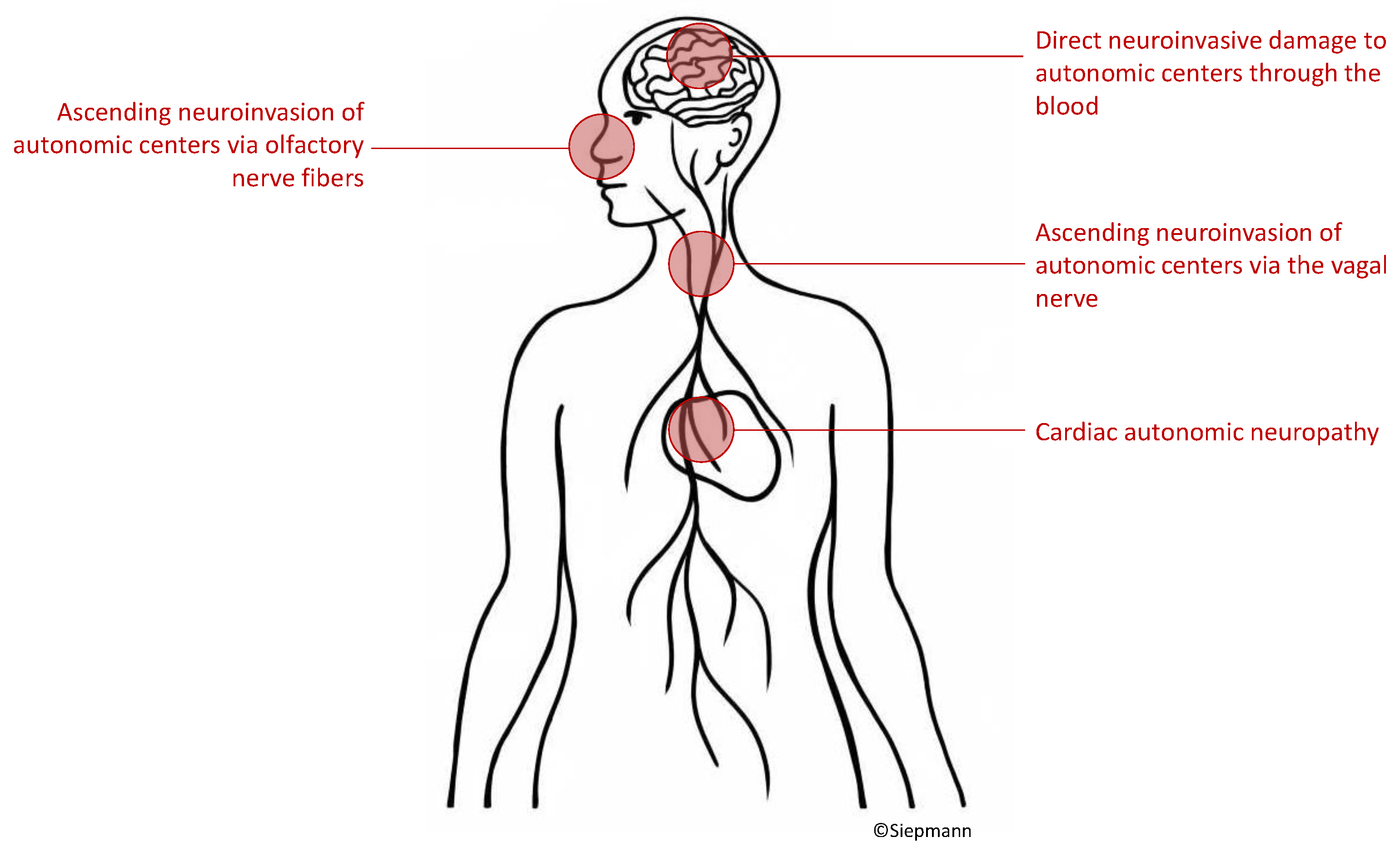
5.1. Possible Pathophysiological Mechanisms of Autonomic Neurocardiac Dysfunction in Patients with COVID-19
5.1.1. Virus Ascent via Cranial Nerves
5.1.2. Hematogenous Invasion of the Central Nervous System
5.1.3. Cardiac Autonomic Neuropathy
6. Crosstalk of the Immune System and the Autonomic Nervous System in the Context of COVID-19
7. Cardiovascular Dysautonomia beyond the Acute Phases of COVID-19
8. HRV Biofeedback and Its Potential to Modify Cardiovascular Outcomes in Patients with COVID-19
8.1. Background
8.2. HRV Biofeedback in Cardiovascular and Cerebrovascular Disease
8.3. Possible Implictaions of HRV Biofeedback in Patients with COVID-19
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-COVID-19—11-march-2020 (accessed on 5 May 2021).
- Rahman, A.; Sathi, N.J. Risk factors of the severity of COVID-19: A meta-analysis. Int. J. Clin. Pract. 2020, 75, e13916. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Kassiri, Z.; Jiang, C.; Liu, P.P.; Poutanen, S.M.; Penninger, J.M.; Butany, J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009, 39, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudowenz, O.; Klemm, P.; Lange, U.; Rolf, A.; Schultheiss, H.-P.; Hamm, C.; Müller-Ladner, U.; Wegner, F. Case report of severe PCR-confirmed COVID-19 myocarditis in a European patient manifesting in mid January 2020. Eur. Heart J. Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Jacobs, W.; Lammens, M.; Kerckhofs, A.; Voets, E.; Van San, E.; Van Coillie, S.; Peleman, C.; Mergeay, M.; Sirimsi, S.; Matheeussen, V.; et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): Autopsy reveals a ferroptosis signature. ESC Heart Fail. 2020, 7, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhang, Z.; Luo, L.; Wu, W.; Jia, T.; Lu, L.; Liu, W.V.; Qin, Y.; Hu, F.; Bs, X.D.; et al. Cardiac T1 and T2 Mapping Showed Myocardial Involvement in Recovered COVID-19 Patients Initially Considered Devoid of Cardiac Damage. J. Magn. Reson. Imaging 2021, 54, 421–428. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Wang, F.; Liu, B.; Li, H.; Tang, G.; Chang, Z.; Liu, A.; Fu, C.; Lv, Y.; et al. Cardiac damage in patients with the severe type of coronavirus disease 2019 (COVID-19). BMC Cardiovasc. Disord. 2020, 20, 479. [Google Scholar] [CrossRef]
- Magadum, A.; Kishore, R. Cardiovascular Manifestations of COVID-19 Infection. Cells 2020, 9, 2508. [Google Scholar] [CrossRef]
- Unudurthi, S.D.; Luthra, P.; Bose, R.J.; McCarthy, J.R.; Kontaridis, M.I. Cardiac inflammation in COVID-19: Lessons from heart failure. Life Sci. 2020, 260, 118482. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Gheibi Hayat, S.M.; Taghizadeh, H.; Akbari, A.; Inabadi, M.; Savardashtaki, A.; Johnston, T.P.; Sahebkar, A. COVID-19 and cardiac injury: Clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev. Anti-Infect. Ther. 2021, 19, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Song, L.; Wang, X.; Xu, Z.; Wang, S.; Wang, J.; Xu, H.; Zheng, Y.; Wang, Y. Cardiac injury prediction and lymphocyte immunity and inflammation analysis in hospitalized patients with coronavirus disease 2019 (COVID-19). Int. J. Cardiol. 2020, 326, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Crackower, M.A.; Sarao, R.; Oudit, G.Y.; Yagil, C.; Kozieradzki, I.; Scanga, S.E.; Oliveira-Dos-Santos, A.J.; da Costa, J.; Zhang, L.; Pei, Y.; et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002, 417, 822–828. [Google Scholar] [CrossRef]
- Rangon, C.-M.; Krantic, S.; Moyse, E.; Fougère, B. The Vagal Autonomic Pathway of COVID-19 at the Crossroad of Alzheimer’s Disease and Aging: A Review of Knowledge. J. Alzheimer’s Dis. Rep. 2020, 4, 537–551. [Google Scholar] [CrossRef]
- Chigr, F.; Merzouki, M.; Najimi, M. Autonomic Brain Centers and Pathophysiology of COVID-19. ACS Chem. Neurosci. 2020, 11, 1520–1522. [Google Scholar] [CrossRef]
- Hasty, F.; García, G.; Dávila, C.H.; Wittels, S.H.; Hendricks, S.; Chong, S. Heart Rate Variability as a Possible Predictive Marker for Acute Inflammatory Response in COVID-19 Patients. Mil. Med. 2020, 186, e34–e38. [Google Scholar] [CrossRef]
- Kaliyaperumal, D.; Rk, K.; Alagesan, M.; Ramalingam, S. Characterization of cardiac autonomic function in COVID-19 using heart rate variability: A hospital based preliminary observational study. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 247–253. [Google Scholar] [CrossRef]
- Aragón-Benedí, C.; Oliver-Forniés, P.; Galluccio, F.; Altinpulluk, E.Y.; Ergonenc, T.; Allam, A.E.S.; Salazar, C.; Fajardo-Pérez, M. Is the heart rate variability monitoring using the analgesia nociception index a predictor of illness severity and mortality in critically ill patients with COVID-19? A pilot study. PLoS ONE 2021, 16, e0249128. [Google Scholar] [CrossRef]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021, 31, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hinduja, A.; Moutairou, A.; Calvet, J.-H. Sudomotor dysfunction in patients recovered from COVID-19. Neurophysiol. Clin. 2021, 51, 193–196. [Google Scholar] [CrossRef]
- Buchhorn, R.; Willaschek, C.; Baumann, C. SARS-CoV-2 Infektionen und das autonome Nervensystem [SARS-CoV-2 infections and the autonomic nervous system]. Mon. Kinderheilkd. 2021, 169, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Goodman, B.P.; Khoury, J.A.; Blair, J.E.; Grill, M.F. COVID-19 Dysautonomia. Front. Neurol. 2021, 12, 624968. [Google Scholar] [CrossRef]
- Ghosh, R.; Roy, D.; Sengupta, S.; Benito-León, J. Autonomic dysfunction heralding acute motor axonal neuropathy in COVID-19. J. Neurovirol. 2020, 26, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Blitshteyn, S.; Whitelaw, S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: A case series of 20 patients. Immunol. Res. 2021, 69, 205–211. [Google Scholar] [CrossRef]
- Leitzke, M.; Stefanovic, D.; Meyer, J.-J.; Schimpf, S.; Schönknecht, P. Autonomic balance determines the severity of COVID-19 courses. Bioelectron. Med. 2020, 6, 22. [Google Scholar] [CrossRef]
- Del Rio, R.; Marcus, N.J.; Inestrosa, N.C. Potential Role of Autonomic Dysfunction in COVID-19 Morbidity and Mortality. Front. Physiol. 2020, 11, 561749. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Boutjdir, M.; Capecchi, P.L. COVID-19, Arrhythmic Risk, and Inflammation. Circulation 2020, 142, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T., Jr.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef]
- Malik, M.; Farrell, T.; Cripps, T.; Camm, A.J. Heart rate variability in relation to prognosis after myocardial infarction: Selection of optimal processing techniques. Eur. Heart J. 1989, 10, 1060–1074. [Google Scholar] [CrossRef]
- Bigger, J.T., Jr.; Fleiss, J.L.; Steinman, R.C.; Rolnitzky, L.M.; Kleiger, R.E.; Rottman, J.N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992, 85, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Jose, A.D.; Taylor, R.R. Autonomic blockade by propranolol and atropine to study intrinsic myocardial function in man. J. Clin. Investig. 1969, 48, 2019–2031. [Google Scholar] [CrossRef]
- Takahashi, N.; Barber, M.J.; Zipes, D.P. Efferent vagal innervation of canine ventricle. Am. J. Physiol. Circ. Physiol. 1985, 248, H89–H97. [Google Scholar] [CrossRef]
- Levy, M.N.; Schwartz, P.J. Vagal Control of the Heart: Experimental Basis & Clinical Implications, 1st ed.; Futura Publishing: Armonk, NY, USA, 1994; p. 613. [Google Scholar]
- Bailey, J.C.; Watanabe, A.M.; Besch, H.R., Jr.; Lathrop, D.A. Acetylcholine antagonism of the electrophysiological effects of isoproterenol on canine cardiac Purkinje fibers. Circ. Res. 1979, 44, 378–383. [Google Scholar] [CrossRef]
- Kolman, B.S.; Verrier, R.L.; Lown, B. Effect of vagus nerve stimulation upon excitability of the canine ventricle: Role of sympathetic-parasympathetic interactions. Am. J. Cardiol. 1976, 37, 1041–1045. [Google Scholar] [CrossRef]
- Ziemssen, T.; Siepmann, T. The Investigation of the Cardiovascular and Sudomotor Autonomic Nervous System—A Review. Front. Neurol. 2019, 10, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seely, A.J.; Macklem, P.T. Complex systems and the technology of variability analysis. Crit. Care 2004, 8, R367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassi, R.; Cerutti, S.; Lombardi, F.; Malik, M.; Huikuri, H.V.; Peng, C.-K.; Schmidt, G.; Yamamoto, Y.; Gorenek, B.; Lip, G.Y.; et al. Advances in heart rate variability signal analysis: Joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1341–1353. [Google Scholar] [CrossRef]
- Kristal-Boneh, E.; Raifel, M.; Froom, P.; Ribak, J. Heart rate variability in health and disease. Scand. J. Work Environ. Health 1995, 21, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Stuckey, M.I.; Tulppo, M.P.; Kiviniemi, A.M.; Petrella, R.J. Heart rate variability and the metabolic syndrome: A systematic review of the literature. Diabetes/Metab. Res. Rev. 2014, 30, 784–793. [Google Scholar] [CrossRef]
- Low, P.A. Testing the Autonomic Nervous System. Semin. Neurol. 2003, 23, 407–422. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Pinna, G.D.; Raczak, G. Baroreflex Sensitivity: Measurement and Clinical Implications. Ann. Noninvasive Electrocardiol. 2008, 13, 191–207. [Google Scholar] [CrossRef]
- Murthy, S.; Gomersall, C.D.; Fowler, R.A. Care for Critically Ill Patients with COVID-19. JAMA 2020, 323, 1499–1500. [Google Scholar] [CrossRef] [Green Version]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, T.; Sedghi, A.; Barlinn, J.; de With, K.; Mirow, L.; Wolz, M.; Gruenewald, T.; Helbig, S.; Schroettner, P.; Winzer, S.; et al. Association of history of cerebrovascular disease with severity of COVID-19. J. Neurol. 2020, 268, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A Novel Angiotensin-Converting Enzyme–Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9. Circ. Res. 2000, 87, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Zhong, J.C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef]
- Prabhu, S.D. Cytokine-Induced Modulation of Cardiac Function. Circ. Res. 2004, 95, 1140–1153. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T.; Büller, H.R. Bidirectional relation between inflammation and coagulation. Circulation 2004, 109, 2698–2704. [Google Scholar] [CrossRef] [Green Version]
- Nunan, D.; Sandercock, G.R.H.; Brodie, D.A. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin. Electrophysiol. 2010, 33, 1407–1417. [Google Scholar] [CrossRef]
- Pincherle, A.; Jöhr, J.; Pancini, L.; Leocani, L.; Vecchia, L.D.; Ryvlin, P.; Schiff, N.D.; Diserens, K. Intensive Care Admission and Early Neuro-Rehabilitation. Lessons for COVID-19? Front. Neurol. 2020, 11, 880. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2020, 24, 168–175. [Google Scholar] [CrossRef]
- Yachou, Y.; El Idrissi, A.; Belapasov, V.; Benali, S.A. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: Understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 2020, 41, 2657–2669. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Yang, N.; Han, D.; Mi, X.; Li, Y.; Liu, K.; Vuylsteke, A.; Xiang, H.; Guo, X. Neurological manifestations of patients with COVID-19: Potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front. Med. 2020, 14, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Abboud, H.; Abboud, F.Z.; Kharbouch, H.; Arkha, Y.; El Abbadi, N.; El Ouahabi, A. COVID-19 and SARS-Cov-2 Infection: Pathophysiology and Clinical Effects on the Nervous System. World Neurosurg. 2020, 140, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2020, 21, e63–e67. [Google Scholar] [CrossRef]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. Lancet Neurol. 2020, 19, 767–783. [Google Scholar] [CrossRef]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.-E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef]
- Chen, M.; Shen, W.; Rowan, N.R.; Kulaga, H.; Hillel, A.; Ramanathan, M.; Lane, A.P. Elevated ACE-2 expression in the olfactory neuroepithelium: Implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020, 56, 2001948. [Google Scholar] [CrossRef]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van Den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.-J.; Fletcher, R.B.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef]
- South, A.M.; Diz, D.I.; Chappell, M.C.; Krishnamurthy, S.; Lockey, R.F.; Kolliputi, N.; Habashi, N.M.; Camporota, L.; Gatto, L.A.; Nieman, G.; et al. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Chen, Y.; Zhang, W.; Chen, A.F.; Lin, S.; Morris, M. RNA interference shows interactions between mouse brainstem angiotensin AT1receptors and angiotensin-converting enzyme 2. Exp. Physiol. 2008, 93, 676–684. [Google Scholar] [CrossRef]
- Mendelsohn, F.A.; Allen, A.M.; Chai, S.-Y.; McKinley, M.J.; Oldfield, B.J.; Paxinos, G. The brain angiotensin system Insights from mapping its components. Trends Endocrinol. Metab. 1990, 1, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.-R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gourine, A.V.; Machhada, A.; Trapp, S.; Spyer, K.M. Cardiac vagal preganglionic neurones: An update. Auton. Neurosci. 2016, 199, 24–28. [Google Scholar] [CrossRef]
- Chakraborty, T.; Kramer, C.L.; Wijdicks, E.F.M.; Rabinstein, A.A. Dysautonomia in guillain-barré syndrome: Prevalence, clinical spectrum, and outcomes. Neurocrit. Care 2019, 32, 113–120. [Google Scholar] [CrossRef]
- Young, R.R.; Asbury, A.K.; Corbett, J.L.; Adams, R.D. Pure pan-dysautonomia with recovery. Brain 1975, 98, 613–636. [Google Scholar] [CrossRef]
- Yamamoto, V.; Bolanos, J.F.; Fiallos, J.; Strand, S.E.; Morris, K.; Shahrokhinia, S.; Cushing, T.R.; Hopp, L.; Tiwari, A.; Hariri, R.; et al. COVID-19: Review of a 21st Century Pandemic from Etiology to Neuro-psychiatric Implications. J. Alzheimer’s Dis. 2020, 77, 459–504. [Google Scholar] [CrossRef]
- Schoene, D.; Schnekenberg, L.G.; Pallesen, L.-P.; Barlinn, J.; Puetz, V.; Barlinn, K.; Siepmann, T. Pathophysiology of Cardiac Injury in COVID-19 Patients with Acute Ischaemic Stroke: What Do We Know So Far?—A Review of the Current Literature. Life 2022, 12, 75. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef] [Green Version]
- UR, A.; Verma, K. Happy Hypoxemia in COVID-19—A Neural Hypothesis. ACS Chem. Neurosci. 2020, 11, 1865–1867. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, J.J.; Arora, R.; Buckley, U.; Shivkumar, K. Autonomic Nervous System Dysfunction: JACC Focus Seminar. J. Am. Coll. Cardiol. 2019, 73, 1189–1206. [Google Scholar] [CrossRef] [PubMed]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotan, A.; David, P.; Arnheim, D.; Shoenfeld, Y. The autonomic aspects of the post-COVID19 syndrome. Autoimmun. Rev. 2022, 21, 103071. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Carneiro, D.R.; Rocha, I.; Habek, M.; Helbok, R.; Sellner, J.; Struhal, W.; Wenning, G.; Fanciulli, A. Clinical presentation and management strategies of cardiovascular autonomic dysfunction following a COVID-19 infection—A systematic review. Eur. J. Neurol. 2023, 30, 1528–1539. [Google Scholar] [CrossRef]
- Hanson, S.W.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; Borzakova, S.; et al. Estimated global proportions of individ-uals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar]
- Hilz, M.J. Cardiovascular autonomic dysfunction: A cause of acute COVID-19 complications and persistent post-COVID-19 complaints? Eur. J. Neurol. 2023, 30, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Norcliffe-Kaufmann, L.; Palma, J.-A.; Martinez, J.; Camargo, C.; Kaufmann, H. Fear conditioning as a pathogenic mechanism in the postural tachycardia syndrome. Brain 2022, 145, 3763–3769. [Google Scholar] [CrossRef]
- Koczulla, A.R.; Ankermann, T.; Behrends, U.; Berlit, P.; Böing, S.; Brinkmann, F.; Franke, C.; Glöckl, R.; Gogoll, C.; Hummel, T.; et al. S1-Leitlinie Post-COVID/Long-COVID [S1 Guideline Post-COVID/Long-COVID]. Pneumologie 2021, 75, 869–900. [Google Scholar] [CrossRef]
- Mehandru, S.; Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022, 23, 194–202. [Google Scholar] [CrossRef]
- Perrin, R.; Riste, L.; Hann, M.; Walther, A.; Mukherjee, A.; Heald, A. Into the looking glass: Post-viral syndrome post COVID-19. Med. Hypotheses 2020, 144, 110055. [Google Scholar] [CrossRef]
- Andrade, B.S.; Siqueira, S.; Soares, W.R.d.A.; Rangel, F.D.S.; Santos, N.O.; Freitas, A.d.S.; da Silveira, P.R.; Tiwari, S.; Alzahrani, K.J.; Góes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Fürst, J.; Schulze-Rothe, S.; Wallukat, A.; Hönicke, A.-S.; Müller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 2021, 4, 100100. [Google Scholar] [CrossRef]
- Jammoul, M.; Naddour, J.; Madi, A.; Reslan, M.A.; Hatoum, F.; Zeineddine, J.; Abou-Kheir, W.; Lawand, N. Investigating the possible mechanisms of autonomic dysfunction post-COVID-19. Auton. Neurosci. 2022, 245, 103071. [Google Scholar] [CrossRef]
- Barizien, N.; Le Guen, M.; Russel, S.; Touche, P.; Huang, F.; Vallée, A. Clinical characterization of dysautonomia in long COVID-19 patients. Sci. Rep. 2021, 11, 14042. [Google Scholar] [CrossRef] [PubMed]
- Marques, K.C.; Silva, C.C.; Trindade, S.D.S.; Santos, M.C.D.S.; Rocha, R.S.B.; Vasconcelos, P.F.D.C.; Quaresma, J.A.S.; Falcão, L.F.M. Reduction of Cardiac Autonomic Modulation and Increased Sympathetic Activity by Heart Rate Variability in Patients With Long COVID. Front. Cardiovasc. Med. 2022, 9, 862001. [Google Scholar] [CrossRef] [PubMed]
- Burlacu, A.; Brinza, C.; Popa, I.V.; Covic, A.; Floria, M. Influencing Cardiovascular Outcomes through Heart Rate Variability Modulation: A Systematic Review. Diagnostics 2021, 11, 2198. [Google Scholar] [CrossRef]
- Siepmann, T.; Ohle, P.; Sedghi, A.; Simon, E.; Arndt, M.; Pallesen, L.-P.; Ritschel, G.; Barlinn, J.; Reichmann, H.; Puetz, V.; et al. Randomized Sham-Controlled Pilot Study of Neurocardiac Function in Patients With Acute Ischaemic Stroke Undergoing Heart Rate Variability Biofeedback. Front. Neurol. 2021, 12, 669843. [Google Scholar] [CrossRef]
- Del Pozo, J.M.; Gevirtz, R.N.; Scher, B.; Guarneri, E. Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. Am. Heart J. 2004, 147, 545. [Google Scholar] [CrossRef]
- Pinter, A.; Jr, S.S.; Horvath, T.; Penzlin, A.I.; Barlinn, K.; Siepmann, M.; Siepmann, T. Cardiac dysautonomia in depression—Heart rate variability biofeedback as a potential add-on therapy. Neuropsychiatr. Dis. Treat. 2019, 15, 1287–1310. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.-P.; Xiang, Q.; Fu, X.; Wang, S.-Z.; Wang, S.; Chen, S.; Shao, L.; Zhao, Y.; Wang, T.H.; Alayan, N.; et al. Heart Rate Variability Biofeedback Decreases Blood Pressure in Prehypertensive Subjects by Improving Autonomic Function and Baroreflex. J. Altern. Complement. Med. 2012, 18, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Limmer, A.; Laser, M.; Schütz, A. Mobile Heart Rate Variability Biofeedback as a Complementary Intervention After Myocardial Infarction: A Randomized Controlled Study. Int. J. Behav. Med. 2021, 29, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gevirtz, R.N.; Brown, M.; Spira, J.; Guarneri, E.; Stoletniy, L. The Effect of Biofeedback on Function in Patients with Heart Failure. Appl. Psychophysiol. Biofeedback 2009, 34, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-L.; Lee, J.-T.; Li, C.-R.; Davis, A.H.T.; Yang, C.-C.; Chen, Y.-J. Effects of Heart Rate Variability Biofeedback in Patients with Acute Ischemic Stroke: A Randomized Controlled Trial. Biol. Res. Nurs. 2020, 22, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.-M.; Lin, P.-Y.; Fan, S.-Y. The Effects of Heart Rate Variability (HRV) Biofeedback on HRV Reactivity and Recovery During and After Anger Recall Task for Patients with Coronary Artery Disease. Appl. Psychophysiol. Biofeedback 2022, 47, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-C.; Lin, I.-M.; Fan, S.-Y.; Chien, C.-L.; Lin, T.-H. One-Year Cardiovascular Prognosis of the Randomized, Controlled, Short-Term Heart Rate Variability Biofeedback Among Patients with Coronary Artery Disease. Int. J. Behav. Med. 2018, 25, 271–282. [Google Scholar] [CrossRef]
- Peláez-Hernández, V.; Luna-Rodríguez, G.L.; Orea-Tejeda, A.; Mora-Gallegos, J.; Keirns-Davis, C.; González-Islas, D. Heart rate variability disturbances and biofeedback treatment in COVID-19 survivors. e-J. Cardiol. Pract. 2021, 21. [Google Scholar]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long Covid—Mechanisms, Risk Factors, and Management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Corrado, J.; Halpin, S.; Preston, N.; Whiteside, D.; Tarrant, R.; Davison, J.; Simms, A.D.; O’Connor, R.J.; Casson, A.; Sivan, M. HEART rate variability biofeedback for long COVID symptoms (HEARTLOC): Protocol for a feasibility study. BMJ Open 2022, 12, e066044. [Google Scholar] [CrossRef]
- Available online: https://beta.clinicaltrials.gov/study/NCT05793736 (accessed on 21 May 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnekenberg, L.; Sedghi, A.; Schoene, D.; Pallesen, L.-P.; Barlinn, J.; Woitek, F.; Linke, A.; Puetz, V.; Barlinn, K.; Mangner, N.; et al. Assessment and Therapeutic Modulation of Heart Rate Variability: Potential Implications in Patients with COVID-19. J. Cardiovasc. Dev. Dis. 2023, 10, 297. https://doi.org/10.3390/jcdd10070297
Schnekenberg L, Sedghi A, Schoene D, Pallesen L-P, Barlinn J, Woitek F, Linke A, Puetz V, Barlinn K, Mangner N, et al. Assessment and Therapeutic Modulation of Heart Rate Variability: Potential Implications in Patients with COVID-19. Journal of Cardiovascular Development and Disease. 2023; 10(7):297. https://doi.org/10.3390/jcdd10070297
Chicago/Turabian StyleSchnekenberg, Luiz, Annahita Sedghi, Daniela Schoene, Lars-Peder Pallesen, Jessica Barlinn, Felix Woitek, Axel Linke, Volker Puetz, Kristian Barlinn, Norman Mangner, and et al. 2023. "Assessment and Therapeutic Modulation of Heart Rate Variability: Potential Implications in Patients with COVID-19" Journal of Cardiovascular Development and Disease 10, no. 7: 297. https://doi.org/10.3390/jcdd10070297
APA StyleSchnekenberg, L., Sedghi, A., Schoene, D., Pallesen, L.-P., Barlinn, J., Woitek, F., Linke, A., Puetz, V., Barlinn, K., Mangner, N., & Siepmann, T. (2023). Assessment and Therapeutic Modulation of Heart Rate Variability: Potential Implications in Patients with COVID-19. Journal of Cardiovascular Development and Disease, 10(7), 297. https://doi.org/10.3390/jcdd10070297








