Abstract
Cardiovascular disease morbidity/mortality are increasing due to an aging population and the rising prevalence of diabetes and obesity. Therefore, innovative cardioprotective measures are required to reduce cardiovascular disease morbidity/mortality. The role of necroptosis in myocardial ischemia–reperfusion injury (MI–RI) is beyond doubt, but the molecular mechanisms of necroptosis remain incompletely elucidated. Growing evidence suggests that MI–RI frequently results from the superposition of multiple pathways, with autophagy, ferroptosis, and CypD-mediated mitochondrial damage, and necroptosis all contributing to MI–RI. Receptor-interacting protein kinases (RIPK1 and RIPK3) as well as mixed lineage kinase domain-like pseudokinase (MLKL) activation is accompanied by the activation of other signaling pathways, such as Ca2+/calmodulin-dependent protein kinase II (CaMKII), NF-κB, and JNK-Bnip3. These pathways participate in the pathological process of MI–RI. Recent studies have shown that inhibitors of necroptosis can reduce myocardial inflammation, infarct size, and restore cardiac function. In this review, we will summarize the molecular mechanisms of necroptosis, the links between necroptosis and other pathways, and current breakthroughs in pharmaceutical therapies for necroptosis.
Keywords:
necroptosis; myocardial ischemia–reperfusion; RIPK1; RIPK3; MLKL; pharmaceutical therapies 1. Introduction
With the aging of the population, myocardial infarction has become the leading cause of mortality globally, posing a serious threat to human physical and mental health. Acute myocardial infarction (AMI) is a serious and common medical emergency that causes irreversible damage to the heart [1]. Most patients with AMI have a history of coronary artery disease, which leads to atherosclerotic plaque rupture, platelet aggregation, thrombus formation, and eventual blockage of the coronary lumen. The fundamental principles of therapy are the timely restoration of coronary blood flow and the decrease of myocardial infarct size. Reperfusion therapy, whether direct coronary intervention or thrombolytic therapy, has become a relatively standard and mature treatment. However, in the past few years, it has been observed that myocardial reperfusion may cause more myocardial cell death, and that reperfusion itself restores the supply of oxygen and nutrients to myocardial cells, saving the ischemic myocardium while causing additional myocardial injury, known as MI–RI. Studies in animal models have shown that reperfusion injury accounts for 25–50% of eventual myocardial infarction [2]. The series of pathophysiological events following reperfusion include the excessive release of intracellular Ca2+, impairment of the mitochondrial electron transport chain, massive production of reactive oxygen species (ROS), neutrophil recruitment, and inflammation, which can lead to irreversible loss of cardiomyocytes. This results in several fatal consequences, such as arrhythmias, fibrosis, and ventricular systolic and diastolic dysfunction [3]. Preventing or mitigating MI–RI has emerged as one of the most recent hot topics in cardiovascular disease research.
Many studies have investigated the pathogenesis of MI–RI, which is associated with oxidative stress, intracellular calcium overload, impaired energy metabolism [4,5], endoplasmic reticulum stress, apoptosis, necroptosis [6], autophagy [7,8], pyroptosis [9,10], and ferroptosis [11,12,13]. In addition, these mechanisms are interrelated and may contribute directly or indirectly to the exacerbation of cell death. Most previous studies on cardiomyocyte death have focused on apoptosis, whereas necrosis is considered a passive, unregulated process that occurs in response to severe pathological stress and is characterized by cell swelling, rupture of the cell membranes and organelles, and an efflux of cell contents [14]. Recent studies have questioned the role of apoptosis as a major contributor to cardiomyocyte loss during I/R injury [15]. Necroptosis, a form of cell death similar to the necrotic phenotype, has been identified in cardiac pathology. It has been reported that the rate of myocardial cell death by necroptosis is higher than that of apoptosis during the course of heart failure [16], and that I/R-induced myocardial cell death may account for up to 50% of the final myocardial infarct area [17]. Necroptosis is thought to play a role in cardiovascular disease by driving inflammation and inflammasome activation, as well as causing cell death [18]. It was found that ischemic preconditioning and post-treatment attenuates MI–RI-induced necroptosis [19], with inhibitors that have been proven to have cardioprotective effects. Here, we review the current information on the regulatory mechanisms of necroptosis and the involvement of its pharmacological agents in MI–RI.
2. Roles of Necroptosis during Myocardial Ischemia or I/R Injury
Necroptosis is a newly discovered form of regulated cellular necrosis with dual characteristics of necrosis and apoptosis. In terms of cell morphology, necroptosis is mainly characterized by cell swelling and cytoplasmic membrane rupture [20,21]. Both necroptosis and apoptosis are forms of cell death that may be regulated. Necroptosis is a type of caspase-independent programmed cell necrosis that is also negatively regulated by caspase-8 and is triggered by death receptors (such as tumor necrosis factor receptor 1, TNFR1). It also requires the kinase activities of RIPK1 and RIPK3. Necroptosis is often accompanied by the release of damage-associated molecular patterns (DAMP) and cytokines, which induce an immune inflammatory response and exacerbate tissue damage [22,23]. Necroptosis plays a key role in the extent of myocardial cell loss after MI–RI and the development of poor postischemic remodeling and cardiac dysfunction. Recently, there has been growing evidence that necroptosis leads to irreversible loss of cardiomyocytes, ventricular remodeling, and post-MI cardiac dysfunction [24,25]. Necroptosis can be induced by a variety of stimuli, including death ligands l, Toll-like receptors, and certain pathogens. Tumor necrosis factor (TNF)-induced necroptosis is the most common pathogen, mediated mainly by RIPK1 and RIPK3.
RIPK1 and RIPK3 interact through a common RIP homotypic interaction motif (RHIM) to form necrosome vesicles. This leads to phosphorylation and activation of RIPK1 and RIPK3 [26]. MLKL is phosphorylated by activated RIPK3 and oligomerizes as a result, migrating from the cytoplasm to the cell membrane to form open pores that alter ion permeability and lead to necroptosis [23] (Figure 1). RIPK1 is not the only molecule that activates RIPK3. The TIR structural domain junction containing the RHIM structural domain induces interferon-β (TRIF) and DNA-dependent activator of interferon regulatory factor (DAI) can also interact with RIPK3 to form non-classical necroptosis vesicles and participate in the development of necroptosis. The Toll-like receptor ligands mediate the activation of RIPK3 through TRIF, and some viruses not only bind directly to RIPK3 but also promote the binding of DAI to RIPK3, thus participating in necroptosis [27]. Meanwhile, some recent studies have shown that phosphoglycerate mutase 5 (PGAM5) and Ca MKII could also be involved in necroptosis as downstream molecules of RIPK3 [28,29]. In Fritsch’s study, it was shown that caspase-8 induces TNFα-mediated necroptosis, and caspase-8 is the molecular switch for apoptosis, necroptosis, and pyroptosis. Additionally, caspase-8 can cleave RIPK1, which inhibits the development of necroptosis [30]. The RIPK1/RIPK3/MLKL axis is the classical pathway of necroptosis. Activation of RIPK1 and RIPK3 is required during necroptosis, and both CaMKII and MLKL are thought to be executors of programmed cellular necrosis [31].
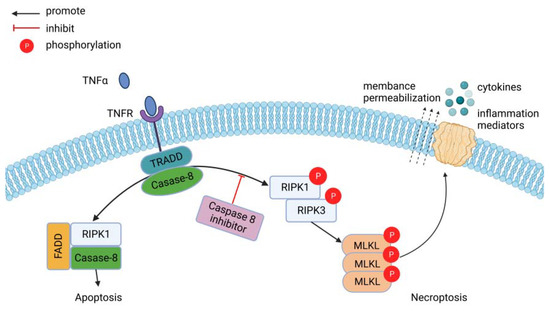
Figure 1.
Simplified version of necroptosis signal transduction events downstream of tumor necrosis factor-α/tumor necrosis factor receptor (TNFα/TNFR) interactions. Soluble TNFα binds TNFR and can trigger the formation of a pro-apoptotic (left, complex I) or pro-necroptotic (right, complex IIb) complex. In the absence of caspase-8, the pro-necroptotic complex IIb forms. After phosphorylation of RIPK1 and RIPK3, RIPK3 phosphorylates MLKL, which subsequently oligomerizes and is thought to be inserted into the cell membrane, forming pores. After cell membrane permeabilization, cytokines and intracellular contents are relapsed into the extracellular environment. Figure created through BioRender.com.
4-Hydroxy-2-Nonenal (4-HNE), one of the most toxic aldehydes, is a major secondary product of lipid peroxidation [32]. It has been found that both necrotrophic apoprotein and 4-HNE are upregulated in MIRI. Further studies revealed that 4-HNE perfusion enhanced necroptosis in a time- and concentration-dependent manner, and that 4-HNE bound directly to RIPK1, leading to a reduction in RIPK1 degradation mediated by K48 polyubiquitination of RIPK1, activation of the necroptotic pathway, and promotion of myocardial necroptosis [33]. RIPK3 is rapidly increased in hypoxia/reoxygenation (H/R)-treated cardiomyocytes and is associated with myocyte necroptosis [34]. It has been recently shown that, in the context of MI–RI, elevated RIPK3-mediated endoplasmic reticulum stress leads to increased mPTP opening through XO-mediated ROS production [35]. RIPK3 deficiency inhibits mitochondrial permeability transition pore (mPTP) opening, reduces mitochondrial oxidative stress, and attenuates H/R-mediated necroptosis in cardiomyocytes [36]. Similarly, activation of RIPK3 is thought to underlie the structural and histological abnormalities of tissues caused by post-infarct heart failure due to the promotion of inflammation and necroptosis [37]. Chen Li et al. showed that in vivo experiments in mice with Necrostatin-1 treatment (RIPK1 inhibitor), RIPK3 deletion, and cardiac p62 knockdown demonstrated that RIPK1-RIPK3-dependent myocardial necroptosis increased senescence-associated myocardial susceptibility to I/R injury vulnerability [38].
However, necroptosis is unlikely to be the cause of impaired cardiac function during early reperfusion, according to research by Csaba Horvath et al. It is believed that RIPK3 regulates early reperfusion injury through oxidative stress and mitochondrial activity-related effects rather than cell loss due to necroptosis [39].
3. Relationship between Necroptosis and Other Associated Mechanisms of MI–RI
In MI–RI, more than one type of regulatory necrosis often plays a role as a result of the superposition of multiple pathways. Although the different types of regulatory necrosis differ in morphological features, the core difference between them lies in the molecular pathways involved. For example, necroptosis is mediated by the RIPK1/RIPK3/MLKL pathway [40], and ferroptosis is closely associated with pathways responsible for iron or cysteine/glutathione homeostasis [2,41]. Meanwhile, CypD-mediated necrosis is dependent on pathways associated with mPTP opening [42]. Parkin can act as a negative regulator of CypD by directly ubiquitinating CypD, which in turn inhibits necrosis, reduces myocardial I/R injury, and improves cardiac function [43].
Research by Csaba Horváth et al. has demonstrated for the first time that early reperfusion inhibits the activation of autophagy and suggests that there may be a positive relationship between autophagy and the necroptotic protein RIPK3. Inhibition of RIPK3 during reperfusion significantly attenuated the loss of plasma membrane integrity but did not improve cardiac function [39]. Autophagy is a protective mechanism in chronic myocardial ischemia, and inhibition of autophagy leads to significant exacerbation of cardiac myocytes in ischemia–reperfusion injury. Conversely, enhancing autophagy has a potential protective effect against ischemia/reperfusion injury in cardiac cells [44]. Necroptosis is considered an upstream inhibitor of autophagy [45], while other studies have shown that overstimulated autophagy initiates the necroptotic pathway; therefore, that necroptosis becomes a subsequent procedural event [46]. It has also been suggested that in acute ischemia–reperfusion injury of the myocardium, inhibitors of two pathways, apoptosis and ferroptosis, exert cardioprotective effects on cardiac I/R injury by regulating mitochondrial function. In contrast, necroptosis is not involved in the pathogenesis of this acute cardiac I/R setting [47].
ROS is a crucial component of MI–RI [48], and numerous studies have pointed to a connection between ROS and necroptosis. In NIH 3T3 cells, TNF-induced ROS production is RIPK3-dependent [36], and ROS production has been shown to be inhibited in a variety of RIPK3 or MLKL deficient cell lines [49,50]. A study by Zhang et al. showed a direct link between I/R-mediated RIPK3 activation and ROS production in isolated cardiac myocytes, while the potential mechanisms were shown to include CaMKII activation and a subsequent increase in mPTP opening [45]. However, Tait’s experiments showed that mitochondrial ROSs are not necessary for necroptosis and can be bypassed if caspase-8 is inhibited [51]. Non-mitochondrial-derived ROSs are also associated with TNF-induced necroptosis [52]. Therefore, complementary pathways leading to necroptosis may exist. The above evidence provides strong evidence for the involvement of ROS in necrotic signaling; however, the specific mechanisms of ROS involvement in necroptosis still require further exploration.
Some studies have revealed that MAPK and NF-κB are involved in many biological processes [53,54]. p38MAPK-activated protein kinase 2 (MK2), an effector kinase located downstream of MAPK and NF-κB, phosphorylates RIPK1 directly at residue S321 and inhibits RIPK1 integration into the necrosome, thereby suppressing RIPK1 kinase-dependent apoptosis and necroptosis [55]. There is conclusive evidence that RIPK1 phosphorylates signal transducers and activators of transcription 3 (STAT3), contributing to its interaction with the gene associated with retinoid-IFN-induced mortality-19 (GRIM-19) (a subunit of mitochondrial complex I). This causes GRIM-19 translocation to mitochondria, launching the generation of ROS and ultimately bringing on TNF-induced necroptosis [56]. Additionally, Akt (a serine/threonine kinase)/the nuclear factor erythroid-2-related factor 2 (Nrf2) pathway is essential for regulating ROS production [57]. Akt is involved in the positive regulation of Nrf2 antioxidants. Activated Nrf2 further segregates from kelch-like ECH-associated protein 1 (Keap1) and enters the nucleus to form a dimer with Maf, recognizing and inducing transcription of NQO-1 and HO-1 and attenuating oxidative stress injury [58]. Thus, modulation of the Akt/Nrf2 signaling pathway can reduce ROS production-induced necroptosis.
Rho-associated coiled-coil kinase (ROCK), a serine/threonine kinase regulated by the small GTPase RhoA, is involved in regulating cell migration, proliferation, and survival. During MI–RI, RIPK3 mediates the linkage between Rho proteins and substrates and enhances necrotic cell death by activating the RhoA/ROCK pathway [59]. Activation of the RhoA/ROCK pathway can also enhance oxidative stress, inflammation, endothelial dysfunction, fibrosis, and necroptosis in damaged myocardia after MI–RI [60]. CaMKII can act as a RIPK3 substrate, mediating ischemia and oxidative stress-induced myocardial necroptosis [61]. It has also been shown that RIPK3 enhances oxidative stress-induced myocardial necroptosis through the activation of CaMKII [62]. RIPK3 may act as a molecular switch for necroptosis and apoptotic cells involved in myocardial I/R injury during RhoA/ROCK pathway activation [61].
Activation of RIPK3 is accompanied by activation of the JNK pathway and upregulation of Bcl-2 19-kDa interacting protein 3 (BNIP3), a downstream regulator of HIF-1alpha. Blocking the JNK pathway eliminates the deleterious effects of H/R injury on mitochondrial function, energy metabolism, and redox homeostasis. Overexpression of Bnip3 eliminates the protective effect of RIPK3 deficiency on cardiomyocyte survival [36]. In addition, RIPK3 overexpression also activates the NF-κB signaling pathway [63], while N″-(carboxymethyl) lysine (CML) is a major member of the advanced glycation end product (RAGE). CML promotes necroptosis by increasing the phosphorylation of RIPK3 and its downstream proteins [64]. Collectively, this evidence revealed that necroptosis is a new type of cell death. Table 1 summarizes the differences between necroptosis and several other types of regulated cell death. At the same time, the occurrence of necroptosis is often accompanied by a rise in markers of myocardial injury, such as cTnI [65,66,67], CK-MB [68,69], and N-terminal pro-brain natriuretic peptide (NT-pro-BNP) [70]. Further support comes from Rosana’s study showing that major adverse cardiac events (MACE) were associated with higher log copies/mL of SARS-CoV-2, cTnI, and pro-BNP in plasma. An active form of MLKL (phosphorylated MLKL, pMLKL) was also raised in the serum of MACE patients. The presence of necroptosis in the heart was revealed by the elevated pMLKL and cTnI levels [71]. There is further work to be done on necroptosis and novel biological molecules/biomarkers (ST2, GDF-15, syndecan-1).

Table 1.
Comparison of different forms of programmed cell death.
4. Potential Interventions of Necroptosis in Myocardial Ischemia/Reperfusion Injury
4.1. Necroptosis Inhibitor
Inhibition of necroptosis has recently been shown to attenuate reperfusion injury after AMI in mice, rats, and pigs [24,72,73,74]. Nec-1 is a potent allosteric inhibitor of RIPK1 kinase activity that reduces myocardial cell death and maintains myocardial structural integrity, thereby inhibiting the reactive fibrotic process during late myocardial ischemia/reperfusion. In animal models of degenerative disease, Nec-1 prevented cell death, including necroptosis and apoptosis. In addition, administration of Nec-1 to donors and recipients improved graft function in Lewi rats after lung transplantation by reducing necroptosis [75]. Liang et al. showed that administration of NEC-1 (0.6 mg/kg) at the beginning of reperfusion significantly reduced the release of serum creatine kinase and downregulation of autophagy 24 h after reperfusion, whereas higher concentrations of NEC-1 (1.8 mg/kg) increased mortality in rats with chronic myocardial ischemia [69]. Preclinical data from a model of MI–RI showed that the brain also undergoes dendritic spine loss [76,77], which prevents it from forming synapses to maintain normal cognition. However, a study by Liao et al. showed that Nec-1 did not inhibit p-RIPK1, p-RIPK3, and p-MLKL in the brains of rats with MI–RI. The three proteins were not upregulated in the brain, and Nec-1 did not interfere with the physiological functions of these proteins, probably due to the blood–brain barrier (BBB). In addition, all doses of Nec-1 effectively reduced hippocampal apoptosis after MI–RI [78].
In the rat model of persistent coronary blockage, the cardioprotective effects of the necroptosis inhibitor necrostatin-7 were investigated. Necrostatin-7 pretreatment led to a decrease in the plasma level of NT-Pro-BNP, which suggested that the left ventricular function had improved [79]. Compound 547 is a novel RIPK1 inhibitor that promotes cell viability and minimizes mitochondrial damage by decreasing the expression and activation of the necrotic cell group, RIPK1, RIPK3, MLKL, and CaMKII, but not the mRNA level [80]. Advanced glycation end products (AGEs) can be produced more quickly in prolonged hyperglycemic situations as a result of non-enzymatic interactions between glucose and proteins, lipids, or nucleic acids [81]. In a model of AGE-induced cardiomyocyte injury, AGEs raised RIPK3 expression and aggravated the disruption of CaMKIIδ selective splicing. They also accelerated CaMKII activation, increased oxidative stress, triggered necroptosis, and damaged cardiomyocytes. Pretreatment with the RIPK3 inhibitor GSK′872 increased CaMKIIδA and CaMKIIδB expression but significantly decreased AGE-induced ox-CaMKII, p-CaMKII, and CaMKIIδC expression, suggesting that GSK′872 corrected AGE-induced CaMKIIδ selective splicing disorder in cardiomyocytes and attenuated AGE-induced CaMKII activation. Downregulation of RIPK3 or GSK′872 inhibited CaMKII activation, reduced oxidative stress, attenuated necrosis, and ameliorated cellular injury in cardiomyocytes [82].
Nesfatin-1 is a hypothalamic polypeptide produced by the translation of its precursor nuclear histone 2. It plays a key role in the control of water uptake, blood pressure, and glucose homeostasis [83]. Nesfatin-1 is also a novel cardiac peptide, mainly through the RIPK1/RIPK3/MLKL axis and the RhoA/ROCK/RIPK3 signaling pathway, protecting against I/R injury. Nesfatin-1 is able to exert cardioprotective effects against MI/R in a dose-dependent manner. Montesanti G, et al. demonstrated in the same rat I/R model that only high doses of Nesfatin-1 (20 μg/kg) were able to inhibit the expression of RIPK1, RIPK3, MLKL, ROCK1, and ROCK2 proteins, and nesfatin-1 intraperitoneal injection reduced the infarct size by 50% [84]. Later, Sharifi et al. also confirmed that high doses of nesfatin-1 (20 μg/kg) significantly reduced the expression levels of these proteins (RIPK3, MLKL, ROCK1, and ROCK2 proteins, ROS, antioxidant peptide (GSH), and MDA levels), restored superoxide dismutase (SOD) activity, inhibited the elevation of cTnI, creatine kinase myocardial band (CK-MB), and lactate dehydrogenase (LDH) activity, restored ejection fraction (EF) and fractional shortening (FS), reduced myocardial interstitial collagen deposition and scar formation, and inhibited cardiac histopathological damage [25]. However, CaMKII was not measured to determine whether nesfatin-1 could simultaneously affect this pathway. A novel Nec-1 analog, (Z)-5-(3,5-dimethoxybenzyl)2-imine-1-methylimidazolin-4-1 (DIMO), could exert myocardial protective effects by reducing RIPK1 activation, inhibiting the interaction of RIPK1 with RIPK3, and restoring impaired autophagic flow. In the oxygen–glucose deprivation/reoxygenation injury model (OGD/R), a 0.1 μm dose of DIMO reduced LDH leakage and the proportion of PI-positive cells, and a dose of 1 or 2 mg/kg of DIMO reduced cardiomyocyte necroptosis and improved myocardial infarct size, whereas DIMO at a dose of 4 mg/kg was ineffective. In addition, DIMO attenuated myocardial I/R-induced lysosomal injury [85].
Resveratrol (RES) is an edible compound found in grape skins and red wine. RES exerts biological and pharmacological functions such as anti-aging, anti-inflammatory, anti-cancer, and cardioprotective effects [86,87]. A study by Hu et al. showed that resveratrol (RES) treated with different concentrations of RES-treated H/R cardiomyocytes revealed a significant downregulation of TNF-α, RIPK1, RIPK3, and p-MLKL/MLKL expression, a decrease in necroptosis, and an increase in cell viability in a dose-dependent manner. In addition, RES ameliorated the enhanced effect of TNF-α on programmed necrosis in myocardial H/R injured cells and confirmed the effect of RES in MI–RI rats [88]. The natural product oleanolic acid derivative, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO), is a necroptosis inhibitor that inhibits the phosphorylation of RIPK1 and RIPK3 in necrotic cells by targeting Hsp90, thereby blocking the formation of necrosomes. A more active analogue was later discovered, compound 20, which was effective against necroptosis in human and mouse cells. With activity to attenuate TNF-induced systemic inflammatory response syndrome (SIRS) and brain I/R injury after oral administration, this compound can also synergize with other inhibitors of necroptosis, including TAK632 (a RIPK1/3 inhibitor), SZM630 (a RIPK3 inhibitor), Nec-1 (a RIPK1 inhibitor), and NSA (an MLKL inhibitor). They can be used as lead compounds for necroptosis inhibitors in I/R therapy [89]. In mouse L929 cells, we found no protective effect of CDDO against TNF-α and Z-VAD-fmk-induced necroptosis. Zhu et al. showed that polypeptide globular adiponectin treatment attenuated post-hypoxia/reoxygenation cardiomyocyte injury, as evidenced by increased cell viability and reduced LDH release. Immunofluorescence staining and Western blotting results showed that both necroptosis and apoptosis were triggered by H/R and attenuated by globular adiponectin. Moreover, globular adiponectin also attenuated the formation of reactive oxygen species, oxidative stress, and p38 MAPK and NF-B signaling, which are significant contributors to necroptosis and apoptosis [90].
4.2. Chinese Medicine
Salvia miltiorrhiza Bunge, a traditional Chinese medicine, is widely used in the treatment of cardiovascular diseases for its efficacy in activating blood circulation and resolving blood stasis [91]. Tanshinone I (TI) is the main lipid-soluble component of Salvia miltiorrhiza [92]. Zhuo et al. showed that TI pretreatment attenuated tert-butyl hydroperoxide (t-BHP) and MIRI-induced necroptosis by inhibiting the expression of p-RIPK1, p-RIPK3, and p-MLKL. TI activates the Akt/Nrf2 pathway and promotes the expression of antioxidant-related proteins such as Akt phosphorylation, Nrf2, NQO-1, and heme oxygenase-1 (HO-1) in t-BHP-stimulated H9C2 cells. Furthermore, TI reduces ROS production and reverses the loss of mitochondrial membrane potential (MMP) to alleviate oxidative stress; therefore, TI preconditioning plays a role in myocardial injury [93].
Arctiin is one of the main active ingredients extracted from the dried mature fruits of Burdock burdock, commonly known as burdock seeds, from the family Asteraceae, as an ingredient in Chinese pharmacopoeia [94,95]. Arctiin reduces cellular necroptosis by scavenging ROS and restoring mitochondrial function or targeting RIPK1 and/or MLKL. Notably, arctiin can interact with amino acid residues in RIPK1 or MLKL but not RIPK3. Arctiin significantly reduced myocardial I/R injury (myocardial infarction and creatine kinase release), while decreasing the levels of necroptosis-related proteins (RIPK1/p-RIPK1, RIPK3/p-RIPK3, and MLKL/p-MLKL) in the hearts of I/R-treated rats. In the presence of Arctiin, necrosis and LDH release were attenuated in H/R-treated cardiomyocytes. Additionally, arctiin impaired H/R-induced ROS generation and mitochondrial dysfunction (increased MMP and decreased ATP production). Likewise, arctiin reduced H/R-treated H9C2 cells in reactive oxygen species production and improved mitochondrial function [96].
Bauhinia championii (BC) is a well-known folk herb from Taiwan, China, mainly used for the treatment of acute and chronic pain and rheumatoid arthritis [97]. In previous studies, BC was considered to have antioxidant activity and anti-inflammatory potential [98]. According to Chen et al., BC significantly decreased the size of infarcts caused by I/R, as well as the release of myoglobin and oxidation of CaMK. It could also block Na+ channels to lessen action potential depolarization and lessen necroptosis, which prevented I/R-caused ventricular arrhythmias and cardiomyocyte death. BC may be a novel possibility for the treatment of myocardial infarction and ventricular arrhythmias because it also inhibits the oxidation of CaMK, which lowers I/R-induced necroptosis [99].
Baicalin is one of the major flavonoids isolated from the dried root of Scutellaria baicalensis. Due to its various pharmacological effects, such as its antioxidant and antitumor effects, it is widely used in the treatment of various diseases in Chinese medicine. Recently, the protective effect of baicalin on myocardial infarction was reported [100]. Baicalin protects neonatal rat cardiomyocytes from hypoxic injury by significantly increasing nitric oxide (NO) levels [101]. Bai et al. showed that baicalin could promote NO production and inhibit cardiomyocyte apoptosis by activating the PI3K-AKT-eNOS signaling pathway, which in turn reduces the myocardial infarct size and significantly improves cardiac function. Baicalin had a protective effect on myocardial microvasculature and promoted NO production and cGMP levels in rats with MI–RI. The results of in vitro experiments showed that baicalin significantly increased the cellular activity and function of cardiac microvascular endothelial cells (CMEC) in cardiac microvascular endothelial cells exposed to H/R, and RIPK3 and p-MLKL blocked CMEC necroptosis [102]. It was found that TNF-α induced phosphorylation of RIPK1 and RIPK3, and MLKL was negatively correlated with transforming growth factor-activated kinase 1 (TAK1) phosphorylation. Inhibition of TAK1 phosphorylation enhanced necroptosis [103].
Ginsenosides are the main components of ginseng. They have various pharmacological effects, such as vasodilator, antitumor, antidiabetic, anti-inflammatory, and antioxidant effects. Ginsenoside Rg2 is one of the compounds in the original ginseng triol group [104]. Li et al. showed that ginsenoside Rg2 effectively inhibited the phosphorylation of RIPK1, RIPK3, and MLKL in H/R cardiomyocytes and inhibited the formation of the RIPK1/RIPK3 complex (necrosome). More importantly, Ginsenoside Rg2 significantly increased TAK1 phosphorylation and enhanced TAK1 binding to RIPK1 while inhibiting the formation of necrosomes and ultimately reducing I/R-induced necroptosis [103].
4.3. Clinical Medication
Metformin is a classical oral hypoglycemic agent, and its role in other diseases has been extensively studied. In a study of animal models by Li et al., the aging-associated autophagy defect leads to increased I/R-induced myocardial necroptosis. Aggregated p62 forms a complex with RIPK1 and RIPK3, promoting the binding of RIPK1 and RIPK3 and subsequent MLKL phosphorylation. This leads to the development of aging I/R heart necroptosis. Metformin restores autophagy and disrupts the p62–RIPK1–RIPK3 complex, effectively reducing I/R-induced necroptosis in the senescent heart. Impaired autophagosome clearance is one of the cornerstones of I/R-induced necroptosis in cardiac myocytes [38]. However, Techyran et al. showed that in a porcine model (3~4 months of age), intravascular administration of metformin before reperfusion to achieve higher intracoronary plasma levels failed to reduce the infarct size [105]. Recombinant apyrase (AZD3366) significantly attenuated the increase in RIPK1, RIPK3, and P-MLKL after 1 h of reperfusion and significantly inhibited the increase in IL-6 and GSDMD-N (markers of cellular scorching). The above effects were not observed with Tegretol. At 24 h of reperfusion, both drugs attenuated the elevation of these markers to the same extent, and their effects were additive [106].
Sevoflurane is a volatile anesthetic widely used in cardiovascular surgery. Sevoflurane postconditioning (SPC) is protective against MI–RI, and its myocardial protective effects are regulated by multiple signaling pathways [107,108]. Recent studies have shown that O-Glc NAc transferase (OGT) inhibits necroptosis by targeting RIPK3 [109]. In a study by Zhang et al., it was shown that SPC promoted O-GlcNAc transferase OGT-mediated O-GlcNAcylation of RIPK3, reduced RIPK3 expression, and ultimately led to a reduction in RIPK3–MLKL complex formation to inhibit I/R-induced necroptosis. SPC also improved cardiac function and significantly reduced hemodynamic parameters. However, it was not determined whether SPC promotes RIPK3 degradation by directly affecting OGT [110].
Remifentanil is an ultra-short-acting opioid that has been shown to promote cardioprotective effects in selected animal models and some clinical settings [111]. Oxidative protein damage, p21 levels, IL-8-based proinflammatory signaling, and MLKL activity decrease after remifentanil preconditioning. Simultaneously, remifentanil preconditioning reduces phosphorylation signaling of RIPK3 and MLKL and attenuates hypoxia-induced necroptosis in cardiomyocytes [112].
Dexmedetomidine (Dex) is a highly selective α2-adrenoceptor agonist with superior sedative, anxiolytic, analgesic, and anti-sympathetic activity [113]. H9C2 (embryonic rat heart-derived myoblast) cells treated with H2O2 showed a significant decrease in cell viability, an increase in LDH release, and a significant increase in necroptosis. Dex pretreatment attenuated these H2O2-induced injuries, significantly increased the expression of H2O2-induced protein HO-1, and decreased protein RIPK1 and RIPK3 expression. All these protective effects of Dex were reversed by yohimbine hydrochloride (YOH) [114]. According to Chen et al., H/R significantly increased CK-MB, cTnI, TNF-α, IL-1β, and IL-6 levels and significantly decreased cell viability, while H/R dramatically raised the protein levels of RIPK1, RIPK3, MLKL, and high-mobility group box-1 (HMGB1). DEX pre-treatment significantly improved H/R-induced necroptosis, cell injury, and inflammation. Silencing of HMGB1 enhanced the protective effect of DEX pretreatment on myocardium. However, only the role of Dex preconditioning in ameliorating I/R-induced inflammation was investigated, and the relationship between oxidative stress and DEX pre-adaptation also needs to be explored [66]. Furthermore, in an in vivo study, Dex pretreatment inhibited inflammatory responses and reduced myocardial infarct size by activating α2 adrenergic receptors in rats with MI–RI [115,116].
Zhang et al. discovered that a high glucose environment increased hypoxia injury-induced apoptosis, necroptosis, oxidative stress, and endoplasmic reticulum stress. They also found that a high glucose environment combined hypoxic damage suppressed the activation of Janus kinase 2 (JAK2)/signal transducers and activators of the transcription (STAT3) signaling pathway. In contrast, Empagliflozin (EMPA) protected against I/R and H/R-induced cardiomyocyte injury by activating JAK2/STAT3 signaling under high-glucose environmental conditions, while downregulation of STAT3 reversed the protective effect of EMPA, indicating that EMPA had a beneficial effect on cardiomyocyte protection [117]. Long-term administration of EMPA reduces myocardial infarct size by activating STAT3 in microvascular endothelial cells [118].
Simvastatin is a common drug used in the cardiovascular system. Jones et al. reported that in a mouse model of MIRI, simvastatin pretreatment reduced infarct size and preserved myocardial function [119]. Naseroleslami et al. showed that simvastatin pretreatment attenuates injury in a rat heart graft I/R model by targeting caspase-9 and RIPK1 protein activity. Furthermore, during MI–RI, excess ROS production activates the Rho/ROCK pathway, and Rho kinase can accelerate cytokine-mediated inflammatory responses, exacerbating left ventricular remodeling after myocardial infarction. This pathway can be reversed by simvastatin and simvastatin-containing nanovesicles [120]. The Moerke study sought a selection of medicinal drugs to assess their capacity to influence RIPK1-mediated cell death. The anti-epileptic medicine Phenhydan® was found to be a powerful inhibitor of death receptor-induced necroptosis and apoptosis in their small-scale screen. In further research, Phenhydan® inhibited RIPK1’s early activation in the TNF receptor signaling complex-I. It is possible that Phenhydan® also inhibits TNFR1 signaling as a whole. Phenhydan® inhibited pathophysiologic cell death processes by altering cell membrane function, such as lipid raft formation, and blocking necrosome formation/activation as well as death receptor-induced NF-B signaling. Phenhydan® extended this observation to several cell types and species by inhibiting RIPK1-mediated cell death not only in L929 cells but also in murine NIH3T3, murine HT-29, human HT-29, and U937 cells. Hence, Phenhydan® can act as a potent inhibitor of death receptor-induced necroptosis and apoptosis [34]. Tu et al. discovered that the combination of ponatinib and desferrioxamine reduced MI–RI by simultaneously inhibiting necroptosis and ferroptosis. The combination significantly reduced myocardial infarct size and creatine kinase release and was more effective than single-drug therapy [68].
4.4. Enzymes That Regulate Necroptosis
PGAM5, a serine/threonine protein phosphatase localized to the outer mitochondrial membrane, functions as a novel inducer of necroptosis. Zhu et al. revealed that PGAM5 enhances I/R-mediated necroptosis in cardiomyocytes and that PGAM5 expression impairs cardiac function by decreasing cardiomyocyte systolic/diastolic characteristics. At the subcellular level, PGAM5 deficiency boosted the copy number and transcript levels of mitochondrial DNA, restored mitochondrial respiration, reduced the formation of mitochondrial ROS, and stopped aberrant mPTP opening during I/R. Cardiac-specific PGAM5 deletion suppressed cardiac inflammation and decreased the size of myocardial infarctions [121]. A study by She et al. demonstrated for the first time that inhibition of PGAM5 attenuates I/R-induced necroptosis in rat hearts by inhibiting the mitochondrial dynamin-related protein 1 (Drp1). They also found that there is positive feedback between RIPK1 and PGAM5 [40]. In a study by Park et al., both RIPK1 inhibition and RIPK3 knockout did not provide the same level of protection against I/R injury in knockout (KO) of the mitochondrial Ca2+ uniporter (MCU-KO) mice hearts as they did in wild-type (WT) mice hearts. This indicates that the lack of protection cannot be explained by upregulation of necroptosis [122].
SB-706375 is a selective receptor antagonist of human urotensin-II (hU-II) that can inhibit hU-II-induced aortic contraction in rats. SB-706375 significantly reduced necroptosis in cardiac myocytes by decreasing LDH and CK-MB activity, increasing RhoA activity and U-II receptor (UTR), RIPK3, ROCK1, and ROCK2 protein expression, and decreasing cTnI levels in coronary effluent, according to research by Duan et al. [59]. Aldehyde dehydrogenases 2 (ALDH2) may be a key regulator of high-glucose-induced cardiomyocyte injury, and activation of ALDH2 prevented the onset of fibrosis, apoptosis, and necroptosis in a model of high-glucose-induced primary cardiomyocyte injury [123]. Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) can recirculate calcium from the cytoplasm back to the endoplasmic reticulum. In CMEC that had undergone I/R treatment, SERCA overexpression reduced intracellular calcium excess, inhibited the production of the MCU, and stopped the aberrant opening of the mPTP. At the same time, SERCA overexpression significantly reduced I/R-induced luminal narrowing and vessel wall edema, protecting the microcirculation against I/R injury in a calcium/MCU/necroptosis pathway-dependent manner [124].
In I/R-injured hearts, adenosine kinase inhibitors markedly reduced I/R-induced myonodular formation, mitochondrial injury, infarct size, LVEF, and LVFS. Further studies showed that ADK inhibitors suppressed RIPK3 and RIPK3 phosphorylation and cystatins-9, cystatins-8, and cystatins-3 activation, but not cystatins-12 activation. Additionally, ADK inhibitors prevented mPTP opening and CaMKII activation, which are crucial for the protection of cardiomyocytes during I/R [125]. A study by Xiang et al. discovered that the level of mmu circ 000338, a cardiac-necroptosis-associated circRNA (CNEACR), was decreased in cardiomyocytes exposed to H/R and in the hearts of mice with I/R damage. Histone deacetylase (HDAC7) is directly bound by CNEACR in the cytoplasm, preventing it from entering the nucleus. Forkhead box protein A2 (Foxa2), which can repress the RIPK3 gene by attaching to its promoter region, is suppressed less as a result of HDAC7-dependent inhibition of transcription. In addition, RIPK3-dependent necroptotic mortality of cardiomyocytes was reduced by CNEACR-mediated overexpression of FOXA2. CircRNAs like CNEACR, which increase cell survival and enhance cardiac function in the I/R-damaged heart, might thereby regulate the activity of HDACs that are related to cardiomyocyte necroptosis [126]. Mitsugumin 53 (MG53), an E3 ubiquitin ligase, attaches many ubiquitin chains to the K316, K604, and K627 sites of RIPK1 to facilitate RIPK1’s destruction by the proteasome and prevent necroptosis. After reperfusion injury, the production of ROS in the infarct area promotes the interaction between MG53 and RIPK1. Application of N-acetylcysteine (NAC) disrupts the interaction between MG53 and RIPK1 and abolishes the MG53-mediated cardioprotective effect [127].
4.5. Other
In a mouse model, microRNA-325-3p protects the heart from I/R injury after myocardial infarction by inhibiting RIPK3 and necroptosis [128]. Two cannabinoid receptors, CB1R and CB2R, regulate cannabinoids in the myocardium. Previous research has demonstrated that activating the CB1R promotes cell death by inducing the p38 and JNK/MAPK signaling pathways [129]. In a study by Zaafan, a tiny non-coding RNA known as microRNA-103 controls the production of the Fas-associated protein with death domain (FADD) gene, which is a negative regulator of necroptosis during MI progression. CK-MB and troponin-I levels in animals treated with miR-103 power inhibitor greatly improved, and the histology of the cardiac tissue was almost normal. Through targeting the FADD/RIPK pathway, miR-103 inhibitor can be a strong cardioprotective drug and a viable MI treatment [65]. In the ethanol-induced myocardial injury model, CB2R agonist (JWH-133) significantly decreased ethanol-induced production of total phosphorylated protein content, which resulted in elevated expression of RIPK1, RIPK3, and MLKL. Because the JWH-133 significantly decreased the ethanol-induced rise in total phosphorylated protein content expression level and protected against ethanol-induced cardiac injury, it is postulated that CB2R may be an upstream regulator in the RIPK1/RIPK3/MLKL signaling pathway [130].
According to research by Qiu et al., shock wave therapy (SWT) reduced the expression of RIPK1 and RIPK3 and increased cell survival and cytotoxicity in the H/R model. Moreover, SWT reduced the blockage of autophagic flow in response to H/R damage in the fluorescent-tagged LC3 (tfLC3) test. SWT prevented H/R-induced necroptosis, and this action was mediated via the restoration of autophagic flow [131]. Maslinic acid (MA) dramatically reduced cardiac tissue damage, decreased CK-MB and LDH levels, and decreased infarct size, according to research by Lin Li et al. MA also promoted autophagic flow and inhibited apoptosis and necroptosis [132]. High-intensity interval training (HIIT) decreased lipid peroxidation and infarct area in the rat model. On the other hand, HIIT dramatically increased SOD activity and decreased the expression of major mediators of MI-induced necroptosis (MLKL, RIPK3, and RIPK1). HIIT after MI protected LV function and prevented remodeling by restoring EF and FS. In addition, long-term HIIT significantly reduced myocardial interstitial collagen deposition and scar formation [133]. Therefore, the prevention of necroptotic cardiomyocyte death is a new direction for preventing MI–RI. Table 2 summarizes the role of necroptosis and its inhibitors in different models.

Table 2.
Summary of studies investigating necroptosis and its inhibitors in different models.
4.6. Summary and Outlook
In the next ten years, cardiovascular diseases, including AMI, are expected to continue to be the leading cause of death worldwide. According to extant research, necroptosis plays a crucial role in MI–RI, including cardiac inflammation, myocardial infarct size enlargement, cardiac dysfunction, and adverse cardiac remodeling. The main focus of this paper was on the different ways to inhibit necroptosis, all of which greatly decreased the size of myocardial infarctions, diminished the release of inflammatory mediators, and enhanced cardiac function. As a result, preventing necroptotic cardiomyocyte death is a new strategy for preventing MI–RI. For patients with acute coronary syndrome, multi-target strategic therapy can significantly improve their prognosis, and the combined prevention of myocardial ischemia–reperfusion injury will become the best myocardial protection therapy [134]. More than one form of programmed necrosis generally plays a role in MI–RI, usually as a result of the overlapping of multiple pathways. For instance, activation of the JNK/Bnip3 route [36], the RhoA/ROCK pathway [59], CaMKII [62], NF-κB signaling pathway [63], PGAM5/CypD/mPTP [135], and other pathways occurs, along with activation of the classical necroptosis pathway. Many necroptosis activators and inhibitors have been created, and targeted inhibition of necroptosis can supplement traditional reperfusion strategies and existing treatments. The majority of these inhibitors of necroptosis only inhibit a single pathway; hence, pharmacological therapies for the treatment of AMI patients in the future should be developed using medication combinations or pleiotropic drugs. In addition, most studies targeting necroptosis therapy are based on in vitro experiments or animal models, so it is still necessary to assess the feasibility of using these medications in clinical trials and in vivo. In the meantime, special consideration should be given to off-target consequences of necroptosis-targeted medicines to enhance selectivity and safety. Finally, the therapeutic time window for the application of drug therapy plays a crucial role in the successful treatment of reperfusion injuries. In reperfusion-induced cellularity following prolonged ischemia, death can only be avoided if cardioprotective agents are administered at the beginning of reperfusion, ideally before the coronary arteries are reopened [136]. However, even if the time window of administration should precede reperfusion, further studies are needed to determine how long the protective therapy must be applied to completely prevent myocardial ischemia–reperfusion injury.
Author Contributions
Conceptualization, Y.Z. (Yinchang Zhang) and Y.Z. (Yantao Zhang); writing—original draft preparation, Y.Z. (Yinchang Zhang); writing—review and editing, Y.L., Y.Z. (Yinchang Zhang), J.Z., and Y.Z. (Yantao Zhang); funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Natural Sciences Foundation of Gansu (No. 23JRRA0972), the Natural Sciences Foundation of Fujian (No. 2022J05105), the Science and Technology Planning Project of Chengguan District (2022RCCX0023), the Cuiying Graduate Supervisor Applicant Training Program of Lanzhou University Second Hospital (No. 201709), the Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (No. CY2022-MS-A03), the Talent Introduction Plan of the Lanzhou University Second Hospital (No. YJRCKYQDJ-2021-02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest in relation to the subject of this review.
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The Molecular Machinery of Regulated Cell Death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Amani, H.; Habibey, R.; Hajmiresmail, S.J.; Latifi, S.; Pazoki-Toroudi, H.; Akhavan, O. Antioxidant Nanomaterials in Advanced Diagnoses and Treatments of Ischemia Reperfusion Injuries. J. Mater. Chem. B 2017, 5, 9452–9476. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of Cyclophilin D Reveals a Critical Role for Mitochondrial Permeability Transition in Cell Death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Karch, J.; Kwong, J.Q.; Burr, A.R.; Sargent, M.A.; Elrod, J.W.; Peixoto, P.M.; Martinez-Caballero, S.; Osinska, H.; Cheng, E.H.-Y.; Robbins, J.; et al. Bax and Bak Function as the Outer Membrane Component of the Mitochondrial Permeability Pore in Regulating Necrotic Cell Death in Mice. elife 2013, 2, e00772. [Google Scholar] [CrossRef]
- Li, N.; Huang, Y.; He, Q. Vital Role of RIP3 in the Mechanism of Myocardial Cell Necroptosis. Chin. Crit. Care Med. 2019, 31, 1045–1047. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Strasser, A.; McDunn, J.E.; Swanson, P.E. Cell Death. N. Engl. J. Med. 2009, 361, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef] [PubMed]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host Cell Death and Inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Zhang, D.; Yu, P.; Zhang, J.; Yu, S. Research Progress on the Role of Pyroptosis in Myocardial Ischemia-Reperfusion Injury. Cells 2022, 11, 3271. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Hong, M.; Rong, J.; Tao, X.; Xu, Y. The Emerging Role of Ferroptosis in Cardiovascular Diseases. Front. Pharmacol. 2022, 13, 822083. [Google Scholar] [CrossRef] [PubMed]
- Edinger, A.L.; Thompson, C.B. Death by Design: Apoptosis, Necrosis and Autophagy. Curr. Opin. Cell Biol. 2004, 16, 663–669. [Google Scholar] [CrossRef]
- Inserte, J.; Cardona, M.; Poncelas-Nozal, M.; Hernando, V.; Vilardosa, Ú.; Aluja, D.; Parra, V.M.; Sanchis, D.; Garcia-Dorado, D. Studies on the Role of Apoptosis after Transient Myocardial Ischemia: Genetic Deletion of the Executioner Caspases-3 and -7 Does Not Limit Infarct Size and Ventricular Remodeling. Basic Res. Cardiol. 2016, 111, 18. [Google Scholar] [CrossRef] [PubMed]
- Adameova, A.; Goncalvesova, E.; Szobi, A.; Dhalla, N.S. Necroptotic Cell Death in Failing Heart: Relevance and Proposed Mechanisms. Heart Fail Rev. 2016, 21, 213–221. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial Ischemia-Reperfusion Injury: A Neglected Therapeutic Target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- DeRoo, E.; Zhou, T.; Liu, B. The Role of RIPK1 and RIPK3 in Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 8174. [Google Scholar] [CrossRef]
- Szobi, A.; Farkašová-Ledvényiová, V.; Lichý, M.; Muráriková, M.; Čarnická, S.; Ravingerová, T.; Adameová, A. Cardioprotection of Ischaemic Preconditioning Is Associated with Inhibition of Translocation of MLKL within the Plasma Membrane. J. Cell. Mol. Med. 2018, 22, 4183–4196. [Google Scholar] [CrossRef]
- Khan, I.; Yousif, A.; Chesnokov, M.; Hong, L.; Chefetz, I. A Decade of Cell Death Studies: Breathing New Life into Necroptosis. Pharmacol. Ther. 2021, 220, 107717. [Google Scholar] [CrossRef]
- Cao, L.; Mu, W. Necrostatin-1 and Necroptosis Inhibition: Pathophysiology and Therapeutic Implications. Pharmacol. Res. 2021, 163, 105297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ren, Z.; Xu, W.; Jiang, Z. Necroptosis in Atherosclerosis. Clin. Chim. Acta 2022, 534, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Grootjans, S.; Vanden Berghe, T.; Vandenabeele, P. Initiation and Execution Mechanisms of Necroptosis: An Overview. Cell Death Differ. 2017, 24, 1184–1195. [Google Scholar] [CrossRef]
- Koshinuma, S.; Miyamae, M.; Kaneda, K.; Kotani, J.; Figueredo, V.M. Combination of Necroptosis and Apoptosis Inhibition Enhances Cardioprotection against Myocardial Ischemia-Reperfusion Injury. J. Anesth. 2014, 28, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Nazarinia, D.; Ramezani, F.; Azizi, Y.; Naderi, N.; Aboutaleb, N. Necroptosis and RhoA/ROCK Pathways: Molecular Targets of Nesfatin-1 in Cardioprotection against Myocardial Ischemia/Reperfusion Injury in a Rat Model. Mol. Biol. Rep. 2021, 48, 2507–2518. [Google Scholar] [CrossRef]
- Ying, L.; Benjanuwattra, J.; Chattipakorn, S.C.; Chattipakorn, N. The Role of RIPK3-Regulated Cell Death Pathways and Necroptosis in the Pathogenesis of Cardiac Ischaemia-Reperfusion Injury. Acta Physiol. 2021, 231, e13541. [Google Scholar] [CrossRef]
- Choi, M.E.; Price, D.R.; Ryter, S.W.; Choi, A.M.K. Necroptosis: A Crucial Pathogenic Mediator of Human Disease. JCI Insight 2019, 4, e128834. [Google Scholar] [CrossRef]
- Khoury, M.K.; Gupta, K.; Franco, S.R.; Liu, B. Necroptosis in the Pathophysiology of Disease. Am. J. Pathol. 2020, 190, 272–285. [Google Scholar] [CrossRef]
- Chen, J.; Kos, R.; Garssen, J.; Redegeld, F. Molecular Insights into the Mechanism of Necroptosis: The Necrosome as a Potential Therapeutic Target. Cells 2019, 8, 1486. [Google Scholar] [CrossRef]
- Fritsch, M.; Günther, S.D.; Schwarzer, R.; Albert, M.-C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 Is the Molecular Switch for Apoptosis, Necroptosis and Pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef]
- Shi, Z.-W.; Ge, L.-S.; Li, Y.-C. The Role of Necroptosis in Cardiovascular Disease. Front. Pharmacol. 2018, 9, 721. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, W.; Sun, S.; Han, Y.; Li, J.; Cao, S.; Li, R.; Xu, T.; Yuan, Q.; Wang, J.; et al. 4-Hydroxy-2-Nonenal Promotes Cardiomyocyte Necroptosis via Stabilizing Receptor-Interacting Serine/Threonine-Protein Kinase 1. Front. Cell Dev. Biol. 2021, 9, 721795. [Google Scholar] [CrossRef] [PubMed]
- Moerke, C.; Jaco, I.; Dewitz, C.; Müller, T.; Jacobsen, A.V.; Gautheron, J.; Fritsch, J.; Schmitz, J.; Bräsen, J.H.; Günther, C.; et al. The Anticonvulsive Phenhydan(®) Suppresses Extrinsic Cell Death. Cell Death Differ. 2019, 26, 1631–1645. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Hu, S.; Jin, Q.; Li, D.; Tian, F.; Toan, S.; Li, Y.; Zhou, H.; Chen, Y. Ripk3 Promotes ER Stress-Induced Necroptosis in Cardiac IR Injury: A Mechanism Involving Calcium Overload/XO/ROS/MPTP Pathway. Redox Biol. 2018, 16, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, T. Ripk3 Mediates Cardiomyocyte Necrosis through Targeting Mitochondria and the JNK-Bnip3 Pathway under Hypoxia-Reoxygenation Injury. J. Recept. Signal Transduct. Res. 2019, 39, 331–340. [Google Scholar] [CrossRef]
- Lichý, M.; Szobi, A.; Hrdlička, J.; Horváth, C.; Kormanová, V.; Rajtík, T.; Neckář, J.; Kolář, F.; Adameová, A. Different Signalling in Infarcted and Non-Infarcted Areas of Rat Failing Hearts: A Role of Necroptosis and Inflammation. J. Cell. Mol. Med. 2019, 23, 6429–6441. [Google Scholar] [CrossRef]
- Li, C.; Mu, N.; Gu, C.; Liu, M.; Yang, Z.; Yin, Y.; Chen, M.; Wang, Y.; Han, Y.; Yu, L.; et al. Metformin Mediates Cardioprotection against Aging-Induced Ischemic Necroptosis. Aging Cell 2020, 19, e13096. [Google Scholar] [CrossRef]
- Horvath, C.; Young, M.; Jarabicova, I.; Kindernay, L.; Ferenczyova, K.; Ravingerova, T.; Lewis, M.; Suleiman, M.S.; Adameova, A. Inhibition of Cardiac RIP3 Mitigates Early Reperfusion Injury and Calcium-Induced Mitochondrial Swelling without Altering Necroptotic Signalling. Int. J. Mol. Sci. 2021, 22, 7983. [Google Scholar] [CrossRef]
- She, L.; Tu, H.; Zhang, Y.-Z.; Tang, L.-J.; Li, N.-S.; Ma, Q.-L.; Liu, B.; Li, Q.; Luo, X.-J.; Peng, J. Inhibition of Phosphoglycerate Mutase 5 Reduces Necroptosis in Rat Hearts Following Ischemia/Reperfusion Through Suppression of Dynamin-Related Protein 1. Cardiovasc. Drugs Ther. 2019, 33, 13–23. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a Target for Protection against Cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Algieri, C.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Nesci, S. Phenylglyoxal Inhibition of the Mitochondrial F1FO-ATPase Activated by Mg2+ or by Ca2+ Provides Clues on the Mitochondrial Permeability Transition Pore. Arch. Biochem. Biophys. 2020, 681, 108258. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Ding, W.; Xu, T.; Ao, X.; Yu, T.; Li, M.; Liu, Y.; Zhang, X.; Hou, L.; Wang, J. Parkin Regulates Programmed Necrosis and Myocardial Ischemia/Reperfusion Injury by Targeting Cyclophilin-D. Antioxid. Redox Signal. 2019, 31, 1177–1193. [Google Scholar] [CrossRef] [PubMed]
- Hamacher-Brady, A.; Brady, N.R.; Gottlieb, R.A. Enhancing Macroautophagy Protects against Ischemia/Reperfusion Injury in Cardiac Myocytes. J. Biol. Chem. 2006, 281, 29776–29787. [Google Scholar] [CrossRef]
- Ogasawara, M.; Yano, T.; Tanno, M.; Abe, K.; Ishikawa, S.; Miki, T.; Kuno, A.; Tobisawa, T.; Muratsubaki, S.; Ohno, K.; et al. Suppression of Autophagic Flux Contributes to Cardiomyocyte Death by Activation of Necroptotic Pathways. J. Mol. Cell. Cardiol. 2017, 108, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, C.; Zhang, C.; Li, J.; Guo, W.; Yan, D.; Yang, C.; Zhao, J.; Xia, T.; Wang, Y.; et al. Heat Shock Protein 70 Inhibits Cardiomyocyte Necroptosis through Repressing Autophagy in Myocardial Ischemia/Reperfusion Injury. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 690–698. [Google Scholar] [CrossRef]
- Luo, Y.; Apaijai, N.; Liao, S.; Maneechote, C.; Chunchai, T.; Arunsak, B.; Benjanuwattra, J.; Yanpiset, P.; Chattipakorn, S.C.; Chattipakorn, N. Therapeutic Potentials of Cell Death Inhibitors in Rats with Cardiac Ischaemia/Reperfusion Injury. J. Cell. Mol. Med. 2022, 26, 2462–2476. [Google Scholar] [CrossRef]
- Adameova, A.; Horvath, C.; Abdul-Ghani, S.; Varga, Z.V.; Suleiman, M.S.; Dhalla, N.S. Interplay of Oxidative Stress and Necrosis-like Cell Death in Cardiac Ischemia/Reperfusion Injury: A Focus on Necroptosis. Biomedicines 2022, 10, 127. [Google Scholar] [CrossRef]
- Zhao, J.; Jitkaew, S.; Cai, Z.; Choksi, S.; Li, Q.; Luo, J.; Liu, Z.-G. Mixed Lineage Kinase Domain-like Is a Key Receptor Interacting Protein 3 Downstream Component of TNF-Induced Necrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 5322–5327. [Google Scholar] [CrossRef]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor Interacting Protein Kinase-3 Determines Cellular Necrotic Response to TNF-Alpha. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef]
- Tait, S.W.G.; Oberst, A.; Quarato, G.; Milasta, S.; Haller, M.; Wang, R.; Karvela, M.; Ichim, G.; Yatim, N.; Albert, M.L.; et al. Widespread Mitochondrial Depletion via Mitophagy Does Not Compromise Necroptosis. Cell Rep. 2013, 5, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of Reactive Oxygen Species Generation in Cell Signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Jiang, H.; Yang, J.; Chen, J.; Yang, J.; Ding, J.-W.; Li, S.; Wu, H.; Ding, H.-S. Radioprotective 105 KDa Protein Attenuates Ischemia/Reperfusion-Induced Myocardial Apoptosis and Autophagy by Inhibiting the Activation of the TLR4/NF-ΚB Signaling Pathway in Rats. Int. J. Mol. Med. 2016, 38, 885–893. [Google Scholar] [CrossRef]
- Hernández, G.; Lal, H.; Fidalgo, M.; Guerrero, A.; Zalvide, J.; Force, T.; Pombo, C.M. A Novel Cardioprotective P38-MAPK/MTOR Pathway. Exp. Cell Res. 2011, 317, 2938–2949. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Shulga, N.; Pastorino, J.G. Retraction: GRIM-19-Mediated Translocation of STAT3 to Mitochondria Is Necessary for TNF-Induced Necroptosis. J. Cell Sci. 2016, 129, 2686. [Google Scholar] [CrossRef]
- Wen, Z.; Hou, W.; Wu, W.; Zhao, Y.; Dong, X.; Bai, X.; Peng, L.; Song, L. 6’-O-Galloylpaeoniflorin Attenuates Cerebral Ischemia Reperfusion-Induced Neuroinflammation and Oxidative Stress via PI3K/Akt/Nrf2 Activation. Oxid. Med. Cell. Longev. 2018, 2018, 8678267. [Google Scholar] [CrossRef]
- Jiang, L.; Yin, X.; Chen, Y.-H.; Chen, Y.; Jiang, W.; Zheng, H.; Huang, F.-Q.; Liu, B.; Zhou, W.; Qi, L.-W.; et al. Proteomic Analysis Reveals Ginsenoside Rb1 Attenuates Myocardial Ischemia/Reperfusion Injury through Inhibiting ROS Production from Mitochondrial Complex I. Theranostics 2021, 11, 1703–1720. [Google Scholar] [CrossRef]
- Duan, J.-S.; Chen, S.; Sun, X.-Q.; Du, J.; Chen, Z.-W. Urotensin-#receptor Antagonist SB-706375 Protected Isolated Rat Heart from Ischaemia-Reperfusion Injury by Attenuating Myocardial Necrosis via RhoA/ROCK/RIP3 Signalling Pathway. Inflammopharmacology 2019, 27, 1309–1318. [Google Scholar] [CrossRef]
- Dong, M.; Yan, B.P.; Liao, J.K.; Lam, Y.-Y.; Yip, G.W.K.; Yu, C.-M. Rho-Kinase Inhibition: A Novel Therapeutic Target for the Treatment of Cardiovascular Diseases. Drug Discov. Today 2010, 15, 622–629. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Cui, M.; Jin, L.; Wang, Y.; Lv, F.; Liu, Y.; Zheng, W.; Shang, H.; Zhang, J.; et al. CaMKII Is a RIP3 Substrate Mediating Ischemia- and Oxidative Stress-Induced Myocardial Necroptosis. Nat. Med. 2016, 22, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, K.; Liu, X.; Qin, M.; Xiang, Y. CaMKII in Regulation of Cell Death During Myocardial Reperfusion Injury. Front. Mol. Biosci. 2021, 8, 668129. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhou, L.-Y.; Xu, H.-J.; Chen, X.-Y.; Tong, Z.-S.; Liu, X.-D.; Jia, Y.-S.; Chen, Y. RIP3 Overexpression Sensitizes Human Breast Cancer Cells to Parthenolide in Vitro via Intracellular ROS Accumulation. Acta Pharmacol. Sin. 2014, 35, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, F.; Shi, H.; Gao, Y.; Dong, Z.; Ma, L.; Sun, X.; Li, X.; Chang, S.; Wang, Z.; et al. Neutrophil-Derived Advanced Glycation End Products-N Epsilon-(Carboxymethyl) Lysine Promotes RIP3-Mediated Myocardial Necroptosis via RAGE and Exacerbates Myocardial Ischemia/Reperfusion Injury. FASEB J. 2019, 33, 14410–14422. [Google Scholar] [CrossRef]
- Zaafan, M.A.; Abdelhamid, A.M. The Cardioprotective Effect of MicroRNA-103 Inhibitor against Isoprenaline-Induced Myocardial Infarction in Mice through Targeting FADD/RIPK Pathway. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 837–844. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Z.; Zhou, X.; Sun, X.; Cao, J.; Liu, Y.; Wang, X. Dexmedetomidine Preconditioning Protects Cardiomyocytes Against Hypoxia/Reoxygenation-Induced Necroptosis by Inhibiting HMGB1-Mediated Inflammation. Cardiovasc. Drugs Ther. 2019, 33, 45–54. [Google Scholar] [CrossRef]
- Ma, F.; Zhu, Y.; Chang, L.; Gong, J.; Luo, Y.; Dai, J.; Lu, H. Hydrogen Sulfide Protects Against Ischemic Heart Failure by Inhibiting RIP1/RIP3/MLKL-Mediated Necroptosis. Physiol. Res. 2022, 71, 771–781. [Google Scholar] [CrossRef]
- Tu, H.; Zhou, Y.-J.; Tang, L.-J.; Xiong, X.-M.; Zhang, X.-J.; Sheikh, M.S.A.; Zhang, J.-J.; Luo, X.-J.; Yuan, C.; Peng, J. Combination of Ponatinib with Deferoxamine Synergistically Mitigates Ischemic Heart Injury via Simultaneous Prevention of Necroptosis and Ferroptosis. Eur. J. Pharmacol. 2021, 898, 173999. [Google Scholar] [CrossRef]
- Wang, L.; Lv, X.; Tian, J.; Wang, X.; Wu, Y.; Liu, H.R. Cardioprotective Effect of Nec-1 in Rats Subjected to MI/R: Downregulation of Autophagy-Like Cell Death. Cardiovasc. Ther. 2021, 2021, 9956814. [Google Scholar] [CrossRef]
- Dmitriev, Y.; Minasian, S.; Dracheva, A.; Karpov, A.; Chefu, S.; Demchenko, E.; Galagudza, M. Necrostatin 7 Limits Myocardial Infarct Size and Reduces Cardiac Remodeling After Permanent Coronary Occlusion in Rats. Circulation 2014, 130, A17348. [Google Scholar] [CrossRef]
- Wiscovitch-Russo, R.; Ibáñez-Prada, E.D.; Serrano-Mayorga, C.C.; Sievers, B.L.; Engelbride, M.A.; Padmanabhan, S.; Tan, G.S.; Vashee, S.; Bustos, I.G.; Pachecho, C.; et al. Major Adverse Cardiovascular Events Are Associated with Necroptosis during Severe COVID-19. Crit. Care 2023, 27, 155. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, Y.V.; Minasian, S.M.; Demchenko, E.A.; Galagudza, M.M. Study of Cardioprotective Effects of Necroptosis Inhibitors on Isolated Rat Heart Subjected to Global Ischemia-Reperfusion. Bull. Exp. Biol. Med. 2013, 155, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Mao, X.; Li, L.; Tong, Y.; Huang, Y.; Lan, Y.; Jiang, H. Necrostatin-1 Inhibits Hmgb1-IL-23/IL-17 Pathway and Attenuates Cardiac Ischemia Reperfusion Injury. Transpl. Int. 2014, 27, 1077–1085. [Google Scholar] [CrossRef]
- Koudstaal, S.; Oerlemans, M.I.F.J.; Van der Spoel, T.I.G.; Janssen, A.W.F.; Hoefer, I.E.; Doevendans, P.A.; Sluijter, J.P.G.; Chamuleau, S.A.J. Necrostatin-1 Alleviates Reperfusion Injury Following Acute Myocardial Infarction in Pigs. Eur. J. Clin. Investig. 2015, 45, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Lukenaite, B.; Griciune, E.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Necroptosis in Solid Organ Transplantation: A Literature Overview. Int. J. Mol. Sci. 2022, 23, 3677. [Google Scholar] [CrossRef]
- Apaijai, N.; Moisescu, D.M.; Palee, S.; McSweeney, C.M.; Saiyasit, N.; Maneechote, C.; Boonnag, C.; Chattipakorn, N.; Chattipakorn, S.C. Pretreatment with PCSK9 Inhibitor Protects the Brain Against Cardiac Ischemia/Reperfusion Injury Through a Reduction of Neuronal Inflammation and Amyloid Beta Aggregation. J. Am. Heart Assoc. 2019, 8, e010838. [Google Scholar] [CrossRef]
- Benjanuwattra, J.; Apaijai, N.; Chunchai, T.; Kerdphoo, S.; Jaiwongkam, T.; Arunsak, B.; Wongsuchai, S.; Chattipakorn, N.; Chattipakorn, S.C. Metformin Preferentially Provides Neuroprotection Following Cardiac Ischemia/Reperfusion in Non-Diabetic Rats. Biochim. Biophys. Acta. Mol. Basis Dis. 2020, 1866, 165893. [Google Scholar] [CrossRef]
- Liao, S.; Apaijai, N.; Luo, Y.; Wu, J.; Chunchai, T.; Singhanat, K.; Arunsak, B.; Benjanuwattra, J.; Chattipakorn, N.; Chattipakorn, S.C. Cell Death Inhibitors Protect against Brain Damage Caused by Cardiac Ischemia/Reperfusion Injury. Cell Death Discov. 2021, 7, 312. [Google Scholar] [CrossRef]
- Dmitriev, Y.V.; Karpov, A.A.; Dracheva, A.V.; Minasian, S.M.; Chefu, S.G.; Vasina, L.V.; Demchenko, E.A.; Galagudza, I.M.M. Cardioprotfetive Effects of Necrostatin-7 in the Rat Model of Permanent Coronary Occlusion. Ross. Fiziol. Zh. Im. I. M. Sechenova 2015, 101, 408–414. [Google Scholar]
- Peters, M.C.; Neef, K.; Markovska, A.; Oerlemans, M.I.F.; Chamuleau, S.A.J.; Sluijter, J.P.G. A Novel Receptor-Interacting Protein-1 (RIP1) Inhibitor (547) Protects Human Cardiac Cells from Ischemia/Reperfusion-Triggered Necroptotic Cell Death. Cardiovasc. Res. 2022, 118, cvac066-055. [Google Scholar] [CrossRef]
- Yang, P.; Feng, J.; Peng, Q.; Liu, X.; Fan, Z. Advanced Glycation End Products: Potential Mechanism and Therapeutic Target in Cardiovascular Complications under Diabetes. Oxid. Med. Cell. Longev. 2019, 2019, 9570616. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Qian, J.; Cao, J.; Wang, X.; Zhang, W.; Zhang, J. Ca2+/Calmodulin-Dependent Protein Kinase II Regulation by Inhibitor of Receptor Interacting Protein Kinase 3 Alleviates Necroptosis in Glycation End Products-Induced Cardiomyocytes Injury. Int. J. Mol. Sci. 2022, 23, 6988. [Google Scholar] [CrossRef] [PubMed]
- Tekin, T.; Cicek, B.; Konyaligil, N. Regulatory Peptide Nesfatin-1 and Its Relationship with Metabolic Syndrome. Eurasian J. Med. 2019, 51, 18420. [Google Scholar] [CrossRef] [PubMed]
- Angelone, T.; Filice, E.; Pasqua, T.; Amodio, N.; Galluccio, M.; Montesanti, G.; Quintieri, A.M.; Cerra, M.C. Nesfatin-1 as a Novel Cardiac Peptide: Identification, Functional Characterization, and Protection against Ischemia/Reperfusion Injury. Cell. Mol. Life Sci. 2013, 70, 495–509. [Google Scholar] [CrossRef]
- Qiao, S.; Zhao, W.J.; Li, H.Q.; Ao, G.Z.; An, J.Z.; Wang, C.; Zhang, H.L. Necrostatin-1 Analog DIMO Exerts Cardioprotective Effect against Ischemia Reperfusion Injury by Suppressing Necroptosis via Autophagic Pathway in Rats. Pharmacology 2021, 106, 189–201. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health Benefits of Resveratrol: Evidence from Clinical Studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, H.; Peng, J.; He, J.; Tang, M.; Yan, S.; Rong, J.; Li, J.; Zheng, Z.; Wang, H.; et al. Resveratrol Inhibits Necroptosis by Mediating the TNF-Alpha/RIP1/RIP3/MLKL Pathway in Myocardial Hypoxia/Reoxygenation Injury. Acta Biochim. Biophys. Sin. 2021, 53, 430–437. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.; Huang, J.; Yao, Z.; Yu, J.; Zhang, W.; Zhang, L.; Wang, Z.; Zhuang, C. Discovery of Bardoxolone Derivatives as Novel Orally Active Necroptosis Inhibitors. Eur. J. Med. Chem. 2021, 212, 113030. [Google Scholar] [CrossRef]
- Zhu, K.; Guo, J.; Yu, X.; Wang, Q.; Yan, C.; Qiu, Q.; Tang, W.; Huang, X.; Mu, H.; Dou, L.; et al. Polypeptide Globular Adiponectin Ameliorates Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury by Inhibiting Both Apoptosis and Necroptosis. J. Immunol. Res. 2021, 2021, 1815098. [Google Scholar] [CrossRef]
- Ren, J.; Fu, L.; Nile, S.H.; Zhang, J.; Kai, G. Salvia Miltiorrhiza in Treating Cardiovascular Diseases: A Review on Its Pharmacological and Clinical Applications. Front. Pharmacol. 2019, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Liu, Y.; Dong, Z. Tanshinone I Alleviates Motor and Cognitive Impairments via Suppressing Oxidative Stress in the Neonatal Rats after Hypoxic-Ischemic Brain Damage. Mol. Brain 2017, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Yuan, R.; Chen, X.; He, J.; Chen, Y.; Zhang, C.; Sun, K.; Yang, S.; Liu, Z.; Gao, H. Tanshinone I Exerts Cardiovascular Protective Effects in Vivo and in Vitro through Inhibiting Necroptosis via Akt/Nrf2 Signaling Pathway. Chin. Med. 2021, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Lee, J.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Arctiin Is a Pharmacological Inhibitor of STAT3 Phosphorylation at Tyrosine 705 Residue and Potentiates Bortezomib-Induced Apoptotic and Anti-Angiogenic Effects in Human Multiple Myeloma Cells. Phytomedicine 2019, 55, 282–292. [Google Scholar] [CrossRef]
- Li, J.; Yuan, Y.-P.; Xu, S.-C.; Zhang, N.; Xu, C.-R.; Wan, C.-X.; Ren, J.; Zeng, X.-F.; Tang, Q.-Z. Arctiin Protects against Cardiac Hypertrophy through Inhibiting MAPKs and AKT Signaling Pathways. J. Pharmacol. Sci. 2017, 135, 97–104. [Google Scholar] [CrossRef]
- Chen, H.; Tang, L.-J.; Tu, H.; Zhou, Y.-J.; Li, N.-S.; Luo, X.-J.; Peng, J. Arctiin Protects Rat Heart against Ischemia/Reperfusion Injury via a Mechanism Involving Reduction of Necroptosis. Eur. J. Pharmacol. 2020, 875, 173053. [Google Scholar] [CrossRef]
- Xu, W.; Huang, M.; Zhang, Y.; Li, H.; Zheng, H.; Yu, L.; Chu, K.; Lin, Y.; Chen, L. Extracts of Bauhinia championii (Benth.) Benth. Attenuate the Inflammatory Response in a Rat Model of Collagen-Induced Arthritis. Mol. Med. Rep. 2016, 13, 4167–4174. [Google Scholar] [CrossRef]
- Lin, J.M.; Lin, C.C.; Chen, M.F.; Ujiie, T.; Takada, A. Studies on Taiwan Folk Medicine, Thang-Kau-Tin (II): Measurement of Active Oxygen Scavenging Activity Using an ESR Technique. Am. J. Chin. Med. 1995, 23, 43–51. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Chen, W.-Y.; Chung, C.-H.; Kuo, C.-L.; Lee, A.-S. Cardiac Protection of Bauhinia Championii against Reperfusion Injury. Environ. Toxicol. 2020, 35, 774–782. [Google Scholar] [CrossRef]
- Liu, X.; Gu, J.; Fan, Y.; Shi, H.; Jiang, M. Baicalin Attenuates Acute Myocardial Infarction of Rats via Mediating the Mitogen-Activated Protein Kinase Pathway. Biol. Pharm. Bull. 2013, 36, 988–994. [Google Scholar] [CrossRef]
- Liou, S.-F.; Hsu, J.-H.; Liang, J.-C.; Ke, H.-J.; Chen, I.-J.; Wu, J.-R.; Yeh, J.-L. San-Huang-Xie-Xin-Tang Protects Cardiomyocytes against Hypoxia/Reoxygenation Injury via Inhibition of Oxidative Stress-Induced Apoptosis. J. Nat. Med. 2012, 66, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, Q.; Qi, J.; Yu, H.; Wang, C.; Wang, X.; Ren, Y.; Yang, F. Promoting Effect of Baicalin on Nitric Oxide Production in CMECs via Activating the PI3K-AKT-ENOS Pathway Attenuates Myocardial Ischemia-Reperfusion Injury. Phytomedicine 2019, 63, 153035. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, H.; Yu, H.; Yu, L.; Ma, H.; Zhang, H. Ginsenoside Rg2 Ameliorates Myocardial Ischemia/Reperfusion Injury by Regulating TAK1 to Inhibit Necroptosis. Front. Cardiovasc. Med. 2022, 9, 824657. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Wang, R.; Lu, X.; Wang, Y.; Duan, M.; Li, H.; Fan, X.; Wang, S. Pharmacokinetics, Tissue Distribution and Excretion of Saponins after Intravenous Administration of ShenMai Injection in Rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1128, 121777. [Google Scholar] [CrossRef] [PubMed]
- Techiryan, G.; Weil, B.R.; Palka, B.A.; Canty, J.M. Effect of Intracoronary Metformin on Myocardial Infarct Size in Swine. Circ. Res. 2018, 123, 986–995. [Google Scholar] [CrossRef]
- Birnbaum, Y.; Ye, R.; Chen, H.; Carlsson, L.; Whatling, C.; Fjellström, O.; Ryberg, E.; Ye, Y. Recombinant Apyrase (AZD3366) Against Myocardial Reperfusion Injury. Cardiovasc. Drugs Ther. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Yu, S.; Luo, Z.; Chen, Y.; Liu, Q.; Hua, F.; Xu, G.; Yu, P. Sevoflurane Postconditioning Protects Rat Hearts against Ischemia-Reperfusion Injury via the Activation of PI3K/AKT/MTOR Signaling. Sci. Rep. 2014, 4, 7317. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, J.; Yu, S.; Luo, Z.; Hua, F.; Yuan, L.; Zhou, Z.; Liu, Q.; Du, X.; Chen, S.; et al. Protective Effect of Sevoflurane Postconditioning against Cardiac Ischemia/Reperfusion Injury via Ameliorating Mitochondrial Impairment, Oxidative Stress and Rescuing Autophagic Clearance. PLoS ONE 2015, 10, e0134666. [Google Scholar] [CrossRef]
- Li, X.; Gong, W.; Wang, H.; Li, T.; Attri, K.S.; Lewis, R.E.; Kalil, A.C.; Bhinderwala, F.; Powers, R.; Yin, G.; et al. O-GlcNAc Transferase Suppresses Inflammation and Necroptosis by Targeting Receptor-Interacting Serine/Threonine-Protein Kinase 3. Immunity 2019, 50, 576–590.e6. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, P.; Hua, F.; Hu, Y.; Xiao, F.; Liu, Q.; Huang, D.; Deng, F.; Wei, G.; Deng, W.; et al. Sevoflurane Postconditioning Reduces Myocardial Ischemia Reperfusion Injury-Induced Necroptosis by up-Regulation of OGT-Mediated O-GlcNAcylated RIPK3. Aging 2020, 12, 25452–25468. [Google Scholar] [CrossRef]
- Greco, M.; Landoni, G.; Biondi-Zoccai, G.; Cabrini, L.; Ruggeri, L.; Pasculli, N.; Giacchi, V.; Sayeg, J.; Greco, T.; Zangrillo, A. Remifentanil in Cardiac Surgery: A Meta-Analysis of Randomized Controlled Trials. J. Cardiothorac. Vasc. Anesth. 2012, 26, 110–116. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Bloniarz, D.; Horeczy, B.; Zurek, S.; Kurowicki, A.; Woloszczuk-Gebicka, B.; Widenka, K.; Wnuk, M. Remifentanil Preconditioning Protects against Hypoxia-Induced Senescence and Necroptosis in Human Cardiac Myocytes in Vitro. Aging 2020, 12, 13924–13938. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Zhang, X.; Lu, X.; Wu, J.; Li, S. Reduced Mitochondrial Response Sensitivity Is Involved in the Anti-apoptotic Effect of Dexmedetomidine Pretreatment in Cardiomyocytes. Int. J. Mol. Med. 2018, 41, 2328–2338. [Google Scholar] [CrossRef]
- Yin, W.; Wang, C.; Peng, Y.; Yuan, W.; Zhang, Z.; Liu, H.; Xia, Z.; Ren, C.; Qian, J. Dexmedetomidine Alleviates H(2)O(2)-Induced Oxidative Stress and Cell Necroptosis through Activating of A2-Adrenoceptor in H9C2 Cells. Mol. Biol. Rep. 2020, 47, 3629–3639. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Li, Z.; Nguyen, H.; Young, N.; Shi, P.; Fleming, N.; Liu, H. Perioperative Dexmedetomidine Improves Outcomes of Cardiac Surgery. Circulation 2013, 127, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Ibacache, M.; Sanchez, G.; Pedrozo, Z.; Galvez, F.; Humeres, C.; Echevarria, G.; Duaso, J.; Hassi, M.; Garcia, L.; Díaz-Araya, G.; et al. Dexmedetomidine Preconditioning Activates Pro-Survival Kinases and Attenuates Regional Ischemia/Reperfusion Injury in Rat Heart. Biochim. Biophys. Acta 2012, 1822, 537–545. [Google Scholar] [CrossRef]
- Zhang, F.; Cao, X.; Zhao, C.; Chen, L.; Chen, X. Empagliflozin Activates JAK2/STAT3 Signaling and Protects Cardiomyocytes from Hypoxia/Reoxygenation Injury under High Glucose Conditions. J. Thromb. Thrombolysis 2022, 55, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, P.E.; Efentakis, P.; Abu Qourah, F.; Femminò, S.; Makridakis, M.; Kanaki, Z.; Varela, A.; Tsoumani, M.; Davos, C.H.; Dimitriou, C.A.; et al. Chronic Empagliflozin Treatment Reduces Myocardial Infarct Size in Nondiabetic Mice Through STAT-3-Mediated Protection on Microvascular Endothelial Cells and Reduction of Oxidative Stress. Antioxid. Redox Signal. 2021, 34, 551–571. [Google Scholar] [CrossRef]
- Jones, S.P.; Trocha, S.D.; Lefer, D.J. Pretreatment with Simvastatin Attenuates Myocardial Dysfunction after Ischemia and Chronic Reperfusion. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 2059–2064. [Google Scholar] [CrossRef]
- Naseroleslami, M.; Niri, N.M.; Akbarzade, I.; Sharifi, M.; Aboutaleb, N. Simvastatin-Loaded Nano-Niosomes Confer Cardioprotection against Myocardial Ischemia/Reperfusion Injury. Drug Deliv. Transl. Res. 2022, 12, 1423–1432. [Google Scholar] [CrossRef]
- Zhu, H.; Tan, Y.; Du, W.; Li, Y.; Toan, S.; Mui, D.; Tian, F.; Zhou, H. Phosphoglycerate Mutase 5 Exacerbates Cardiac Ischemia-Reperfusion Injury through Disrupting Mitochondrial Quality Control. Redox Biol. 2021, 38, 101777. [Google Scholar] [CrossRef]
- Parks, R.J.; Menazza, S.; Holmström, K.M.; Amanakis, G.; Fergusson, M.; Ma, H.; Aponte, A.M.; Bernardi, P.; Finkel, T.; Murphy, E. Cyclophilin D-Mediated Regulation of the Permeability Transition Pore Is Altered in Mice Lacking the Mitochondrial Calcium Uniporter. Cardiovasc. Res. 2019, 115, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Wang, J.; Fang, D.; Fang, T.; Yu, Y.; Zhang, W.; Shen, L.; Li, Z.; Wang, H.; Ye, H.; et al. Activation of ALDH2 Attenuates High Glucose Induced Rat Cardiomyocyte Fibrosis and Necroptosis. Free Radic. Biol. Med. 2020, 146, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, Q.; Toan, S.; Wang, J.; Zhou, H.; Liang, J. SERCA Overexpression Reduces Reperfusion-Mediated Cardiac Microvascular Damage through Inhibition of the Calcium/MCU/MPTP/Necroptosis Signaling Pathways. Redox Biol. 2020, 36, 101659. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, B.; Sun, S.; Cao, S.; Zhai, X.; Zhang, C.; Zhang, Q.; Yuan, Q.; Sun, Y.; Xue, M.; et al. Inhibition of Adenosine Kinase Attenuates Myocardial Ischaemia/Reperfusion Injury. J. Cell. Mol. Med. 2021, 25, 2931–2943. [Google Scholar] [CrossRef]
- Gao, X.-Q.; Liu, C.-Y.; Zhang, Y.-H.; Wang, Y.-H.; Zhou, L.-Y.; Li, X.-M.; Wang, K.; Chen, X.-Z.; Wang, T.; Ju, J.; et al. The CircRNA CNEACR Regulates Necroptosis of Cardiomyocytes through Foxa2 Suppression. Cell Death Differ. 2022, 29, 527–539. [Google Scholar] [CrossRef]
- Wang, Q.; Park, K.H.; Geng, B.; Chen, P.; Yang, C.; Jiang, Q.; Yi, F.; Tan, T.; Zhou, X.; Bian, Z.; et al. MG53 Inhibits Necroptosis Through Ubiquitination-Dependent RIPK1 Degradation for Cardiac Protection Following Ischemia/Reperfusion Injury. Front. Cardiovasc. Med. 2022, 9, 868632. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Wang, B.-J.; Ma, M.; Yu, K.; Zhang, Q.; Zhang, X.-W. MicroRNA-325-3p Protects the Heart after Myocardial Infarction by Inhibiting RIPK3 and Programmed Necrosis in Mice. BMC Mol. Biol. 2019, 20, 17. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Bátkai, S.; Patel, V.; Kashiwaya, Y.; Liaudet, L.; Evgenov, O.V.; Mackie, K.; Haskó, G.; Pacher, P. CB1 Cannabinoid Receptors Promote Oxidative Stress and Cell Death in Murine Models of Doxorubicin-Induced Cardiomyopathy and in Human Cardiomyocytes. Cardiovasc. Res. 2010, 85, 773–784. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Dong, X.; Zhu, R.; Ye, Y.; Li, L.; Jiang, Y. Pharmacological Activation of CB2 Receptor Protects against Ethanol-Induced Myocardial Injury Related to RIP1/RIP3/MLKL-Mediated Necroptosis. Mol. Cell. Biochem. 2020, 474, 1–14. [Google Scholar] [CrossRef]
- Qiu, Q.; Shen, T.; Yu, X.; Jia, N.; Zhu, K.; Wang, Q.; Liu, B.; He, Q. Cardiac Shock Wave Therapy Alleviates Hypoxia/Reoxygenation-Induced Myocardial Necroptosis by Modulating Autophagy. Biomed. Res. Int. 2021, 2021, 8880179. [Google Scholar] [CrossRef]
- Li, L.; Lin, L.; Lei, S.; Shi, S.; Chen, C.; Xia, Z. Maslinic Acid Inhibits Myocardial Ischemia-Reperfusion Injury-Induced Apoptosis and Necroptosis via Promoting Autophagic Flux. DNA Cell Biol. 2022, 41, 487–497. [Google Scholar] [CrossRef]
- Afousi, A.G.; Gaeini, A.; Rakhshan, K.; Naderi, N.; Azar, A.D.; Aboutaleb, N. Targeting Necroptotic Cell Death Pathway by High-Intensity Interval Training (HIIT) Decreases Development of Post-Ischemic Adverse Remodelling after Myocardial Ischemia/Reperfusion Injury. J. Cell Commun. Signal. 2019, 13, 255–267. [Google Scholar] [CrossRef]
- Davidson, S.M.; Ferdinandy, P.; Andreadou, I.; Bøtker, H.E.; Heusch, G.; Ibáñez, B.; Ovize, M.; Schulz, R.; Yellon, D.M.; Hausenloy, D.J.; et al. Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, D.; Zhu, P.; Ma, Q.; Toan, S.; Wang, J.; Hu, S.; Chen, Y.; Zhang, Y. Inhibitory Effect of Melatonin on Necroptosis via Repressing the Ripk3-PGAM5-CypD-MPTP Pathway Attenuates Cardiac Microvascular Ischemia-Reperfusion Injury. J. Pineal Res. 2018, 65, e12503. [Google Scholar] [CrossRef] [PubMed]
- Boisguérin, P.; Covinhes, A.; Gallot, L.; Barrère, C.; Vincent, A.; Busson, M.; Piot, C.; Nargeot, J.; Lebleu, B.; Barrère-Lemaire, S. A Novel Therapeutic Peptide Targeting Myocardial Reperfusion Injury. Cardiovasc. Res. 2020, 116, 633–644. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).