The Association between Cardiovascular Risk Factors and Carotid Intima-Media Thickness in 42,726 Adults in UK Biobank: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
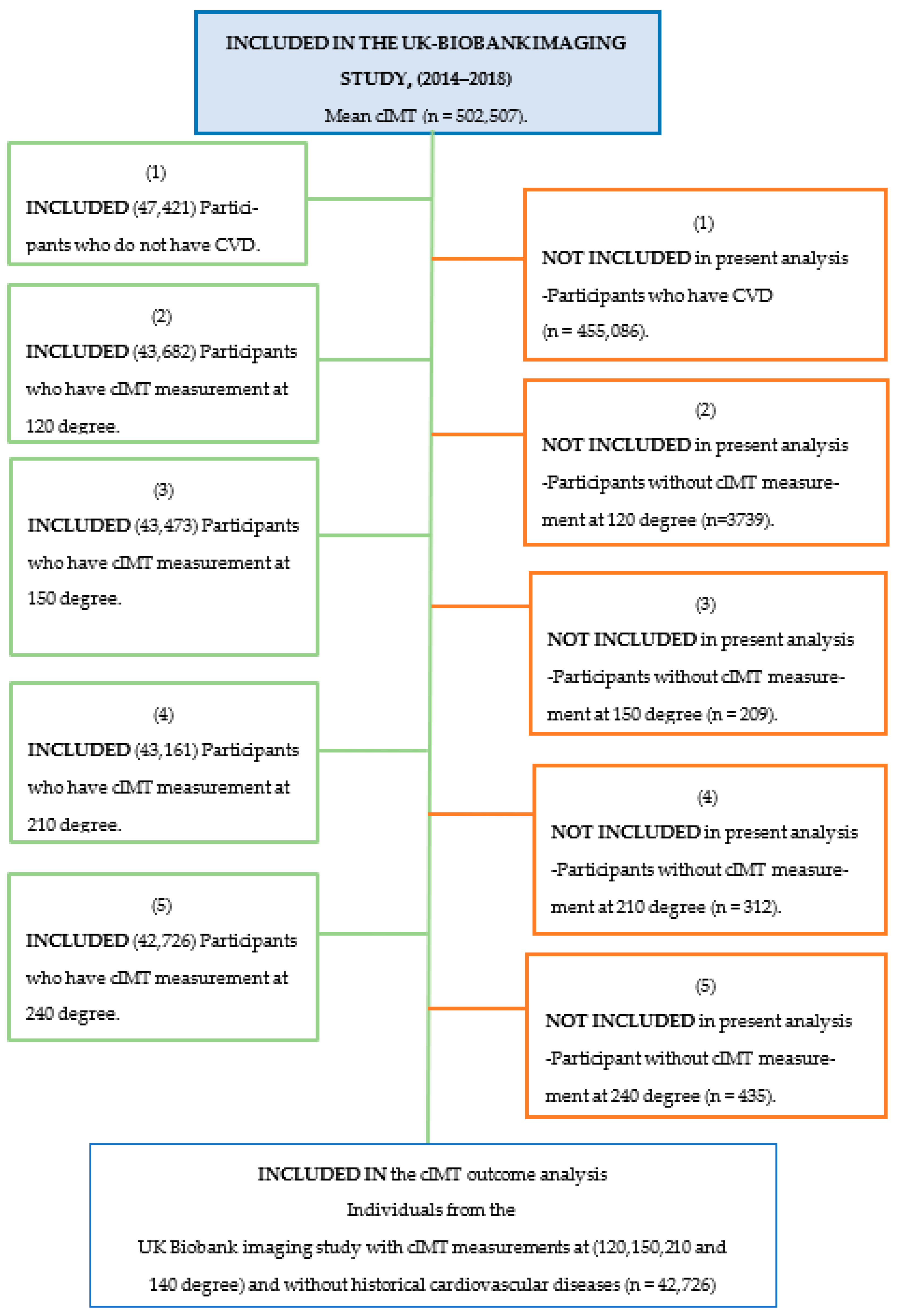
2.1. UK Biobank
2.2. Predictors Measurement (2006–2010)
2.3. Outcomes Measurement (2014–2018)
2.4. Statistical Analyses
2.5. Patient Involvement
3. Results
3.1. Baseline Characteristics
3.2. The Association between cIMT and Cardiovascular Risk Factors
3.2.1. Correlations
3.2.2. CIMT, Single Factor Analysis in the Whole Cohort
3.2.3. Multiple Regression Model of cIMT with Cardiovascular Risk Factors
4. Discussion
4.1. Principal Findings
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chambless, L.E.; Folsom, A.R.; Davis, V.; Sharrett, R.; Heiss, G.; Sorlie, P.; Szklo, M.; Howard, G.; Evans, G.W. Risk factors for progression of common carotid atherosclerosis: The Atherosclerosis Risk in Communities Study, 1987–1998. Am. J. Epidemiol. 2002, 155, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.T.; Charakida, M.; Georgiopoulos, G.; Dangardt, F.; Wade, K.H.; Rapala, A.; Bhowruth, D.J.; Nguyen, H.C.; Muthurangu, V.; Shroff, R.; et al. Determinants of Intima-Media Thickness in the Young: The ALSPAC Study. JACC Cardiovasc. Imaging 2021, 14, 468–478. [Google Scholar] [PubMed]
- Hedblad, B.; Nilsson, P.; Janzon, L.; Berglund, G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabet. Med. A J. Br. Diabet. Assoc. 2000, 17, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.R., 3rd; Raichlen, J.S.; Riley, W.A.; Evans, G.W.; Palmer, M.K.; O’Leary, D.H.; Grobbee, D.E.; Bots, M.L. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: The METEOR Trial. JAMA 2007, 297, 1344–1353. [Google Scholar] [CrossRef]
- Iglseder, B.; Cip, P.; Malaimare, L.; Ladurner, G.; Paulweber, B. The Metabolic Syndrome Is a Stronger Risk Factor for Early Carotid Atherosclerosis in Women Than in Men. Stroke 2005, 36, 1212–1217. [Google Scholar]
- Rundek, T.; Blanton, S.H.; Bartels, S.; Dong, C.; Raval, A.; Demmer, R.T.; Cabral, D.; Elkind, M.S.V.; Sacco, R.L.; Desvarieux, M. Traditional risk factors are not major contributors to the variance in carotid intima-media thickness. Stroke 2013, 44, 2101–2108. [Google Scholar] [CrossRef]
- Baldassarre, D.; De Jong, A.; Amato, M.; Werba, P.J.; Castelnuovo, S.; Frigerio, B.; Veglia, F.; Tremoli, E.; Sirtori, C.R. Carotid intima-media thickness and markers of inflammation, endothelial damage and hemostasis. Ann. Med. 2008, 40, 21–44. [Google Scholar]
- Ernst, E.; Resch, K.L. Fibrinogen as a cardiovascular risk factor: A meta-analysis and review of the literature. Ann. Intern. Med. 1993, 118, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Sekikawa, A.; Sawamura, T.; Kadowaki, T.; Barinas-Mitchell, E.; Mackey, R.H.; Kadota, A.; Evans, R.W.; Edmundowicz, D.; Higashiyama, A.; et al. LOX-1 ligands containing apolipoprotein B and carotid intima-media thickness in middle-aged community-dwelling US Caucasian and Japanese men. Atherosclerosis 2013, 229, 240–245. [Google Scholar]
- Hulthe, J.; Fagerberg, B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study). Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1162–1167. [Google Scholar] [CrossRef]
- Liu, B.; Ni, J.; Shi, M.; Bai, L.; Zhan, C.; Lu, H.; Wu, Y.; Tu, J.; Ning, X.; Hao, J.; et al. Carotid Intima-media Thickness and its Association with Conventional Risk Factors in Low-income Adults: A Population-based Cross-Sectional Study in China. Sci. Rep. 2017, 7, 41500. [Google Scholar]
- Epure, A.M.; Leyvraz, M.; Mivelaz, Y.; Di Bernardo, S.; da Costa, B.R.; Chiolero, A.; Sekarski, N. Risk factors and determinants of carotid intima-media thickness in children: Protocol for a systematic review and meta-analysis. BMJ Open 2018, 8, e019644. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, T.J.; Holliday, J.; Gibson, L.M.; Garratt, S.; Oesingmann, N.; Alfaro-Almagro, F.; Bell, J.D.; Boultwood, C.; Collins, R.; Conroy, M.C.; et al. The UK Biobank imaging enhancement of 100,000 participants: Rationale, data collection, management and future directions. Nat. Commun. 2020, 11, 2624. [Google Scholar] [PubMed]
- UK Biobank. UK Biobank Touch-Screen Questionnaire [Internet]. 2019. Available online: https://www.ukbiobank.ac.uk/wp-content/uploads/2011/06/Touch_screen_questionnaire.pdf?phpMyAdmin=trmKQlYdjjnQIgJ%2CfAzikMhEnx6 (accessed on 1 September 2020).
- Biobank, U. Imaging Modality: Carodtif Ultrasound. Available online: http://biobank.ndph.ox.ac.uk/showcase/showcase/docs/carult_explan_doc.pdf (accessed on 20 August 2020).
- Howard, G.; Burke, G.L.; Evans, G.W.; Crouse, J.R., 3rd; Riley, W.; Arnett, D.; de Lacy, R.; Heiss, G. Relations of intimal-medial thickness among sites within the carotid artery as evaluated by B-mode ultrasound. ARIC Investigators. Atherosclerosis Risk in Communities. Stroke 1994, 25, 1581–1587. [Google Scholar] [CrossRef]
- Price, J.F.; Tzoulaki, I.; Lee, A.J.; Fowkes, F.G. Ankle brachial index and intima media thickness predict cardiovascular events similarly and increased prediction when combined. J. Clin. Epidemiol. 2007, 60, 1067–1075. [Google Scholar] [PubMed]
- Peters, S.A.E.; Lind, L.; Palmer, M.K.; Grobbee, D.E.; Crouse, J.R.; O’leary, D.H.; Evans, G.W.; Raichlen, J.; Bots, M.L.; Ruijter, H.M.D.; et al. Increased age, high body mass index and low HDL-C levels are related to an echolucent carotid intima-media: The METEOR study. J. Intern. Med. 2012, 272, 257–266. [Google Scholar]
- Howard, G.; Sharrett, A.R.; Heiss, G.; Evans, G.W.; Chambless, L.E.; Riley, W.A.; Burke, G.L. Carotid artery intimal-medial thickness distribution in general populations as evaluated by B-mode ultrasound. ARIC Investigators. Stroke 1993, 24, 1297–1304. [Google Scholar] [CrossRef]
- Uchmanowicz, I.; Łoboz-Rudnicka, M.; Jaroch, J.; Bociąga, Z.; Rzyczkowska, B.; Polański, J.; Dudek, K.; Szuba, A.; Łoboz-Grudzień, K. Impact of cardiovascular risk factors on carotid intima-media thickness: Sex differences. Clin. Interv. Aging 2016, 11, 721–731. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Girerd, N.; Bozec, E.; Machu, J.L.; Boivin, J.; London, G.M.; Zannad, F.; Rossignol, P. Intima-Media Thickness Is Linearly and Continuously Associated with Systolic Blood Pressure in a Population-Based Cohort (STANISLAS Cohort Study). J. Am. Heart Assoc. 2016, 5, e003529. [Google Scholar]
- Gao, L.; Bai, L.; Shi, M.; Ni, J.; Lu, H.; Wu, Y.; Tu, J.; Ning, X.; Wang, J.; Li, Y. Association between carotid intima-media thickness and fasting blood glucose level: A population-based cross-sectional study among low-income adults in rural China. J. Diabetes Investig. 2017, 8, 788–797. [Google Scholar]
- Zanchetti Al, C.G.; Bond, M.G.; Gallus, G.V.; Veglia, F.; Ventura, A.; Mancia, G.; Baggio, G.; Sampieri, L.; Rubba, P.; Collatina, S.; et al. Systolic and pulse blood pressures (but not diastolic blood pressure and serum cholesterol) are associated with alterations in carotid intima-media thickness in the moderately hypercholesterolaemic hypertensive patients of the Plaque Hypertension Lipid Lowering Italian Study. J. Hypertens. 2001, 19, 79–88. [Google Scholar]
- Mannami, T.; Konishi, M.; Baba, S.; Nishi, N.; Terao, A. Prevalence of asymptomatic carotid atherosclerotic lesions detected by high-resolution ultrasonography and its relation to cardiovascular risk factors in the general population of a Japanese city: The Suita study. Stroke 1997, 28, 518–525. [Google Scholar] [CrossRef]
- Lakka, T.A.; Salonen, R.; Kaplan, G.A.; Salonen, J.T. Blood pressure and the progression of carotid atherosclerosis in middle-aged men. Hypertension 1999, 34, 51–56. [Google Scholar] [CrossRef]
- Roman, M.J.; Saba, P.S.; Pini, R.; Spitzer, M.; Pickering, T.G.; Rosen, S.; Alderman, M.H.; Devereux, R.B. Parallel cardiac and vascular adaptation in hypertension. Circulation 1992, 86, 1909–1918. [Google Scholar]
- Di Bello, V.; Carerj, S.; Perticone, F.; Benedetto, F.; Palombo, C.; Talini, E.; Giannini, D.; La Carrubba, S.; Antonini-Canterin, F.; Di Salvo, G.; et al. Carotid intima-media thickness in asymptomatic patients with arterial hypertension without clinical cardiovascular disease: Relation with left ventricular geometry and mass and coexisting risk factors. Angiology 2009, 60, 705–713. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar]
- Suurkula, M.A.S.; Fagerberg, B.; Wendelhang, I.; Widgren, B.; Wickstrand, J. Ultrasound evaluation of atherosclerotic manifestation in carotid artery in high- risk hypertensive patients. Risk Intervention Study (RIS) Group. Arterioscler. Thromb. 1994, 14, 1297–1304. [Google Scholar]
- Ku, D.N.; Giddens, D.P.; Zarins, C.K.; Glagov, S. Pulsatile flow and atherosclerosis in the human carotid bifurcation: Positive plaque location and low oscillating shear stress. Arteriosclerosis 1985, 5, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.T.; Salonen, R. Association of Serum Low Density Lipoprotein Cholesterol, Smoking and Hypertension with Different Manifestations of Atherosclerosis. Int. J. Epidemiol. 1990, 19, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Salonen, R.; Salonen, J.T. Determinants of carotid intima-media thickness: A population-based ultrasonography study in eastern Finnish men. J. Intern. Med. 1991, 229, 225–231. [Google Scholar]
- Veldsman, T.; Swanepoel, M.; Monyeki, M.A.; Brits, J.S.; Malan, L. The Role of Physical Activity Status in the Relationship between Obesity and Carotid Intima-Media Thickness (CIMT) in Urban South African Teachers: The SABPA Study. Int. J. Environ. Res. Public Health 2022, 19, 6348. [Google Scholar] [CrossRef]
- Xu, W.; Hang, J.; Cao, T.; Shi, R.; Zeng, W.; Deng, Y.; Gao, W.; Zhao, Y.; Guo, L. Job stress and carotid intima-media thickness in Chinese workers. J. Occup. Health 2010, 52, 257–262. [Google Scholar]
- Vibha, D.; Prasad, K.; Dwivedi, S.N.; Kant, S.; Pandit, A.K.; Tiemeier, H.; Srivastava, A.K.; Karthikeyan, G.; Garg, A.; Verma, V.; et al. Carotid Intima-Media Thickness (cIMT) and Cognitive Performance: A Population-Based Cross-Sectional Study from North India. Alzheimer Dis. Assoc. Disord. 2023, 37, 35–41. [Google Scholar] [PubMed]
- Bots, M.L.; den Ruijter, H.M. Should we indeed measure carotid intima-media thickness for improving prediction of cardiovascular events after IMPROVE? J. Am. Coll. Cardiol. 2012, 60, 1500–1502. [Google Scholar] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2007, 25, 1105–1187. [Google Scholar]
- Bots, M.L.; Groenewegen, K.A.; Anderson, T.J.; Britton, A.R.; Dekker, J.M.; Engström, G.; Evans, G.W.; de Graaf, J.; Grobbee, D.E.; Hedblad, B.; et al. Common Carotid Intima-Media Thickness Measurements Do Not Improve Cardiovascular Risk Prediction in Individuals with Elevated Blood Pressure. Hypertension 2014, 63, 1173–1181. [Google Scholar]
| Continuous Variables | Mean cIMT (150.5–594) µm Number (mean ± SD) | Mean cIMT (594.25–665) µm Number (mean ± SD) | Mean cIMT (665.25–756.75) µm Number (mean ± SD) | Mean cIMT (757–2126.25) µm Number (mean ± SD) | |
|---|---|---|---|---|---|
| Non-invasive measurement of atherosclerosis | |||||
| Mean cIMT | (n = 10,701), 546 ± 35.1 | (n = 10,663), 629 ± 20 | (n = 10,692), 708 ± 26 | (n = 10,670), 856 ± 91 | |
| Pulse wave arterial stiffness index * | (n = 9058), 9.03 (7.26, 10.86) | (n = 8982), 9.35 (7.38, 11.20) | (n = 8910), 9.61 (7.56, 11.54) | (n = 8913), 9.83 (7.74, 11.86) | |
| Demographic data | |||||
| Age (year) | (n = 10,701), 59.3 ± 7 | (n = 10,663), 62.7 ± 7.1 | (n = 10,692), 65.5 ± 7 | (n = 10,670), 67.9 ± 6.6 | |
| Townsend deprivation index | (n = 10,686), −1.77 ± 2.78 | (n = 10,650), −1.86 ± 2.72 | (n = 10,685), −1.97 ± 2.70 | (n = 10,663), −1.98 ± 2.69 | |
| Anthropometric measurements | |||||
| Waist circumference (cm) | (n = 10,417), 85.5 ± 12.25 | (n = 10,368), 86.8 ± 12.12 | (n = 10,358), 88.3 ± 12.55 | (n = 10,299), 91 ± 12.19 | |
| Hip circumference (cm) | (n = 10,418), 99.89 ± 8.75 | (n = 10,368), 100.47 ± 8.62 | (n = 10,358), 100.88 ± 8.68 | (n = 10,299), 101.8 ± 8.36 | |
| BMI (kg/m2) | (n = 10,385), 25.9 ± 4.29 | (n = 10,344), 26.3 ± 4.25 | (n = 10,324), 26.5 ± 4.32 | (n = 10,265), 26.9 ± 4.25 | |
| Blood pressure measurements | |||||
| Systolic blood pressure (mmHg) | (n = 9522), 130 ± 16.4 | (n = 9466), 136 ± 17.4 | (n = 9351), 140 ± 18 | (n = 9325), 146 ± 18.9 | |
| Diastolic blood pressure | (n = 9522), 78 ± 9.7 | (n = 9466), 78.9 ± 10 | (n = 9351), 78.9 ± 10 | (n = 9325), 78.9 ± 10 | |
| Diabetic measurements | |||||
| Glucose *(mmol/mol) | (n = 9069), 4.83 (4.53, 5.16) | (n = 9128), 4.87 (4.55, 5.21) | (n = 9096), 4.89 (4.58, 5.23) | (n = 9134), 4.92 (4.60, 5.28) | |
| HbA1c (mmol/mol) | (n = 9931), 34 ± 4.41 | (n = 9877), 34.77 ± 4.87 | (n = 9962), 35.22 ± 5.20 | (n = 9906), 35.78 ± 5.67 | |
| Enzyme measurements | |||||
| Alanine aminotransferase * (U/L) | (n = 9987), 18.31 (14, 25.22) | (n = 9979), 18.9 (14.67, 25.86) | (n = 10,004), 19.98 (15.36, 26.83) | (n = 9988), 21 (16.43, 28.09) | |
| Aspartate aminotransferase * (U/L) | (n = 9938), 23.2 (20.10, 27.50) | (n = 9943), 23.80 (20.5, 28) | (n = 9966), 24.40 (21, 28.50) | (n = 9957), 25 (21.70, 29.20) | |
| Lipid measurements | |||||
| Total cholesterol (mmol/L) | (n = 9988), 5.6 ± 1.04 | (n = 9983), 5.7 ± 1.07 | (n = 10,006), 5.78 ± 1.07 | (n = 9988), 5.83 ± 1.12 | |
| HDL-cholesterol (mmol/L) | (n = 9080), 1.50 ± 0.37 | (n = 9136), 1.51 ± 0.38 | (n = 9105), 1.49 ± 0.38 | (n = 9143), 1.42 ± 0.36 | |
| LDL-cholesterol (mmol/L) | (n = 9969), 3.47 ± 0.80 | (n = 9962), 3.56 ± 0.82 | (n = 9980), 3.61 ± 0.82 | (n = 9964), 3.73 ± 0.85 | |
| Triglyceride * | (n ≈ 9980), 1.26 (0.91, 1.86) | (n ≈ 9975), 1.34 (0.95, 1.92) | (n ≈ 10,001), 1.42 (1.02, 2.04) | (n = 9979), 1.53 (1.10, 2.19) | |
| Apolipoprotein-A(g/L) | (n = 9024), 1.56 ± 0.26 | (n = 9086), 1.56 ± 0.27 | (n = 9055), 1.56 ± 0.27 | (n = 9102), 1.51 ± 0.25 | |
| Apolipoprotein-B (g/L) | (n = 9933), 1 ± 0.22 | (n = 9927), 1.02 ± 0.23 | (n = 9956), 1.04 ± 0.23 | (n = 9959), 1.07 ± 0.23 | |
| Inflammatory markers | |||||
| C-reactive protein (mg/L) * | (n = 9969), 0.95 (0.48, 1.94) | (n = 9960), 1.02 (0.53, 2.06) | (n = 9983), 1.11 (0.57, 2.20) | (n = 9968), 2.18 (1.2, 3.63) | |
| Vitamins | |||||
| Vitamin D * (nmol/L) | (n = 9680), 46.20 (32.30, 62.10) | (n = 9611), 48.20 (33.80, 63.20) | (n = 9589), 48.80 (34.60, 63.50) | (n = 9519), 48.90 (34.40, 64.10) | |
| Categorical variables | (number,%) | (number,%) | (number,%) | (number,%) | |
| Diabetes | No | 10,327 (96.5%) | 10,198 (95.6%) | 10,089 (94.4%) | 9953 (93.3%) |
| Yes | 374 (3.5%) | 465 (4.4%) | 603 (5.6%) | 717 (6.7%) | |
| Hypertension | No | 6130 (57.3%) | 4839 (45.4%) | 3946 (36.9%) | 2787 (26.1%) |
| Yes | 4571 (42.7%) | 5824 (54.6%) | 6746 (63.1%) | 7883 (73.9%) | |
| Smoking | Never | 7237 (68.1%) | 6802 (64.4%) | 6519 (61.6%) | 5955 (56.6%) |
| Former | 3002 (28.2%) | 3386 (32.1%) | 3728 (35.2%) | 4183 (39.8%) | |
| Current | 388 (3.7%) | 376 (3.6%) | 336 (3.2%) | 381 (3.6%) | |
| Statin | No | 9122 (85.2%) | 8498 (79.7%) | 8003 (74.9%) | 7284 (68.3%) |
| Yes | 1579 (14.8%) | 2165 (20.3%) | 2689 (25.1%) | 3386 (31.7%) | |
| Ethnicity | White | 10,280 (96.3%) | 10,316 (97%) | 10,395 (97.5%) | 10,350 (97.3%) |
| Black | 52 (0.5%) | 67 (0.6%) | 68 (0.6%) | 94 (0.9%) | |
| South Asian | 134 (1.3%) | 99 (0.9%) | 78 (0.7%) | 72 (0.7%) | |
| Other | 211 (2%) | 156 (1.5%) | 124 (1.2%) | 122 (1.1%) | |
| Sex | Female | 4305 (61.7%) | 4715 (58%) | 3658 (43.1%) | 108 (24.8%) |
| Male | 2673 (38.3%) | 3414 (42%) | 4831 (56.9%) | 328 (75.2%) | |
| Continuous Variables | Mean cIMT | ||
|---|---|---|---|
| Pearson Correlation (r) | p-Value | ||
| Age (year) | 0.4129 | <0.001 | 1 |
| Systolic blood pressure (mmHg) | 0.3074 | <0.001 | |
| Waist circumference (cm) | 0.1648 | <0.001 | |
| Log-Triglycerides(mmol/L) | 0.129 | <0.001 | |
| Apolipoprotein-B (g/L) | 0.1248 | <0.001 | |
| HbA1c (mmol/mol) | 0.1221 | <0.001 | |
| LDL cholesterol (mmol/L) | 0.1041 | <0.001 | |
| Log-Alanine aminotransferase (U/L) | 0.1035 | <0.001 | |
| Log-Aspartate aminotransferase (U/L) | 0.0974 | <0.001 | |
| BMI (kg/m2) | 0.0847 | <0.001 | |
| Log-C-reactive protein(mg/L) | 0.0801 | <0.001 | |
| Total cholesterol (mmol/L) | 0.0766 | <0.001 | |
| log-Glucose (mmol/L) | 0.0663 | <0.001 | |
| Hip circumference (cm) | 0.059 | <0.001 | |
| Pulse wave velocity at baseline (m/s) | 0.055 | <0.001 | |
| Log Vitamin D (nmol/L) | 0.0414 | <0.001 | |
| Diastolic blood pressure (mmHg) | 0.0264 | <0.001 | |
| Townsend deprivation index at recruitment | −0.0309 | <0.001 | |
| Apolipoprotein-A (g/L) | −0.0675 | <0.001 | |
| HDL cholesterol (mmol/L) | −0.0925 | <0.001 | −1 |
| Variables | Simple Linear Regression | Stepwise Regression Analysis | |||
|---|---|---|---|---|---|
| B (95% CI) | (b) | B (95% CI) | (b) | ||
| Age (year) | 6.773, (6.631, 6.915) | 0.413 | 5.87, (5.66, 6.09) | 0.357 | |
| Anthropometric measurements | |||||
| Waist circumference(cm) | 1.648, (1.553, 1.743) | 0.164 | |||
| Hip circumference(cm) | 0.854, (0.715, 0.993) | 0.058 | 0.744, (0.544, 0.945) | 0.049 | |
| BMI (kg/m2) | 2.465, (2.185, 2.745) | 0.084 | |||
| Blood pressure measurements | |||||
| Systolic blood pressure (mmHg) | 2.06, (1.997, 2.126) | 0.307 | 0.77, (0.678, 0.862) | 0.115 | |
| Diastolic blood pressure(mmHg) | 0.327, (0.201, 0.452) | 0.026 | |||
| Diabetic measurements | |||||
| Log-Glucose * (mmol/L) | 55.21, (46.67, 63.74) | 0.066 | |||
| HbA1c (mmol/mol) | 2.999, (2.75, 3.23) | 0.122 | |||
| Enzymes measurements | |||||
| Log-Alanine aminotransferase * (U/L) | 28.69, (25.98, 31.39) | 0.103 | |||
| Log-Aspartate aminotransferase * (U/L) | 46.14, (41.51, 50.77) | 0.097 | |||
| Lipid measurements | |||||
| Total cholesterol (mmol/L) | 8.86, (7.73, 9.99) | 0.076 | |||
| HDL cholesterol (mmol/L) | −30.70, (−34.10, −27.31) | −0.092 | |||
| LDL cholesterol (mmol/L) | 15.73, (14.25, 17.20) | 0.104 | |||
| Apolipoprotein-A (g/L) | −31.94, (−36.79, −27.08) | −0.067 | −25.224 (−31.970, −18.47) | −0.053 | |
| Apolipoprotein-B (g/L) | 68.240, (62.906, 73.574) | 0.124 | 32.99, (25.27, 40.71) | 0.055 | |
| Log-Triglycerides (mmol/L) | 70, (69, 72) | 0.129 | |||
| Other markers | |||||
| Log-C-reactive- Protein (mg/L) * | 9.847, (8.644, 11.051) | 0.080 | |||
| Log-Vitamin D * (nmol/L) | −1.420, (−1.855, −0.984) | −0.031 | 0.169, (0.093, 0.245) | 0.029 | |
| Categorical variables | |||||
| Diabetes | Reference | 683, (681, 684.37) | 0.0634 | 10.97, (3.50, 18.45) | 0.019 |
| Yes | 36.232, (30.82, 41.63) | ||||
| Hypertension | Reference | 650, (648.37, 651.95) | 0.234 | 8.76, (5.11, 12.40) | 0.034 |
| Yes | 59.46, (57.12, 61.80) | ||||
| Smoking | Reference | 675.42, (673.93, 676.91) | 0.099 | 10.96, (8.33, 13.59) | 0.0418 |
| Former | 26.13, (23.61, 28.66) | 0.0151 | |||
| Current | 10.28, (3.78, 28.66) | 21.49, (14.77, 28.20) | 0.0317 | ||
| Ethnicity | Reference | 685.43, (684.22, 684.22) | 0.0162 | ||
| Black | 25.06, (10.40, 39.72) | ||||
| South Asian | −28.35, (−40.93, −15.78) | −0.0214 | |||
| Other | −27.31, (−37.28, −17.35) | −0.026 | |||
| Statin | Reference | 674, (673.38, 676.05) | 0.150 | ||
| Yes | 44.69, (41.90, 47.48) | ||||
| Gender | Reference | 663, (661.76, 664.98) | 0.18 | 25.50, (21.04, 27.04) | 0.124 |
| Male | 45.52, (43.18, 47.85) | ||||
| R2% | 21.6% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlGhibiwi, H.K.; Sarawi, W.S.; Alosaimi, M.E.; Alhusaini, A.M.; Assiri, M.A.; Algarzae, N.K. The Association between Cardiovascular Risk Factors and Carotid Intima-Media Thickness in 42,726 Adults in UK Biobank: A Cross-Sectional Study. J. Cardiovasc. Dev. Dis. 2023, 10, 358. https://doi.org/10.3390/jcdd10090358
AlGhibiwi HK, Sarawi WS, Alosaimi ME, Alhusaini AM, Assiri MA, Algarzae NK. The Association between Cardiovascular Risk Factors and Carotid Intima-Media Thickness in 42,726 Adults in UK Biobank: A Cross-Sectional Study. Journal of Cardiovascular Development and Disease. 2023; 10(9):358. https://doi.org/10.3390/jcdd10090358
Chicago/Turabian StyleAlGhibiwi, Hanan K., Wedad S. Sarawi, Manal E. Alosaimi, Ahlam M. Alhusaini, Mohammed A. Assiri, and Norah K. Algarzae. 2023. "The Association between Cardiovascular Risk Factors and Carotid Intima-Media Thickness in 42,726 Adults in UK Biobank: A Cross-Sectional Study" Journal of Cardiovascular Development and Disease 10, no. 9: 358. https://doi.org/10.3390/jcdd10090358






