Design and Harmonization Approach for the Multi-Institutional Neurocognitive Discovery Study (MINDS) of Adult Congenital Heart Disease (ACHD) Neuroimaging Ancillary Study: A Technical Note
Abstract
:1. Introduction
2. Methods and Designs
2.1. Introduction to Parent and Ancillary Study
2.2. Enrollment for MINDS Ancillary Neuroimaging Study
2.3. Neurocognitive Measures—NIH Toolbox (From the Parent MINDS Study) and Questionnaire (New in the Ancillary Study)
2.4. MR Imaging Protocols and Pulse Sequences
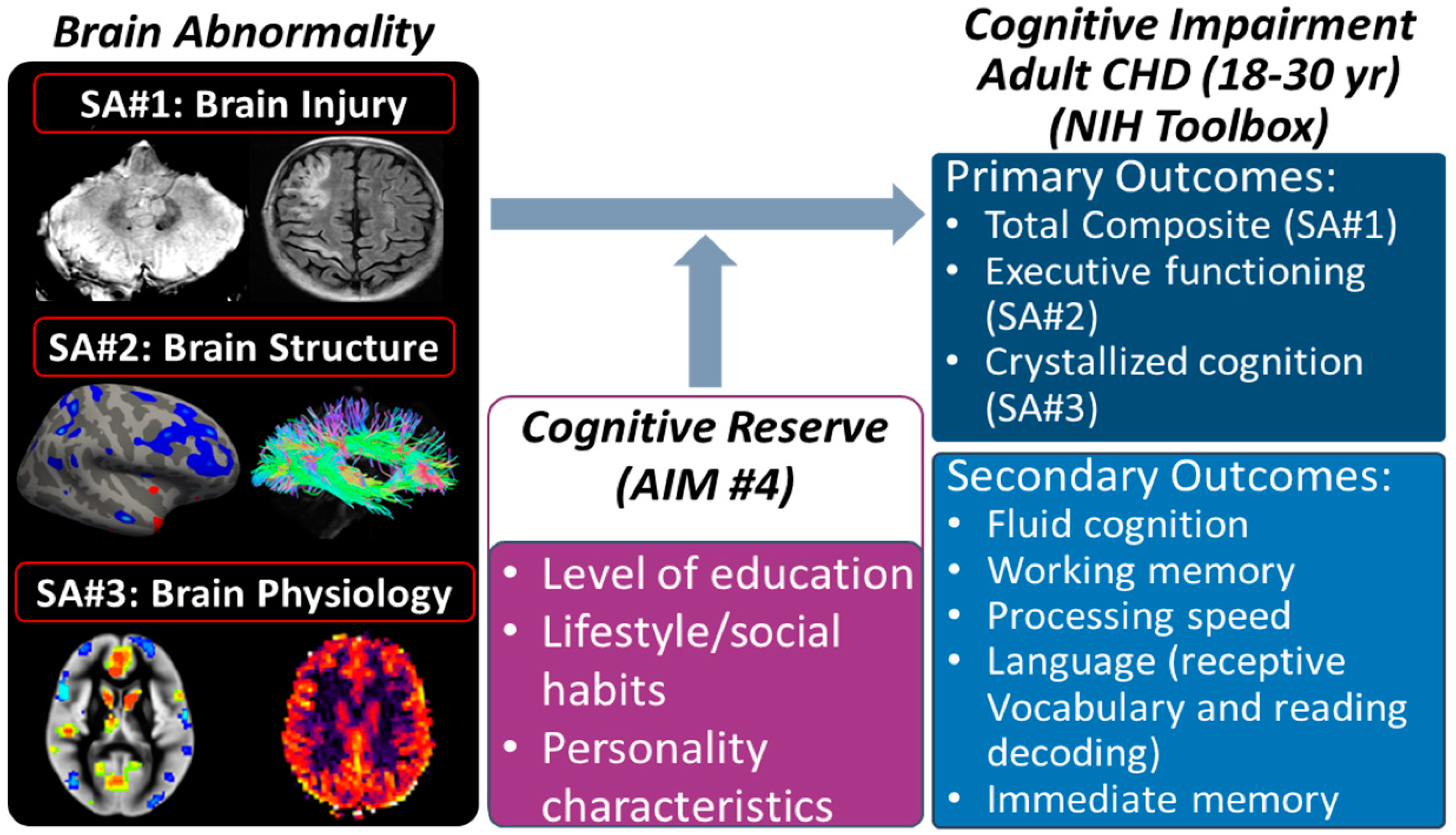
2.5. Specific Aim 1: Vascular-Related Brain Injury—Is It Associated with Neurocognitive Deficits?
2.5.1. Rationale
2.5.2. Analysis Plan
2.5.3. Aim 1 Power Analysis
2.6. Specific Aim 2: Brain Structure—Is It Associated with Neurocognitive Deficits?
2.6.1. Justification
2.6.2. Interpretation
2.6.3. Methodology
2.6.4. Aim 2 Analysis Plan and Brain–Outcome Relationship
2.6.5. Aim 2 Power Analysis
2.7. Specific Aim 3: Brain Physiology—Is It Associated with Neurocognitive Deficits?
2.7.1. Rationale
2.7.2. Methodology
2.7.3. Aim 3 Analysis Plan
2.7.4. Aim 3 Power Analysis
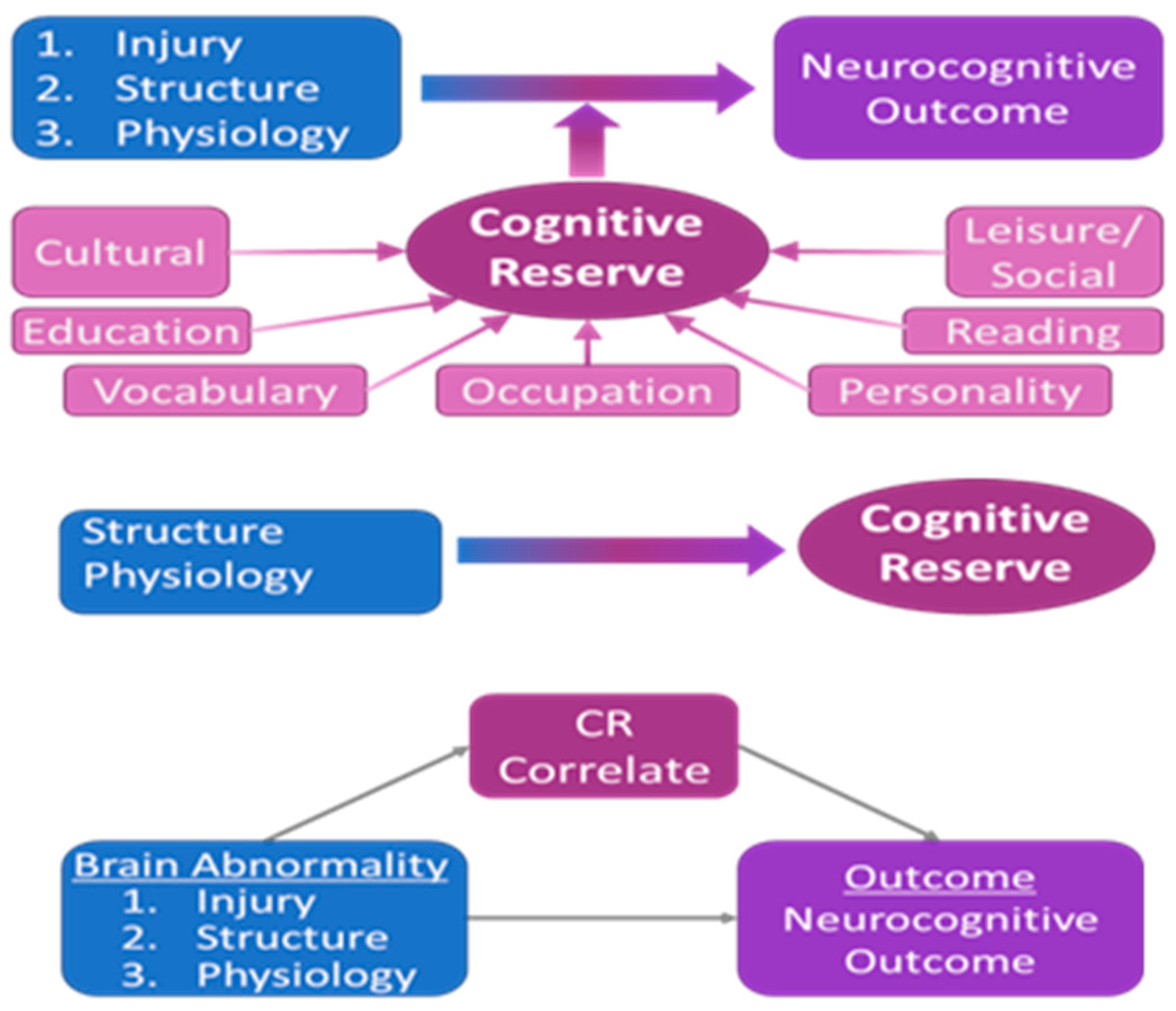
2.8. Specific Aim 4: Cognitive Reserve—Does It Modify Associations between Imaging Biomarkers and Cognitive Outcome?
2.8.1. Rationale
2.8.2. Methodology
2.9. Missing Data
2.10. Multi-Center MRI Quality Assurance and Quality Control (QA/QC)
2.11. Data Transfer—See Supplemental Methods
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandalenakis, Z.; Giang, K.W.; Eriksson, P.; Liden, H.; Synnergren, M.; Wåhlander, H.; Fedchenko, M.; Rosengren, A.; Dellborg, M. Survival in children with congenital heart disease: Have we reached a peak at 97%? J. Am. Heart Assoc. 2020, 9, e017704. [Google Scholar] [CrossRef]
- Cohen, S.; Earing, M.G. Neurocognitive Impairment and Its Long-Term Impact on Adults with Congenital Heart Disease. Prog. Cardiovasc. Dis. 2018, 61, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Daliento, L.; Mapelli, D.; Russo, G.; Scarso, P.; Limongi, F.; Iannizzi, P.; Melendugno, A.; Mazzotti, E.; Volpe, B. Health related quality of life in adults with repaired tetralogy of Fallot: Psychosocial and cognitive outcomes. Heart 2005, 91, 213–218. [Google Scholar] [CrossRef]
- Utens, E.; Versluis-Den Bieman, H.; Verhulst, F.; Meijboom, F.; Erdman, R.; Hess, J. Psychopathology in young adults with congenital heart disease. Follow-Up Results. Eur. Heart J. 1998, 19, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Utens, E.M.; Verhulst, F.C.; Erdman, R.A.; Meijboom, F.J.; Duivenvoorden, H.J.; Bos, E.; Roelandt, J.R.; Hess, J. Psychosocial functioning of young adults after surgical correction for congenital heart disease in childhood: A follow-up study. J. Psychosom. Res. 1994, 38, 745–758. [Google Scholar] [CrossRef]
- Ilardi, D.; Ono, K.E.; McCartney, R.; Book, W.; Stringer, A.Y. Neurocognitive functioning in adults with congenital heart disease. Congenit. Heart Dis. 2017, 12, 166–173. [Google Scholar] [CrossRef]
- Murphy, L.K.; Compas, B.E.; Reeslund, K.L.; Gindville, M.C.; Mah, M.L.; Markham, L.W.; Jordan, L.C. Cognitive and attentional functioning in adolescents and young adults with Tetralogy of Fallot and d-transposition of the great arteries. Child Neuropsychol. 2017, 23, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L.; Franklin, W.J.; Saraf, A.; Parekh, D.R.; Schwartz, D.D. Neurocognitive and executive functioning in adult survivors of congenital heart disease. Congenit. Heart Dis. 2017, 12, 91–98. [Google Scholar] [CrossRef]
- Marelli, A.; Miller, S.P.; Marino, B.S.; Jefferson, A.L.; Newburger, J.W. Brain in congenital heart disease across the lifespan: The cumulative burden of injury. Circulation 2016, 133, 1951–1962. [Google Scholar] [CrossRef]
- Gagliardi, M.G.; Formigari, R.; Perrone, M.A.; Pomiato, E.; Fanisio, F.; Panebianco, M.; Barracano, R.; Guccione, P.; Palmieri, R.; Raponi, M. Changes in the Cath Lab in the Treatment of Adult Patients with Congenital Heart Disease: A 12-Year Experience in a Single Referral Center with the Establishment of a Dedicated Working Group. J. Cardiovasc. Dev. Dis. 2023, 10, 314. [Google Scholar] [CrossRef]
- Bagge, C.N.; Henderson, V.W.; Laursen, H.B.; Adelborg, K.; Olsen, M.; Madsen, N.L. Risk of Dementia in Adults with Congenital Heart Disease: Population-Based Cohort Study. Circulation 2018, 137, 1912–1920. [Google Scholar] [CrossRef]
- Arenaza-Urquijo, E.M.; Vemuri, P. Resistance vs resilience to Alzheimer disease: Clarifying terminology for preclinical studies. Neurology 2018, 90, 695–703. [Google Scholar] [CrossRef]
- Barulli, D.; Stern, Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [CrossRef]
- Ferreira, D.; Bartrés-Faz, D.; Nygren, L.; Rundkvist, L.J.; Molina, Y.; Machado, A.; Junqué, C.; Barroso, J.; Westman, E. Different reserve proxies confer overlapping and unique endurance to cortical thinning in healthy middle-aged adults. Behav. Brain Res. 2016, 311, 375–383. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017, 18, 419. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, P.; Seo, S.W.; Roh, J.H.; Oh, M.; Oh, J.S.; Oh, S.J.; Kim, J.S.; Jeong, Y. Neural substrates of cognitive reserve in Alzheimer’s disease spectrum and normal aging. NeuroImage 2019, 186, 690–702. [Google Scholar] [CrossRef]
- McGarrigle, L.; Irving, K.; van Boxtel, M.P.; Boran, L. Cognitive reserve capacity: Exploring and validating a theoretical model in healthy ageing. J. Int. Neuropsychol. Soc. 2019, 25, 603–617. [Google Scholar] [CrossRef]
- Mebius, M.J.; Roofthooft, M.T.; Bos, A.F. Editorial based on:“Risk of dementia in adults with congenital heart disease: Population-based cohort study”. J. Thorac. Dis. 2018, 10, S2048. [Google Scholar] [CrossRef]
- Medaglia, J.D.; Pasqualetti, F.; Hamilton, R.H.; Thompson-Schill, S.L.; Bassett, D.S. Brain and cognitive reserve: Translation via network control theory. Neurosci. Biobehav. Rev. 2017, 75, 53–64. [Google Scholar] [CrossRef]
- Serra, L.; Cercignani, M.; Petrosini, L.; Basile, B.; Perri, R.; Fadda, L.; Spano, B.; Marra, C.; Giubilei, F.; Carlesimo, G.A. Neuroanatomical correlates of cognitive reserve in Alzheimer disease. Rejuvenation Res. 2011, 14, 143–151. [Google Scholar] [CrossRef]
- Steffener, J.; Stern, Y. Exploring the neural basis of cognitive reserve in aging. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 467–473. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Stern, Y.; Zarahn, E.; Hilton, H.J.; Flynn, J.; DeLaPaz, R.; Rakitin, B. Exploring the neural basis of cognitive reserve. J. Clin. Exp. Neuropsychol. 2003, 25, 691–701. [Google Scholar] [CrossRef]
- Cohen, S.; Gurvitz, M.; Burns, K.M.; Wheaton, O.; Panigrahy, A.; Umfleet, L.; Loman, M.; Brown, N.; Cotts, T.; Ermis, P. Design of a multi-institutional neurocognitive discovery study in adult congenital heart disease (MINDS-ACHD). Am. Heart J. 2023, 262, 131–139. [Google Scholar] [CrossRef]
- Querbes, O.; Aubry, F.; Pariente, J.; Lotterie, J.-A.; Démonet, J.-F.; Duret, V.; Puel, M.; Berry, I.; Fort, J.-C.; Celsis, P. Early diagnosis of Alzheimer’s disease using cortical thickness: Impact of cognitive reserve. Brain 2009, 132, 2036–2047. [Google Scholar] [CrossRef]
- Whalley, L.J.; Staff, R.T.; Fox, H.C.; Murray, A.D. Cerebral correlates of cognitive reserve. Psychiatry Res. Neuroimaging 2016, 247, 65–70. [Google Scholar] [CrossRef]
- Hodgetts, C.J.; Shine, J.P.; Williams, H.; Postans, M.; Sims, R.; Williams, J.; Lawrence, A.D.; Graham, K.S. Increased posterior default mode network activity and structural connectivity in young adult APOE-ε4 carriers: A multimodal imaging investigation. Neurobiol. Aging 2019, 73, 82–91. [Google Scholar] [CrossRef]
- Jagust, W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 687. [Google Scholar] [CrossRef]
- Teipel, S.; Grothe, M.J.; Zhou, J.; Sepulcre, J.; Dyrba, M.; Sorg, C.; Babiloni, C. Measuring cortical connectivity in Alzheimer’s disease as a brain neural network pathology: Toward clinical applications. J. Int. Neuropsychol. Soc. 2016, 22, 138–163. [Google Scholar] [CrossRef]
- Benson, G.; Hildebrandt, A.; Lange, C.; Schwarz, C.; Köbe, T.; Sommer, W.; Flöel, A.; Wirth, M. Functional connectivity in cognitive control networks mitigates the impact of white matter lesions in the elderly. Alzheimer’s Res. Ther. 2018, 10, 109. [Google Scholar] [CrossRef]
- Shaw, P.; Lerch, J.P.; Pruessner, J.C.; Taylor, K.N.; Rose, A.B.; Greenstein, D.; Clasen, L.; Evans, A.; Rapoport, J.L.; Giedd, J.N. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: An observational study. Lancet Neurol. 2007, 6, 494–500. [Google Scholar] [CrossRef]
- Bookheimer, S.Y.; Strojwas, M.H.; Cohen, M.S.; Saunders, A.M.; Pericak-Vance, M.A.; Mazziotta, J.C.; Small, G.W. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 2000, 343, 450–456. [Google Scholar] [CrossRef]
- Ewers, M.; Sperling, R.A.; Klunk, W.E.; Weiner, M.W.; Hampel, H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer’s disease dementia. Trends Neurosci. 2011, 34, 430–442. [Google Scholar] [CrossRef]
- Crivello, F.; Lemaître, H.; Dufouil, C.; Grassiot, B.; Delcroix, N.; Tzourio-Mazoyer, N.; Tzourio, C.; Mazoyer, B. Effects of ApoE-ɛ4 allele load and age on the rates of grey matter and hippocampal volumes loss in a longitudinal cohort of 1186 healthy elderly persons. Neuroimage 2010, 53, 1064–1069. [Google Scholar] [CrossRef]
- Sheline, Y.I.; Morris, J.C.; Snyder, A.Z.; Price, J.L.; Yan, Z.; D’Angelo, G.; Liu, C.; Dixit, S.; Benzinger, T.; Fagan, A. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J. Neurosci. 2010, 30, 17035–17040. [Google Scholar] [CrossRef]
- Dean, D.C.; Jerskey, B.A.; Chen, K.; Protas, H.; Thiyyagura, P.; Roontiva, A.; O’muircheartaigh, J.; Dirks, H.; Waskiewicz, N.; Lehman, K. Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: A cross-sectional imaging study. JAMA Neurol. 2014, 71, 11–22. [Google Scholar] [CrossRef]
- Gershon, R.C.; Wagster, M.V.; Hendrie, H.C.; Fox, N.A.; Cook, K.F.; Nowinski, C.J. NIH toolbox for assessment of neurological and behavioral function. Neurology 2013, 80, S2–S6. [Google Scholar] [CrossRef]
- Weintraub, S.; Dikmen, S.S.; Heaton, R.K.; Tulsky, D.S.; Zelazo, P.D.; Slotkin, J.; Carlozzi, N.E.; Bauer, P.J.; Wallner-Allen, K.; Fox, N. The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: Validation in an adult sample. J. Int. Neuropsychol. Soc. 2014, 20, 567–578. [Google Scholar] [CrossRef]
- Carlozzi, N.; Goodnight, S.; Casaletto, K.; Goldsmith, A.; Heaton, R.; Wong, A.; Baum, C.; Gershon, R.; Heinemann, A.; Tulsky, D. Validation of the NIH toolbox in individuals with neurologic disorders. Arch. Clin. Neuropsychol. 2017, 32, 555–573. [Google Scholar] [CrossRef]
- Tulsky, D.S.; Heinemann, A.W. The clinical utility and construct validity of the NIH Toolbox Cognition Battery (NIHTB-CB) in individuals with disabilities. Rehabil. Psychol. 2017, 62, 409. [Google Scholar] [CrossRef]
- Hackett, K.; Krikorian, R.; Giovannetti, T.; Melendez-Cabrero, J.; Rahman, A.; Caesar, E.E.; Chen, J.L.; Hristov, H.; Seifan, A.; Mosconi, L. Utility of the NIH Toolbox for assessment of prodromal Alzheimer’s disease and dementia. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018, 10, 764–772. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef]
- Tucker, A.M.; Stern, Y. Cognitive reserve in aging. Curr. Alzheimer Res. 2011, 8, 354–360. [Google Scholar] [CrossRef]
- Xu, W.; Yu, J.-T.; Tan, M.-S.; Tan, L. Cognitive Reserve and Alzheimer’s Disease. Mol. Neurobiol. 2015, 51, 187–208. [Google Scholar] [CrossRef]
- Xu, H.; Yang, R.; Qi, X.; Dintica, C.; Song, R.; Bennett, D.A.; Xu, W. Association of Lifespan Cognitive Reserve Indicator with Dementia Risk in the Presence of Brain Pathologies. JAMA Neurol. 2019, 76, 1184–1191. [Google Scholar] [CrossRef]
- Fay, T.B.; Yeates, K.O.; Taylor, H.G.; Bangert, B.; Dietrich, A.; Nuss, K.E.; Rusin, J.; Wright, M. Cognitive reserve as a moderator of postconcussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. J. Int. Neuropsychol. Soc. JINS 2010, 16, 94–105. [Google Scholar] [CrossRef]
- Levi, Y.; Rassovsky, Y.; Agranov, E.; Sela-Kaufman, M.; Vakil, E. Cognitive reserve components as expressed in traumatic brain injury. J. Int. Neuropsychol. Soc. JINS 2013, 19, 664–671. [Google Scholar] [CrossRef]
- Nunnari, D.; Bramanti, P.; Marino, S. Cognitive reserve in stroke and traumatic brain injury patients. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2014, 35, 1513–1518. [Google Scholar] [CrossRef]
- Sumowski, J.F.; Chiaravalloti, N.; Krch, D.; Paxton, J.; Deluca, J. Education attenuates the negative impact of traumatic brain injury on cognitive status. Arch. Phys. Med. Rehabil. 2013, 94, 2562–2564. [Google Scholar] [CrossRef]
- Farias, S.T.; Chand, V.; Bonnici, L.; Baynes, K.; Harvey, D.; Mungas, D.; Simon, C.; Reed, B. Idea density measured in late life predicts subsequent cognitive trajectories: Implications for the measurement of cognitive reserve. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2012, 67, 677–686. [Google Scholar] [CrossRef]
- Iacono, D.; Markesbery, W.; Gross, M.; Pletnikova, O.; Rudow, G.; Zandi, P.; Troncoso, J.C. The Nun study: Clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology 2009, 73, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Kemper, S.; Thompson, M.; Marquis, J. Longitudinal change in language production: Effects of aging and dementia on grammatical complexity and propositional content. Psychol. Aging 2001, 16, 600. [Google Scholar] [CrossRef] [PubMed]
- Snowdon, D.A. Healthy aging and dementia: Findings from the Nun Study. Ann. Intern. Med. 2003, 139, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.J.; Cannonier, T.; Conley, M.I.; Cohen, A.O.; Barch, D.M.; Heitzeg, M.M.; Soules, M.E.; Teslovich, T.; Dellarco, D.V.; Garavan, H. The adolescent brain cognitive development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018, 32, 43–54. [Google Scholar] [CrossRef]
- Debette, S.; Schilling, S.; Duperron, M.-G.; Larsson, S.C.; Markus, H.S. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-analysis. JAMA Neurol. 2018, 76, 81–94. [Google Scholar] [CrossRef]
- Melazzini, L.; Codari, M.; Vitali, P.; Sardanelli, F. Brain vascular changes in adults with congenital heart disease: A systematic review. NeuroImage Clin. 2019, 23, 101873. [Google Scholar] [CrossRef]
- Pinter, D.; Enzinger, C.; Fazekas, F. Cerebral small vessel disease, cognitive reserve and cognitive dysfunction. J. Neurol. 2015, 262, 2411–2419. [Google Scholar] [CrossRef]
- Soul, J.S.; Robertson, R.L.; Wypij, D.; Bellinger, D.C.; Visconti, K.J.; du Plessis, A.J.; Kussman, B.D.; Scoppettuolo, L.A.; Pigula, F.; Jonas, R.A. Subtle hemorrhagic brain injury is associated with neurodevelopmental impairment in infants with repaired congenital heart disease. J. Thorac. Cardiovasc. Surg. 2009, 138, 374–381. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Wypij, D.; Rivkin, M.J.; DeMaso, D.R.; Robertson, R.L., Jr.; Dunbar-Masterson, C.; Rappaport, L.A.; Wernovsky, G.; Jonas, R.A.; Newburger, J.W. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: Neuropsychological assessment and structural brain imaging. Circulation 2011, 124, 1361–1369. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Rivkin, M.J.; Demaso, D.; Robertson, R.L.; Stopp, C.; Dunbar-Masterson, C.; Wypij, D.; Newburger, J.W. Adolescents with tetralogy of Fallot: Neuropsychological assessment and structural brain imaging. Cardiol. Young 2014, 25, 338–347. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Watson, C.G.; Rivkin, M.J.; Robertson, R.L.; Roberts, A.E.; Stopp, C.; Dunbar-Masterson, C.; Bernson, D.; DeMaso, D.R.; Wypij, D. Neuropsychological status and structural brain imaging in adolescents with single ventricle who underwent the Fontan procedure. J. Am. Heart Assoc. 2015, 4, e002302. [Google Scholar] [CrossRef]
- Haller, S.; Montandon, M.-L.; Lazeyras, F.; Scheffler, M.; Meckel, S.; Herrmann, F.R.; Giannakopoulos, P.; Kövari, E. Radiologic-histopathologic correlation of cerebral microbleeds using pre-mortem and post-mortem MRI. PLoS ONE 2016, 11, e0167743. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Han, S.-H.; Moon, W.-J. Patterns of brain iron accumulation in vascular dementia and Alzheimer’s dementia using quantitative susceptibility mapping imaging. J. Alzheimer’s Dis. 2016, 51, 737–745. [Google Scholar] [CrossRef]
- De Reuck, J.; Deramecourt, V.; Auger, F.; Durieux, N.; Cordonnier, C.; Devos, D.; Defebvre, L.; Moreau, C.; Caparros-Lefebvre, D.; Leys, D. Iron deposits in post-mortem brains of patients with neurodegenerative and cerebrovascular diseases: A semi-quantitative 7.0 T magnetic resonance imaging study. Eur. J. Neurol. 2014, 21, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Paradise, M.; Seruga, A.; Crawford, J.D.; Chaganti, J.; Thalamuthu, A.; Kochan, N.A.; Brodaty, H.; Wen, W.; Sachdev, P.S. The relationship of cerebral microbleeds to cognition and incident dementia in non-demented older individuals. Brain Imaging Behav. 2018, 13, 750–761. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Kwok, C.; Benavente, O. Cerebral microbleeds: Histopathological correlation of neuroimaging. Cerebrovasc. Dis. 2011, 32, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Schrag, M.; McAuley, G.; Pomakian, J.; Mueller, C.; Vinters, H.V.; Haacke, E.M.; Holshouser, B.; Kido, D.; Kirsch, W. Correlation of hypointensities in susceptibility weighted magnetic resonance images to tissue histology in dementia patients with cerebral amyloid angiopathy. Cereb. Amyloid Angiopathy Transit. Met. Alzheimer’s Dis. 2010, 95. [Google Scholar]
- Duijn, S.V. MRI and Histologic Studies on Early Markers of Alzheimer’s Disease. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 2018. [Google Scholar]
- Acosta-Cabronero, J.; Betts, M.J.; Cardenas-Blanco, A.; Yang, S.; Nestor, P.J. In vivo MRI mapping of brain iron deposition across the adult lifespan. J. Neurosci. 2016, 36, 364–374. [Google Scholar] [CrossRef]
- Latal, B.; Patel, P.; Liamlahi, R.; Knirsch, W.; Tuura, R.O.G.; von Rhein, M. Hippocampal volume reduction is associated with intellectual functions in adolescents with congenital heart disease. Pediatr. Res. 2016, 80, 531. [Google Scholar] [CrossRef]
- Lim, J.M.; Porayette, P.; Marini, D.; Chau, V.; Au-Young, S.H.; Saini, A.; Ly, L.G.; Blaser, S.; Shroff, M.; Branson, H.M. Associations between age at arterial switch operation, brain growth, and development in infants with transposition of the great arteries. Circulation 2019, 139, 2728–2738. [Google Scholar] [CrossRef]
- Panigrahy, A.; Schmithorst, V.J.; Wisnowski, J.L.; Watson, C.G.; Bellinger, D.C.; Newburger, J.W.; Rivkin, M.J. Relationship of white matter network topology and cognitive outcome in adolescents with d-transposition of the great arteries. NeuroImage Clin. 2015, 7, 438–448. [Google Scholar] [CrossRef]
- Schmithorst, V.J.; Panigrahy, A.; Gaynor, J.W.; Watson, C.G.; Lee, V.; Bellinger, D.C.; Rivkin, M.J.; Newburger, J.W. Organizational topology of brain and its relationship to ADHD in adolescents with d-transposition of the great arteries. Brain Behav. 2016, 6, e00504. [Google Scholar] [CrossRef]
- Schmithorst, V.J.; Votava-Smith, J.K.; Tran, N.; Kim, R.; Lee, V.; Ceschin, R.; Lai, H.; Johnson, J.A.; De Toledo, J.S.; Blüml, S. Structural network topology correlates of microstructural brain dysmaturation in term infants with congenital heart disease. Hum. Brain Mapp. 2018, 39, 4593–4610. [Google Scholar] [CrossRef]
- von Rhein, M.; Buchmann, A.; Hagmann, C.; Huber, R.; Klaver, P.; Knirsch, W.; Latal, B. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain 2013, 137, 268–276. [Google Scholar] [CrossRef]
- Watson, C.G.; Stopp, C.; Newburger, J.W.; Rivkin, M.J. Graph theory analysis of cortical thickness networks in adolescents with d-transposition of the great arteries. Brain Behav. 2018, 8, e00834. [Google Scholar] [CrossRef]
- Watson, C.G.; Stopp, C.; Wypij, D.; Bellinger, D.C.; Newburger, J.W.; Rivkin, M.J. Altered white matter microstructure correlates with IQ and processing speed in children and adolescents post-fontan. J. Pediatr. 2018, 200, 140–149.e4. [Google Scholar] [CrossRef]
- Zaidi, A.H.; Newburger, J.W.; Wypij, D.; Stopp, C.; Watson, C.G.; Friedman, K.G.; Rivkin, M.J.; Rollins, C.K. Ascending Aorta Size at Birth Predicts White Matter Microstructure in Adolescents Who Underwent Fontan Palliation. J. Am. Heart Assoc. 2018, 7, e010395. [Google Scholar] [CrossRef] [PubMed]
- Cordina, R.; Grieve, S.; Barnett, M.; Lagopoulos, J.; Malitz, N.; Celermajer, D.S. Brain volumetrics, regional cortical thickness and radiographic findings in adults with cyanotic congenital heart disease. NeuroImage Clin. 2014, 4, 319–325. [Google Scholar] [CrossRef]
- Brossard-Racine, M.; Panigrahy, A. Structural brain alterations and their associations with function in children, adolescents, and young adults with congenital heart disease. Can. J. Cardiol. 2022, 39, 123–132. [Google Scholar] [CrossRef]
- Yonas, H.; Pindzola, R.R. Physiological determination of cerebrovascular reserves and its use in clinical management. Cerebrovasc. Brain Metab. Rev. 1994, 6, 325–340. [Google Scholar] [PubMed]
- Liu, P.; De Vis, J.B.; Lu, H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: A technical review. NeuroImage 2019, 187, 104–115. [Google Scholar] [CrossRef]
- Gupta, A.; Chazen, J.L.; Hartman, M.; Delgado, D.; Anumula, N.; Shao, H.; Mazumdar, M.; Segal, A.Z.; Kamel, H.; Leifer, D.; et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: A systematic review and meta-analysis. Stroke 2012, 43, 2884–2891. [Google Scholar] [CrossRef]
- Juttukonda, M.R.; Donahue, M.J. Neuroimaging of vascular reserve in patients with cerebrovascular diseases. NeuroImage 2019, 187, 192–208. [Google Scholar] [CrossRef]
- Kosinski, P.D.; Croal, P.L.; Leung, J.; Williams, S.; Odame, I.; Hare, G.M.; Shroff, M.; Kassner, A. The severity of anaemia depletes cerebrovascular dilatory reserve in children with sickle cell disease: A quantitative magnetic resonance imaging study. Br. J. Haematol. 2017, 176, 280–287. [Google Scholar] [CrossRef]
- McKetton, L.; Cohn, M.; Tang-Wai, D.F.; Sobczyk, O.; Duffin, J.; Holmes, K.R.; Poublanc, J.; Sam, K.; Crawley, A.P.; Venkatraghavan, L.; et al. Cerebrovascular Resistance in Healthy Aging and Mild Cognitive Impairment. Front. Aging Neurosci. 2019, 11, 79. [Google Scholar] [CrossRef]
- Richiardi, J.; Monsch, A.U.; Haas, T.; Barkhof, F.; Van de Ville, D.; Radu, E.W.; Kressig, R.W.; Haller, S. Altered cerebrovascular reactivity velocity in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2015, 36, 33–41. [Google Scholar] [CrossRef]
- Votava-Smith, J.K.; Statile, C.J.; Taylor, M.D.; King, E.C.; Pratt, J.M.; Nelson, D.P.; Michelfelder, E.C. Impaired cerebral autoregulation in preoperative newborn infants with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2017, 154, 1038–1044. [Google Scholar] [CrossRef]
- Spaeder, M.C.; Klugman, D.; Skurow-Todd, K.; Glass, P.; Jonas, R.A.; Donofrio, M.T. Perioperative Near-Infrared Spectroscopy Monitoring in Neonates with Congenital Heart Disease: Relationship of Cerebral Tissue Oxygenation Index Variability with Neurodevelopmental Outcome. Pediatr. Crit. Care Med. A J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2017, 18, 213–218. [Google Scholar] [CrossRef]
- Pedersen, M.G.B.; Olsen, M.S.; Schmidt, M.; Johnsen, S.P.; Learn, C.; Laursen, H.B.; Madsen, N.L. Ischemic Stroke in Adults With Congenital Heart Disease: A Population-Based Cohort Study. J. Am. Heart Assoc. 2019, 8, e011870. [Google Scholar] [CrossRef]
- Ghofrani, M.; Tonekaboni, H.; Karimzadeh, P.; Nasiri, J.; Pirzadeh, Z.; Ghazzavi, M.; Yghini, O. Risk Factors of Pediatric Arterial Ischemic Stroke; A Regional Survey. Int. J. Prev. Med. 2018, 9, 69. [Google Scholar] [CrossRef]
- Mandalenakis, Z.; Rosengren, A.; Lappas, G.; Eriksson, P.; Hansson, P.O.; Dellborg, M. Ischemic Stroke in Children and Young Adults with Congenital Heart Disease. J. Am. Heart Assoc. 2016, 5, e003071. [Google Scholar] [CrossRef]
- Matsuda, H.; Higashi, S.; Kinuya, K.; Tsuji, S.; Nozaki, J.; Sumiya, H.; Hisada, K.; Yamashita, J. SPECT evaluation of brain perfusion reserve by the acetazolamide test using Tc-99m HMPAO. Clin. Nucl. Med. 1991, 16, 572–579. [Google Scholar] [CrossRef]
- Dahl, A.; Russell, D.; Rootwelt, K.; Nyberg-Hansen, R.; Kerty, E. Cerebral vasoreactivity assessed with transcranial Doppler and regional cerebral blood flow measurements. Dose, serum concentration, and time course of the response to acetazolamide. Stroke 1995, 26, 2302–2306. [Google Scholar] [CrossRef]
- Pillai, J.J.; Mikulis, D.J. Cerebrovascular reactivity mapping: An evolving standard for clinical functional imaging. AJNR. Am. J. Neuroradiol. 2015, 36, 7–13. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Pinho, M.; Park, D.C.; Welch, B.G.; Lu, H. Cerebrovascular reactivity mapping without gas challenges. NeuroImage 2017, 146, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Golestani, A.M.; Wei, L.L.; Chen, J.J. Quantitative mapping of cerebrovascular reactivity using resting-state BOLD fMRI: Validation in healthy adults. NeuroImage 2016, 138, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Lipp, I.; Murphy, K.; Caseras, X.; Wise, R.G. Agreement and repeatability of vascular reactivity estimates based on a breath-hold task and a resting state scan. NeuroImage 2015, 113, 387–396. [Google Scholar] [CrossRef]
- Setsompop, K.; Gagoski, B.A.; Polimeni, J.R.; Witzel, T.; Wedeen, V.J.; Wald, L.L. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn. Reson. Med. 2012, 67, 1210–1224. [Google Scholar] [CrossRef]
- Liang, X.; Hsu, L.M.; Lu, H.; Sumiyoshi, A.; He, Y.; Yang, Y. The Rich-Club Organization in Rat Functional Brain Network to Balance between Communication Cost and Efficiency. Cereb Cortex 2018, 28, 924–935. [Google Scholar] [CrossRef]
- Collin, G.; Sporns, O.; Mandl, R.C.; van den Heuvel, M.P. Structural and functional aspects relating to cost and benefit of rich club organization in the human cerebral cortex. Cereb Cortex 2014, 24, 2258–2267. [Google Scholar] [CrossRef]
- Griffa, A.; Van den Heuvel, M.P. Rich-club neurocircuitry: Function, evolution, and vulnerability. Dialogues Clin. Neurosci. 2018, 20, 121–132. [Google Scholar]
- Liang, X.; Zou, Q.; He, Y.; Yang, Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc. Natl. Acad. Sci. USA 2013, 110, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhuo, C.; Xu, L.; Liu, F.; Qin, W.; Yu, C. Altered Coupling between Resting-State Cerebral Blood Flow and Functional Connectivity in Schizophrenia. Schizophr. Bull. 2017, 43, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Galiano, A.; Mengual, E.; Garcia de Eulate, R.; Galdeano, I.; Vidorreta, M.; Recio, M.; Riverol, M.; Zubieta, J.L.; Fernandez-Seara, M.A. Coupling of cerebral blood flow and functional connectivity is decreased in healthy aging. Brain Imaging Behav. 2019, 14, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Facal, D.; Valladares, S.; Lojo-Seoane, C.; Pereiro, A.X.; Anido-Rifón, L.; Juncos-Rabadán, O. Machine learning approaches to studying the role of cognitive reserve in conversion from mild cognitive impairment to dementia. Int. J. Geriatr. Psychiatry 2019, 34, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Weiler, M.; Casseb, R.F.; de Campos, B.M.; de Ligo Teixeira, C.V.; Carletti-Cassani, A.F.M.K.; Vicentini, J.E.; Magalhães, T.N.C.; de Almeira, D.Q.; Talib, L.L.; Forlenza, O.V.; et al. Cognitive Reserve Relates to Functional Network Efficiency in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 255. [Google Scholar] [CrossRef]
- Roe, C.M.; Xiong, C.; Miller, J.P.; Morris, J.C. Education and Alzheimer disease without dementia: Support for the cognitive reserve hypothesis. Neurology 2007, 68, 223–228. [Google Scholar] [CrossRef]
- Adam, S.; Bonsang, E.; Grotz, C.; Perelman, S. Occupational activity and cognitive reserve: Implications in terms of prevention of cognitive aging and Alzheimer’s disease. Clin. Interv. Aging 2013, 8, 377–390. [Google Scholar] [CrossRef]
- Myung, W.; Lee, C.; Park, J.H.; Woo, S.Y.; Kim, S.; Chung, J.W.; Kang, H.S.; Lim, S.W.; Choi, J.; Na, D.L.; et al. Occupational Attainment as Risk Factor for Progression from Mild Cognitive Impairment to Alzheimer’s Disease: A CREDOS Study. J. Alzheimer’s Dis. JAD 2017, 55, 283–292. [Google Scholar] [CrossRef]
- Contador, I.; Del Ser, T.; Llamas, S.; Villarejo, A.; Benito-Leon, J.; Bermejo-Pareja, F. Impact of literacy and years of education on the diagnosis of dementia: A population-based study. J. Clin. Exp. Neuropsychol. 2017, 39, 112–119. [Google Scholar] [CrossRef]
- Wilson, R.S.; Barnes, L.L.; Krueger, K.R.; Hoganson, G.; Bienias, J.L.; Bennett, D.A. Early and late life cognitive activity and cognitive systems in old age. J. Int. Neuropsychol. Soc. JINS 2005, 11, 400–407. [Google Scholar] [CrossRef]
- Wilson, R.S.; Schneider, J.A.; Arnold, S.E.; Bienias, J.L.; Bennett, D.A. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch. Gen. Psychiatry 2007, 64, 1204–1212. [Google Scholar] [CrossRef]
- Mondini, S.; Madella, I.; Zangrossi, A.; Bigolin, A.; Tomasi, C.; Michieletto, M.; Villani, D.; Di Giovanni, G.; Mapelli, D. Cognitive Reserve in Dementia: Implications for Cognitive Training. Front. Aging Neurosci. 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Malloy, P.; Tremont, G.; Grace, J.; Frakey, L. The Frontal Systems Behavior Scale discriminates frontotemporal dementia from Alzheimer’s disease. Alzheimer’s Dement. 2007, 3, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Donders, J.; Strong, C.-A.H. Latent structure of the Behavior Rating Inventory of Executive Function—Adult Version (BRIEF–A) after mild traumatic brain injury. Arch. Clin. Neuropsychol. 2016, 31, 29–36. [Google Scholar] [CrossRef]
- Rodriguez, C.P.; Clay, E.; Jakkam, R.; Gauvreau, K.; Gurvitz, M. Cognitive impairment in adult CHD survivors: A pilot study. Int. J. Cardiol. Congenit. Heart Dis. 2021, 6, 100290. [Google Scholar] [CrossRef]
- Kwak, S.; Shin, M.; Kim, H.; Cho, B.; Ha, J.-H.; Han, G.; Kim, H.; Koo, Y.; Kwon, S.; Lee, C.; et al. Moderating effect of cognitive reserve on the association between grey matter atrophy and memory varies with age in older adults. Psychogeriatrics 2019, 20, 87–95. [Google Scholar] [CrossRef]
- Sapkota, S.; Ramirez, J.; Stuss, D.T.; Masellis, M.; Black, S.E. Clinical dementia severity associated with ventricular size is differentially moderated by cognitive reserve in men and women. Alzheimer’s Res. Ther. 2018, 10, 89. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef]
- Martínez, J.H.; López, M.E.; Ariza, P.; Chavez, M.; Pineda-Pardo, J.A.; López-Sanz, D.; Gil, P.; Maestú, F.; Buldú, J.M. Functional brain networks reveal the existence of cognitive reserve and the interplay between network topology and dynamics. Sci. Rep. 2018, 8, 10525. [Google Scholar] [CrossRef] [PubMed]
- Bartres-Faz, D.; Arenaza-Urquijo, E.M. Structural and functional imaging correlates of cognitive and brain reserve hypotheses in healthy and pathological aging. Brain Topogr. 2011, 24, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Esiri, M.M.; Chance, S.A. Cognitive reserve, cortical plasticity and resistance to Alzheimer’s disease. Alzheimer’s Res. Ther. 2012, 4, 7. [Google Scholar] [CrossRef]
- Hayes, A.F.; Scharkow, M. The Relative Trustworthiness of Inferential Tests of the Indirect Effect in Statistical Mediation Analysis: Does Method Really Matter? Psychol. Sci. 2013, 24, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- DiCiccio, T.; Efron, B. Bootstrap confidence intervals. Stat. Sci. 1996, 11, 189–228. [Google Scholar] [CrossRef]
- Efron, B. Better Bootstrap Confidence Intervals. J. Am. Stat. Assoc. 1987, 89, 171–185. [Google Scholar] [CrossRef]
- Fortin, J.-P.; Sweeney, E.M.; Muschelli, J.; Crainiceanu, C.M.; Shinohara, R.T.; The Alzheimer’s Disease Neuroimaging Initiative. Removing inter-subject technical variability in magnetic resonance imaging studies. NeuroImage 2016, 132, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.-P.; Parker, D.; Tunc, B.; Watanabe, T.; Elliott, M.A.; Ruparel, K.; Roalf, D.R.; Satterthwaite, T.D.; Gur, R.C.; Gur, R.E. Harmonization of multi-site diffusion tensor imaging data. NeuroImage 2017, 161, 149–170. [Google Scholar] [CrossRef]
- Fortin, J.-P.; Cullen, N.; Sheline, Y.I.; Taylor, W.D.; Aselcioglu, I.; Cook, P.A.; Adams, P.; Cooper, C.; Fava, M.; McGrath, P.J. Harmonization of cortical thickness measurements across scanners and sites. NeuroImage 2018, 167, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.D.; Lee, V.K.; Dennis, L.G.; Wallace, J.; Schmithorst, V.; Votava-Smith, J.K.; Rajagopalan, V.; Herrup, E.; Baust, T.; Tran, N.N. Harmonization of Multi-Center Diffusion Tensor Tractography in Neonates with Congenital Heart Disease: Optimizing Post-Processing and Application of ComBat. Neuroimage Rep. 2022, 2, 100114. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Newburger, J.W.; Wypij, D.; Kuban, K.C.; duPlesssis, A.J.; Rappaport, L.A. Behaviour at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiol. Young 2009, 19, 86–97. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Wypij, D.; Kuban, K.C.; Rappaport, L.A.; Hickey, P.R.; Wernovsky, G.; Jonas, R.A.; Newburger, J.W. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation 1999, 100, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C.; Wypij, D.; duPlessis, A.J.; Rappaport, L.A.; Jonas, R.A.; Wernovsky, G.; Newburger, J.W. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J. Thorac. Cardiovasc. Surg. 2003, 126, 1385–1396. [Google Scholar] [CrossRef]
- Goldberg, C.S.; Lu, M.; Sleeper, L.A.; Mahle, W.T.; Gaynor, J.W.; Williams, I.A.; Mussatto, K.A.; Ohye, R.G.; Graham, E.M.; Frank, D.U.; et al. Factors Associated with Neurodevelopment for Children with Single Ventricle Lesions. J. Pediatr. 2014, 165, 490–496.e8. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.R.; White, M.T.; DeMaso, D.R.; Newburger, J.W.; Bellinger, D.C. Executive Function in Children and Adolescents with Critical Cyanotic Congenital Heart Disease. J. Int. Neuropsychol. Soc. JINS 2014, 21, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C.; Bernstein, J.H.; Kirkwood, M.W.; Rappaport, L.A.; Newburger, J.W. Visual-spatial skills in children after open-heart surgery. J. Dev. Behav. Pediatr. JDBP 2003, 24, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Schmithorst, V.J.; Adams, P.S.; Badaly, D.; Lee, V.K.; Wallace, J.; Beluk, N.; Votava-Smith, J.K.; Weinberg, J.G.; Beers, S.R.; Detterich, J. Impaired Neurovascular Function Underlies Poor Neurocognitive Outcomes and Is Associated with Nitric Oxide Bioavailability in Congenital Heart Disease. Metabolites 2022, 12, 882. [Google Scholar] [CrossRef]
- Schmithorst, V.J.; Badaly, D.; Beers, S.R.; Lee, V.K.; Weinberg, J.; Lo, C.W.; Panigrahy, A. Relationships between regional cerebral blood flow and neurocognitive outcomes in children and adolescents with congenital heart disease. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 1285–1295. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panigrahy, A.; Schmithorst, V.; Ceschin, R.; Lee, V.; Beluk, N.; Wallace, J.; Wheaton, O.; Chenevert, T.; Qiu, D.; Lee, J.N.; et al. Design and Harmonization Approach for the Multi-Institutional Neurocognitive Discovery Study (MINDS) of Adult Congenital Heart Disease (ACHD) Neuroimaging Ancillary Study: A Technical Note. J. Cardiovasc. Dev. Dis. 2023, 10, 381. https://doi.org/10.3390/jcdd10090381
Panigrahy A, Schmithorst V, Ceschin R, Lee V, Beluk N, Wallace J, Wheaton O, Chenevert T, Qiu D, Lee JN, et al. Design and Harmonization Approach for the Multi-Institutional Neurocognitive Discovery Study (MINDS) of Adult Congenital Heart Disease (ACHD) Neuroimaging Ancillary Study: A Technical Note. Journal of Cardiovascular Development and Disease. 2023; 10(9):381. https://doi.org/10.3390/jcdd10090381
Chicago/Turabian StylePanigrahy, Ashok, Vanessa Schmithorst, Rafael Ceschin, Vince Lee, Nancy Beluk, Julia Wallace, Olivia Wheaton, Thomas Chenevert, Deqiang Qiu, James N Lee, and et al. 2023. "Design and Harmonization Approach for the Multi-Institutional Neurocognitive Discovery Study (MINDS) of Adult Congenital Heart Disease (ACHD) Neuroimaging Ancillary Study: A Technical Note" Journal of Cardiovascular Development and Disease 10, no. 9: 381. https://doi.org/10.3390/jcdd10090381
APA StylePanigrahy, A., Schmithorst, V., Ceschin, R., Lee, V., Beluk, N., Wallace, J., Wheaton, O., Chenevert, T., Qiu, D., Lee, J. N., Nencka, A., Gagoski, B., Berman, J. I., Yuan, W., Macgowan, C., Coatsworth, J., Fleysher, L., Cannistraci, C., Sleeper, L. A., ... Gurvitz, M., on behalf of the Pediatric Heart Network MINDS Neuroimaging Ancillary Study Investigators. (2023). Design and Harmonization Approach for the Multi-Institutional Neurocognitive Discovery Study (MINDS) of Adult Congenital Heart Disease (ACHD) Neuroimaging Ancillary Study: A Technical Note. Journal of Cardiovascular Development and Disease, 10(9), 381. https://doi.org/10.3390/jcdd10090381





