Emerging Stroke Risk Factors: A Focus on Infectious and Environmental Determinants
Abstract
1. Introduction
2. Methodology
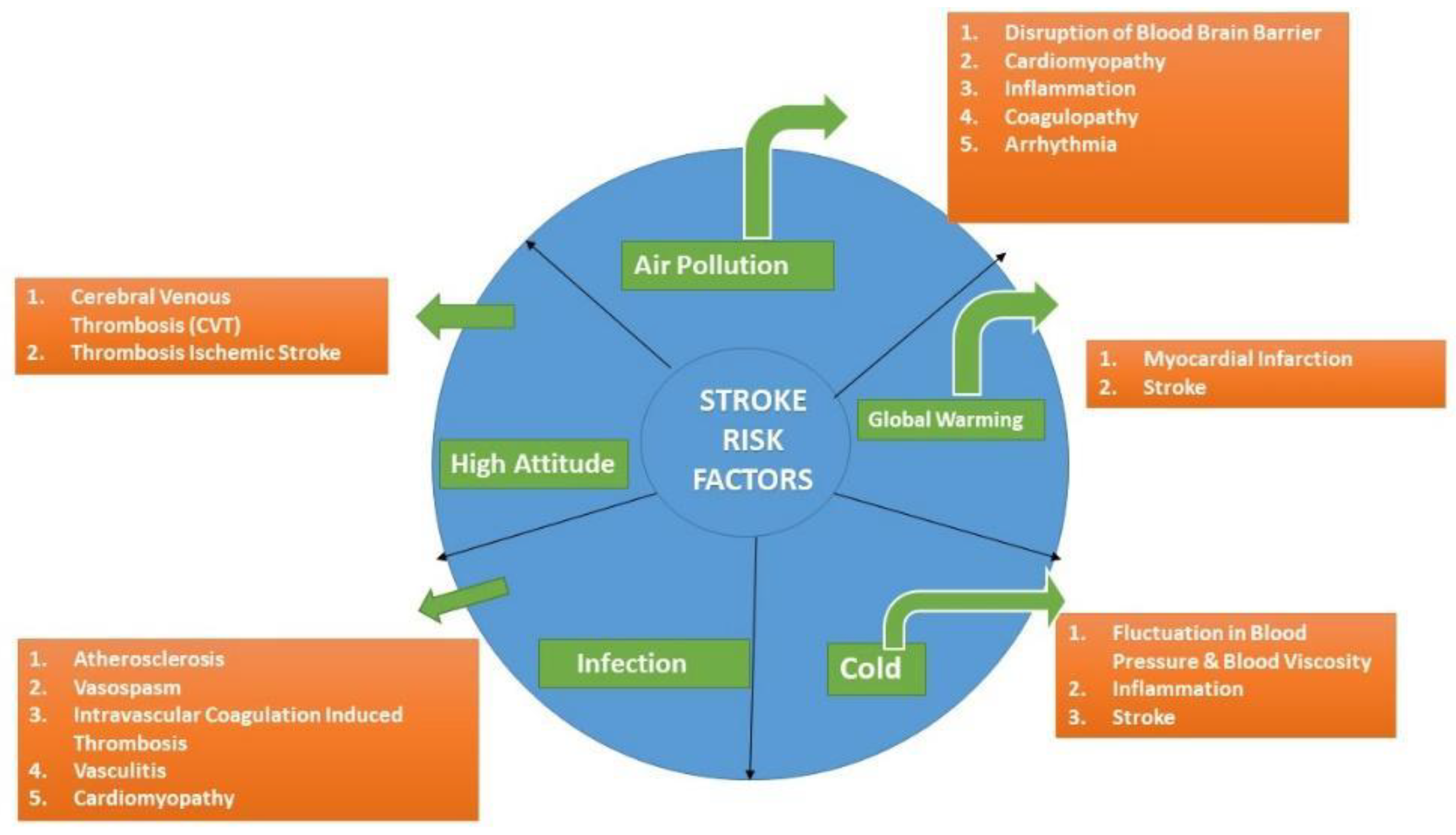
3. Air Pollution
4. Climate Change
5. Microbiota–Gut–Brain Axis
6. High Altitude
7. Systemic Infections
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owolabi, M.O.; Thrift, A.G.; Mahal, A.; Ishida, M.; Martins, S.; Johnson, W.D.; Pandian, J.; Abd-Allah, F.; Yaria, J.; Phan, H.T.; et al. Primary stroke prevention worldwide: Translating evidence into action. Lancet Public Health 2022, 7, e74–e85, Erratum in Lancet Public Health 2022, 7, e14. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthi, R.V.; Moran, A.E.; Feigin, V.L.; Barker-Collo, S.; Norrving, B.; Mensah, G.A.; Taylor, S.; Naghavi, M.; Forouzanfar, M.H.; Nguyen, G.; et al. Stroke Prevalence, Mortality and Disability-Adjusted Life Years in Adults Aged 20–64 Years in 1990–2013: Data from the Global Burden of Disease 2013 Study. Neuroepidemiology 2015, 45, 190–202, Erratum in Neuroepidemiology 2016, 46, 181. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.I.; Allach, Y.; Vaartjes, I.C.H.; Klijn, C.J.M.; de Leeuw, F.E. Ambient air pollution and the risk of ischemic and haemorrhagic stroke. Lancet Planet Health 2021, 5, e542–e552. [Google Scholar] [CrossRef]
- Wellenius, G.A.; Burger, M.R.; Coull, B.A.; Schwartz, J.; Suh, H.H.; Koutrakis, P.; Schlaug, G.; Gold, D.R.; Mittleman, M.A. Ambient air pollution and the risk of acute ischemic stroke. Arch. Intern. Med. 2012, 172, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Roth, G.A.; Naghavi, M.; Parmar, P.; Krishnamurthi, R.; Chugh, S.; Mensah, G.A.; Norrving, B.; Shiue, I.; Ng, M.; et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016, 15, 913–924. [Google Scholar] [CrossRef]
- Wellenius, G.A.; Schwartz, J.; Mittleman, M.A. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among Medicare beneficiaries. Stroke 2005, 36, 2549–2553. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, H.; Zhao, Z.; Xiang, X.; Li, M.; Juan, J.; Song, J.; Cao, Y.; Wang, X.; Chen, L.; et al. Association between ambient air pollution and daily hospital admissions for ischemic stroke: A nationwide time-series analysis. PLoS Med. 2018, 15, e1002668. [Google Scholar] [CrossRef]
- Chiu, H.F.; Chang, C.C.; Yang, C.Y. Relationship between hemorrhagic stroke hospitalization and exposure to fine particulate air pollution in Taipei, Taiwan. J. Toxicol. Environ. Health A 2014, 77, 1154–1163. [Google Scholar] [CrossRef]
- Nzwalo, H.; Guilherme, P.; Nogueira, J.; Félix, C.; André, A.; Teles, J.; Mouzinho, M.; Ferreira, F.; Marreiros, A.; Logallo, N.; et al. Fine particulate air pollution and occurrence of spontaneous intracerebral hemorrhage in an area of low air pollution. Clin. Neurol. Neurosurg. 2019, 176, 67–72. [Google Scholar] [CrossRef]
- Guo, L.; Li, B.; Miao, J.J.; Yun, Y.; Li, G.K.; Sang, N. Seasonal variation in air particulate matter (PM10) exposure-induced ischemia-like injuries in the rat brain. Chem. Res. Toxicol. 2015, 28, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Akopian, G.; Walsh, J.P.; Sioutas, C.; Morgan, T.E.; Finch, C.E. Urban air pollutants reduce synaptic function of CA1 neurons via an NMDA/NȮ pathway in vitro. J. Neurochem. 2013, 127, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.E.; Davis, D.A.; Iwata, N.; Tanner, J.A.; Snyder, D.; Ning, Z.; Kam, W.; Hsu, Y.T.; Winkler, J.W.; Chen, J.C.; et al. Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ. Health Perspect. 2011, 119, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Mustafic, H.; Jabre, P.; Caussin, C.; Murad, M.H.; Escolano, S.; Tafflet, M.; Périer, M.C.; Marijon, E.; Vernerey, D.; Empana, J.P.; et al. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA 2012, 307, 713–721. [Google Scholar] [CrossRef]
- Vogel, C.F.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors–Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Biswas, N.; Alam, M.N. Implications of xenobiotic-response element(s) and aryl hydrocarbon receptor in health and diseases. Hum. Cell 2023, 36, 1638–1655. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, H.; Kurokawa, Y.; Yamamoto, S.; Satoh, M. Distinct requirements for interleukin-6 in airway inflammation induced by diesel exhaust in mice. Immunopharmacol. Immunotoxicol. 2006, 28, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, H.A.; Lucero, J.; Guyot, A.C.; Herbert, L.M.; McDonald, J.D.; Mabondzo, A.; Lund, A.K. Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part. Fibre Toxicol. 2013, 10, 62. [Google Scholar] [CrossRef]
- Lucking, A.J.; Lundback, M.; Mills, N.L.; Faratian, D.; Barath, S.L.; Pourazar, J.; Cassee, F.R.; Donaldson, K.; Boon, N.A.; Badimon, J.J.; et al. Diesel exhaust inhalation increases thrombus formation in man. Eur. Heart J. 2008, 29, 3043–3051. [Google Scholar] [CrossRef]
- Pope, C.A.; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated with Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef]
- O’Toole, T.E.; Hellmann, J.; Wheat, L.; Haberzettl, P.; Lee, J.; Conklin, D.J.; Bhatnagar, A.; Pope, C.A., 3rd. Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ. Res. 2010, 107, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Maheswaran, R.; Pearson, T.; Beevers, S.D.; Campbell, M.J.; Wolfe, C.D. Air Pollution and Subtypes, Severity and Vulnerability to Ischemic Stroke-A Population Based Case-Crossover Study. PLoS ONE 2016, 11, e0158556. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.S.; Brainin, M. COVID-19 and stroke-A global World Stroke Organization perspective. Int. J. Stroke 2020, 15, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Khan, M.; Fatmi, Z.; Wasay, M. Exploring the relationship between air quality and ischemic stroke admissions during the COVID-19 pandemic. J. Stroke Cerebrovasc. Dis. 2021, 30, 105860. [Google Scholar] [CrossRef]
- Libruder, C.; Ram, A.; Hershkovitz, Y.; Tanne, D.; Bornstein, N.M.; Leker, R.R.; Horev, A.; Hallevi, H.; Peretz, S.; Orion, D.; et al. Reduction in acute stroke admissions during the COVID-19 pandemic: Data from a National Stroke Registry. Neuroepidemiology 2021, 55, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Wilker, E.H.; Mostofsky, E.; Fossa, A.; Koutrakis, P.; Warren, A.; Charidimou, A.; Mittleman, M.A.; Viswanathan, A. Ambient Pollutants and Spontaneous Intracerebral Hemorrhage in Greater Boston. Stroke 2018, 18, 2764–2766. [Google Scholar] [CrossRef]
- The Lancet. The Lancet Countdown on Health and Climate Change. 2023. Available online: https://www.thelancet.com/countdown-health-climate (accessed on 5 December 2023).
- Watts, N.; Amann, M.; Arnell, N.; Ayeb-Karlsson, S.; Beagley, J.; Belesova, K.; Boykoff, M.; Byass, P.; Cai, W.; Campbell-Lendrum, D.; et al. The 2020 report of the Lancet Countdown on health and climate change: Responding to converging crises. Lancet 2021, 397, 129–170. [Google Scholar] [CrossRef]
- Atwoli, L.; Baqui, A.H.; Benfield, T.; Bosurgi, R.; Godlee, F.; Hancocks, S.; Horton, R.C.; Laybourn-Langton, L.; Monteiro, C.A.; Normanet, I.; et al. Call for emergency action to limit global temperature increases, restore biodiversity, and protect health. BMJ Nutr. Prev. Health 2021, 4, 362–364. [Google Scholar] [CrossRef]
- Chu, S.Y.; Cox, M.; Fonarow, G.C.; Smith, E.E.; Schwamm, L.; Bhatt, D.L.; Matsouaka, R.A.; Xian, Y.; Sheth, K.N. Temperature and Precipitation Associate with Ischemic Stroke Outcomes in the United States. J. Am. Heart Assoc. 2018, 7, e010020. [Google Scholar] [CrossRef]
- Yang, J.; Yin, P.; Zhou, M.; Ou, C.-Q.; Li, M.; Li, J.; Liu, X.; Gao, J.; Liu, Y.; Qin, R.; et al. The burden of stroke mortality attributable to cold and hot ambient temperatures: Epidemiological evidence from China. Environ. Int. 2016, 92–93, 232–238. [Google Scholar] [CrossRef]
- Feigin, V.L.; Parmar, P.G.; Barker-Collo, S.; Bennett, D.A.; Anderson, C.S.; Thrift, A.G.; Kasabov, N. Geomagnetic storms can trigger stroke: Evidence from 6 large population-based studies in Europe and Australasia. Stroke 2014, 45, 1639–1645. [Google Scholar] [CrossRef]
- Chen, H.; Samet, J.M.; Bromberg, P.A.; Tong, H. Cardiovascular health impacts of wildfire smoke exposure. Part. Fibre Toxicol. 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.E.; Brauer, M.; Johnston, F.H.; Jerrett, M.; Balmes, J.R.; Elliott, C.T. Critical Review of Health Impacts of Wildfire Smoke Exposure. Environ. Health Perspect. 2016, 124, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Brauer, M.; Wei, J.; McGrail, K.M.; Johnston, F.H.; Henderson, S.B. Sub-Daily Exposure to Fine Particulate Matter and Ambulance Dispatches during Wildfire Seasons: A Case-Crossover Study in British Columbia, Canada. Environ. Health Perspect. 2020, 128, 67006. [Google Scholar] [CrossRef] [PubMed]
- Cascio, W.E. Wildland fire smoke and human health. Sci. Total Environ. 2018, 624, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Lavados, P.M.; Olavarría, V.V.; Hoffmeister, L. Ambient temperature and stroke risk: Evidence supporting a short-term effect at a population level from acute environmental exposures. Stroke 2018, 49, 255–261. [Google Scholar] [CrossRef]
- Louis, S.; Carlson, A.K.; Suresh, A.; Rim, J.; Mays, M.; Ontaneda, D.; Dhawan, A. Impacts of Climate Change and Air Pollution on Neurologic Health, Disease, and Practice: A Scoping Review. Neurology 2022, 100, 474–483. [Google Scholar] [CrossRef]
- Amiri, M.; Peinkhofer, C.; Othman, M.H.; De Vecchi, T.; Nersesjan, V.; Kondziella, D. Global warming and neurological practice: Systematic review. PeerJ 2021, 9, e11941. [Google Scholar] [CrossRef]
- Mazidi, M.; Speakman, J.R. Predicted impact of increasing average ambient temperature over the coming century on mortality from cardiovascular disease and stroke in the USA. Atherosclerosis 2020, 313, 1–7. [Google Scholar] [CrossRef]
- Zhou, L.; He, C.; Kim, H.; Honda, Y.; Lee, W.; Hashizume, M.; Chen, R.; Kan, H. The burden of heat-related stroke mortality under climate change scenarios in 22 East Asian cities. Environ. Int. 2022, 170, 107602. [Google Scholar] [CrossRef]
- Lei, J.; Chen, R.; Yin, P.; Meng, X.; Zhang, L.; Liu, C.; Qiu, Y.; Ji, J.S.; Kan, H.; Zhou, M. Association between cold spells and mortality risk and burden: A nationwide study in China. Environ. Health Perspect. 2022, 130, 027006. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Qin, W.; Pan, R.; Yi, W.; Song, S.; Cheng, J.; Su, H. A global comprehensive analysis of ambient low temperature and non-communicable diseases burden during 1990–2019. Environ. Sci. Pollut. Res. 2022, 29, 66136–66147. [Google Scholar] [CrossRef]
- Kang, Y.; Tang, H.; Zhang, L.; Wang, S.; Wang, X.; Chen, Z.; Zheng, C.; Yang, Y.; Wang, Z.; Huang, G. Long-term temperature variability and the incidence of cardiovascular diseases: A large, representative cohort study in China. Environ. Pollut. 2021, 278, 116831. [Google Scholar] [CrossRef] [PubMed]
- Khraishah, H.; Alahmad, B.; Ostergard, R.L., Jr.; AlAshqar, A.; Albaghdadi, M.; Vellanki, N.; Chowdhury, M.M.; Al-Kindi, S.G.; Zanobetti, A.; Gasparrini, A. Climate change and cardiovascular disease: Implications for global health. Nat. Rev. Cardiol. 2022, 19, 798–812. [Google Scholar] [CrossRef]
- Abrignani, M.G.; Lombardo, A.; Braschi, A.; Renda, N.; Abrignani, V. Climatic influences on cardiovascular diseases. World J. Cardiol. 2022, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Kobzeva, K.A.; Soldatova, M.O.; Stetskaya, T.A.; Soldatov, V.O.; Deykin, A.V.; Freidin, M.B.; Bykanova, M.A.; Churnosov, M.I.; Polonikov, A.V.; Bushueva, O.Y. Association between HSPA8 Gene Variants and Ischemic Stroke: A Pilot Study Providing Additional Evidence for the Role of Heat Shock Proteins in Disease Pathogenesis. Genes 2023, 14, 1171. [Google Scholar] [CrossRef] [PubMed]
- Peh, A.; O’Donnell, J.A.; Broughton, B.R.; Marques, F.Z. Gut microbiota and their metabolites in stroke: A double-edged sword. Stroke 2022, 53, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Jama, H.A.; Beale, A.; Shihata, W.A.; Marques, F.Z. The effect of diet on hypertensive pathology: Is there a link via gut microbiota-driven immunometabolism? Cardiovasc. Res. 2019, 115, 1435–1447. [Google Scholar] [CrossRef]
- Benakis, C.; Liesz, A. The gut-brain axis in ischemic stroke: Its relevance in pathology and as a therapeutic target. Neurol. Res. Pract. 2022, 4, 57. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, L.; Guo, Y.; Xiao, J.; Zhang, J.; Xu, S. New Insights into Stroke Prevention and Treatment: Gut Microbiome. Cell Mol. Neurobiol. 2022, 42, 455–472. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Winek, K.; Engel, O.; Koduah, P.; Heimesaat, M.M.; Fischer, A.; Bereswill, S.; Dames, C.; Kershaw, O.; Gruber, A.D.; Curato, C.; et al. Depletion of Cultivatable Gut Microbiota by Broad-Spectrum Antibiotic Pretreatment Worsens Outcome After Murine Stroke. Stroke 2016, 47, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, K.; Kurita, N.; Urabe, T.; Hattori, N. Role of the gut microbiota in stroke pathogenesis and potential therapeutic implications. Ann. Nutr. Metab. 2021, 77 (Suppl. S2), 36–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nwe, P.K.; Yang, Y.; Rosen, C.E.; Bielecka, A.A.; Kuchroo, M.; Cline, G.W.; Kruse, A.C.; Ring, A.M.; Crawford, J.M.; et al. A Forward Chemical Genetic Screen Reveals Gut Microbiota Metabolites That Modulate Host Physiology. Cell 2019, 177, 1217–1231.e18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Svingen, G.F.; Zuo, H.; Ueland, P.M.; Seifert, R.; Løland, K.H.; Pedersen, E.R.; Schuster, P.M.; Karlsson, T.; Tell, G.S.; Schartum-Hansen, H.; et al. Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. Int. J. Cardiol. 2018, 267, 100–106. [Google Scholar] [CrossRef]
- Tang, W.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Sun, C.; Wu, X.; Lu, M.; Si, Y.; Ye, X.; Wang, T.; Yu, X.; Zhao, X.; et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019, 19, 191. [Google Scholar] [CrossRef]
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Dichgans, M.; Liesz, A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J. Neurosci. 2016, 36, 7428–7440. [Google Scholar] [CrossRef]
- Benakis, C.; Poon, C.; Lane, D.; Brea, D.; Sita, G.; Moore, J.; Murphy, M.; Racchumi, G.; Iadecola, C.; Anrather, J. Distinct commensal bacterial signature in the gut is associated with acute and long-term protection from ischemic stroke. Stroke 2020, 51, 1844–1854. [Google Scholar] [CrossRef]
- Chernevskaya, E.; Klimenko, N.; Pautova, A.; Buyakova, I.; Tyakht, A.; Beloborodova, N. Host-microbiome interactions mediated by phenolic metabolites in chronically critically ill patients. Metabolites 2021, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Shekarforoush, S.S.; Hosseinzadeh, S.; Nazifi, S.; Sajedianfard, J.; Eskandari, M.H. The effects of orally administered Bacillus coagulans and inulin on prevention and progression of rheumatoid arthritis in rats. Food Nutr. Res. 2016, 60, 30876. [Google Scholar] [CrossRef]
- Spychala, M.S.; Venna, V.R.; Jandzinski, M.; Doran, S.J.; Durgan, D.J.; Ganesh, B.P.; Ajami, N.J.; Putluri, N.; Graf, J.; Bryan, R.M.; et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 2018, 84, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, Y.; Wu, P.; Zhou, H.; Lasanajak, Y.; Fang, Y.; Tang, L.; Ye, L.; Li, X.; Cai, Z.; et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol. Res. 2019, 148, 104403. [Google Scholar] [CrossRef]
- Syed, M.J.; Khatri, I.A.; Alamgir, W.; Wasay, M. Stroke at Moderate and High Altitude. High. Alt. Med. Biol. 2022, 23, 1–7. [Google Scholar] [CrossRef]
- Hassan, W.U.; Syed, M.J.; Alamgir, W.; Awan, S.; Bell, S.M.; Majid, A.; Wasay, M. Cerebral Venous Thrombosis at High Altitude: Analysis of 28 Cases. Cerebrovasc. Dis. 2019, 48, 184–192. [Google Scholar] [CrossRef]
- Khattar, N.K.; Sumardi, F.; Zemmar, A.; Liang, Q.; Li, H.; Xing, Y.; Andrade-Barazarte, H.; Fleming, J.L.; Cherian, I.; Hernesniemi, J.; et al. Cerebral Venous Thrombosis at High Altitude: A Retrospective Cohort of Twenty-one Consecutive Patients. Cureus 2019, 11, e4940. [Google Scholar] [CrossRef]
- Imray, C.; Booth, A.; Wright, A.; Bradwell, A. Acute altitude illnesses. BMJ 2011, 343, d4943. [Google Scholar] [CrossRef]
- Jansen, G.F.; Krins, A.; Basnyat, B.; Bosch, A.; Odoom, J.A. Cerebral autoregulation in subjects adapted and not adapted to high altitude. Stroke 2000, 31, 2314–2318. [Google Scholar] [CrossRef] [PubMed]
- Claydon, V.E.; Gulli, G.; Slessarev, M.; Appenzeller, O.; Zenebe, G.; Gebremedhin, A.; Hainsworth, R. Cerebrovascular responses to hypoxia and hypocapnia in Ethiopian high altitude dwellers. Stroke 2008, 39, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Niaz, A.; Nayyar, S. Cerebrovascular stroke at high altitude. J. Coll. Phys. Surg. Pak. 2003, 13, 446–448. [Google Scholar]
- Jha, S.K.; Anand, A.C.; Sharma, V.; Kumar, N.; Adya, C.M. Stroke at high altitude: Indian experience. High. Alt. Med. Biol. 2002, 3, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.C.; Jha, S.K.; Saha, A.; Sharma, V.; Adya, C.M. Thrombosis as a complication of extended stay at high altitude. Natl. Med. J. India 2001, 14, 197–201. [Google Scholar] [PubMed]
- Jaillard, A.S.; Hommel, M.; Mazetti, P. Prevalence of stroke at high altitude (3380 m) in Cuzco, a town of Peru. A population-based study. Stroke 1995, 26, 562–568. [Google Scholar] [CrossRef]
- Liu, M.; Yan, M.; Guo, Y.; Xie, Z.; Li, R.; Li, J.; Ren, C.; Ji, X.; Guo, X. Acute Ischemic Stroke at High Altitudes in China: Early Onset and Severe Manifestations. Cells 2021, 10, 809. [Google Scholar] [CrossRef]
- Lu, Y.; Zhuoga, C.; Jin, H.; Zhu, F.; Zhao, Y.; Ding, Z.; He, S.; Du, A.; Xu, J.; Luo, J.; et al. Characteristics of acute ischemic stroke in hospitalized patients in Tibet: A retrospective comparative study. BMC Neurol. 2020, 20, 380. [Google Scholar] [CrossRef]
- Wilson, M.H.; Newman, S.; Imray, C.H. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009, 8, 175–191. [Google Scholar] [CrossRef]
- Faeh, D.; Gutzwiller, F.; Bopp, M.; Swiss National Cohort Study Group. Lower mortality from coronary heart disease and stroke at higher altitudes in Switzerland. Circulation 2009, 120, 495–501. [Google Scholar] [CrossRef]
- Ezzati, M.; Horwitz, M.E.; Thomas, D.S.; Friedman, A.B.; Roach, R.; Clark, T.; Murray, C.J.; Honigman, B. Altitude, life expectancy and mortality from ischaemic heart disease, stroke, COPD, and cancers: National population-based analysis of US counties. J. Epidemiol. Community Health 2012, 66, e17. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Millet, G.P.; Burtscher, M. Does living at moderate altitudes in Austria affect mortality rates of various causes? An ecological study. BMJ Open 2021, 11, e048520. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Espinosa, P.S.; Borrero, A.; Cordovez, S.P.; Vasconez, J.E.; Barreto-Grimales, A.; Coral-Almeida, M.; Henriquez-Trujillo, A.R.; Simbaña-Rivera, K.; Gomez-Barreno, L.; et al. Stroke-Related Mortality at Different Altitudes: A 17-Year Nationwide Population-Based Analysis from Ecuador. Front. Physiol. 2021, 12, 733928. [Google Scholar] [CrossRef]
- Dhiman, D.; Mahajan, S.K.; Sharma, S.; Raina, R. The evolving pattern and outcome of stroke at moderate altitude. J. Neurosci. Rural. Pract. 2018, 9, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Mitroshina, E.V.; Savyuk, M.O.; Ponimaskin, E.; Vedunova, M.V. Hypoxia-Inducible Factor (HIF) in Ischemic Stroke and Neurodegenerative Disease. Front. Cell Dev. Biol. 2021, 9, 703084. [Google Scholar] [CrossRef]
- Humaidan, H.; Yassi, N.; Weir, L.; Davis, S.M.; Meretoja, A. Airplane stroke syndrome. J. Clin. Neurosci. 2016, 29, 77–80. [Google Scholar] [CrossRef]
- Mohrman, E. What Is the Altitude of a Plane in Flight? 2023. Available online: https://www.allgetaways.com/flight-booking/altitude-plane-flight.html (accessed on 25 December 2023).
- Kakkos, S.K.; Geroulakos, G. Economy class stroke syndrome: Case report and review of the literature. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 239–243. [Google Scholar] [CrossRef][Green Version]
- Álvarez-Velasco, R.; Masjuan, J.; DeFelipe, A.; Corral, I.; Estévez-Fraga, C.; Crespo, L.; Alonso-Cánovas, A. Stroke in commercial flights. Stroke 2016, 47, 1117–1119. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Cordovez, S.P.; Vasconez, E.; Viscor, G.; Roderick, P. Chronic high-altitude exposure, and the epidemiology of ischaemic stroke: A systematic review. BMJ Open 2022, 12, e051777. [Google Scholar] [CrossRef]
- Wasay, M.; Khan, M.; Farooq, S.; Khowaja, Z.A.; Bawa, Z.A.; Mansoor Ali, S.; Awan, S.; Beg, M.A. Frequency and Impact of Cerebral Infarctions in Patients with Tuberculous Meningitis. Stroke 2018, 49, 2288–2293. [Google Scholar] [CrossRef]
- Sotero, F.D.; Rosário, M.; Fonseca, A.C.; Ferro, J.M. Neurological Complications of Infective Endocarditis. Curr. Neurol. Neurosci. Rep. 2019, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Lage, T.A.R.; Tupinambás, J.T.; Pádua, L.B.; Ferreira, M.O.; Ferreira, A.C.; Teixeira, A.L.; Nunes, M.C.P. Stroke in Chagas disease: From pathophysiology to clinical practice. Rev. Soc. Bras. Med. Trop. 2022, 55, e0575. [Google Scholar] [CrossRef]
- Grau, A.J.; Urbanek, C.; Palm, F. Common infections, and the risk of stroke. Nat. Rev. Neurol. 2010, 6, 681–694. [Google Scholar] [CrossRef]
- Sung, J.; Song, Y.M.; Choi, Y.H.; Ebrahim, S.; Davey Smith, G. Hepatitis B virus seropositivity and the risk of stroke and myocardial infarction. Stroke 2007, 38, 1436–1441. [Google Scholar] [CrossRef]
- Cojocaru, I.M.; Cojocaru, M.; Iacob, S.A.; Burcin, C. Cerebral ischemic attack secondary to hepatitis C virus infection. Rom. J. Intern. Med. 2005, 43, 255–260. [Google Scholar]
- Khan, M.; Hameed, S.; Soomro, B.A.; Mairaj, S.; Malik, A.; Farooq, S.; Rukn, S.A.; Wasay, M. COVID-19 independently predicts poor outcomes in Acute Ischemic Stroke-Insights from a multicenter study from Pakistan and United Arab Emirates. J. Stroke Cerebrovasc. Dis. 2023, 32, 106903. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Stein, L.K.; Dhamoon, M.S. Infection as a Stroke Trigger. Stroke 2019, 50, 2216–2218. [Google Scholar] [CrossRef] [PubMed]
- Boehme, A.K.; Ranawat, P.; Luna, J.; Kamel, H.; Elkind, M.S. Risk of Acute Stroke After Hospitalization for Sepsis: A Case-Crossover Study. Stroke 2017, 48, 574–580. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis–Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Morris, N.A.; Matiello, M.; Lyons, J.L.; Samuels, M.A. Neurologic complications in infective endocarditis: Identification, management, and impact on cardiac surgery. Neurohospitalist 2014, 4, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Sakaguchi, M.; Hyun, B.; Nagano, K.; Tagaya, M.; Sakata, Y.; Sakaguchi, T.; Kitagawa, K. Cerebral microbleeds predict impending intracranial hemorrhage in infective endocarditis. Cerebrovasc. Dis. 2011, 32, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.A.; Jones, D.; Wyborny, A. Varicella zoster virus vasculopathy: The expanding clinical spectrum and pathogenesis. J. Neuroimmunol. 2017, 308, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.Y.; Chia, L.G.; Shen, W.C. Locations of cerebral infarctions in tuberculous meningitis. Neuroradiology 1992, 34, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Panackal, A.A.; Williamson, P.R. Fungal infections of the central nervous system. Continuum 2015, 21, 1662–1678. [Google Scholar] [CrossRef]
- Hier, D.B.; Caplan, L.R. Stroke Due to Fungal Infections. In Uncommon Causes of Stroke, 2nd ed.; Caplan, L.R., Bogousslavsky, J., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 47–52. [Google Scholar] [CrossRef]
- Smith, R.M.; Schaefer, M.K.; Kainer, M.A.; Wise, M.; Finks, J.; Duwve, J.; Fontaine, E.; Chu, A.; Carothers, B.; Reilly, A.; et al. Fungal infections associated with contaminated methylprednisolone injections. N. Engl. J. Med. 2013, 369, 1598–1609. [Google Scholar] [CrossRef]
- Samuel, J.; Oliveira, M.; Correa De Araujo, R.R.; Navarro, M.A.; Muccillo, G. Cardiac thrombosis, and thromboembolism in chronic Chagas’ heart disease. Am. J. Cardiol. 1983, 52, 147–151. [Google Scholar] [CrossRef]
- Aras, R.; Matta, J.A.; Mota, G.; Gomes, I.; Melo, A. Cerebral infarction in autopsies of chagasic patients with heart failure. Arq. Bras. Cardiol. 2003, 81, 414–416. [Google Scholar] [CrossRef]
- Rosenfeld, M.E.; Campbell, L.A. Pathogens, and atherosclerosis: Update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb. Haemost. 2011, 106, 858–867. [Google Scholar] [CrossRef]
- Kiechl, S.; Egger, G.; Mayr, M.; Wiedermann, C.J.; Bonora, E.; Oberhollenzer, F.; Muggeo, M.; Xu, Q.; Wick, G.; Poewe, W.; et al. Chronic infections, and the risk of carotid atherosclerosis: Prospective results from a large population study. Circulation 2001, 103, 1064–1070. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameed, S.; Karim, N.; Wasay, M.; Venketasubramanian, N. Emerging Stroke Risk Factors: A Focus on Infectious and Environmental Determinants. J. Cardiovasc. Dev. Dis. 2024, 11, 19. https://doi.org/10.3390/jcdd11010019
Hameed S, Karim N, Wasay M, Venketasubramanian N. Emerging Stroke Risk Factors: A Focus on Infectious and Environmental Determinants. Journal of Cardiovascular Development and Disease. 2024; 11(1):19. https://doi.org/10.3390/jcdd11010019
Chicago/Turabian StyleHameed, Sajid, Nurose Karim, Mohammad Wasay, and Narayanaswamy Venketasubramanian. 2024. "Emerging Stroke Risk Factors: A Focus on Infectious and Environmental Determinants" Journal of Cardiovascular Development and Disease 11, no. 1: 19. https://doi.org/10.3390/jcdd11010019
APA StyleHameed, S., Karim, N., Wasay, M., & Venketasubramanian, N. (2024). Emerging Stroke Risk Factors: A Focus on Infectious and Environmental Determinants. Journal of Cardiovascular Development and Disease, 11(1), 19. https://doi.org/10.3390/jcdd11010019







