Ruptured Sinus of Valsalva Aneurysm with Resultant Myocardial Pouch Formation in the Fetal Heart—A Diagnostic Challenge
Abstract
:1. Introduction
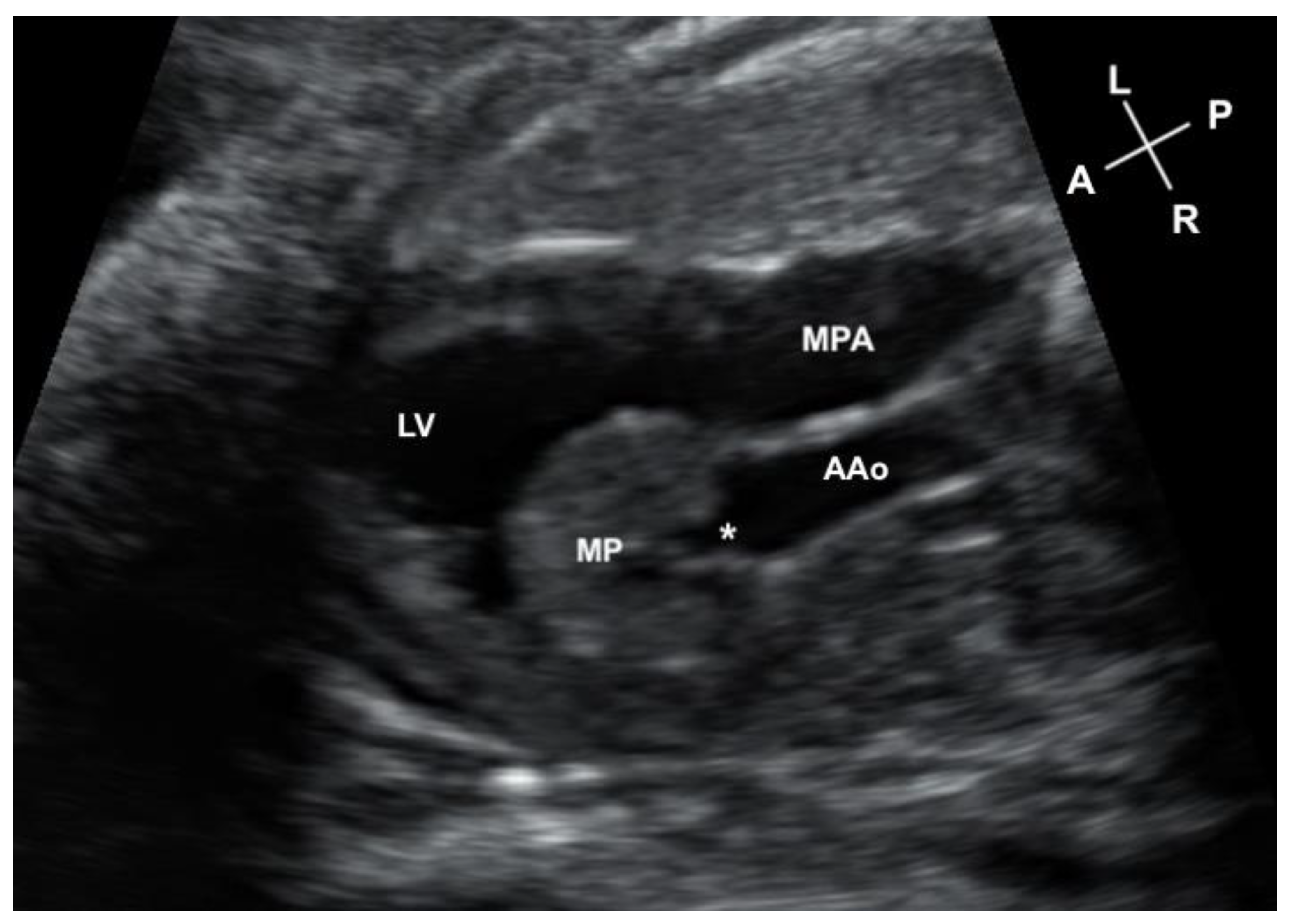
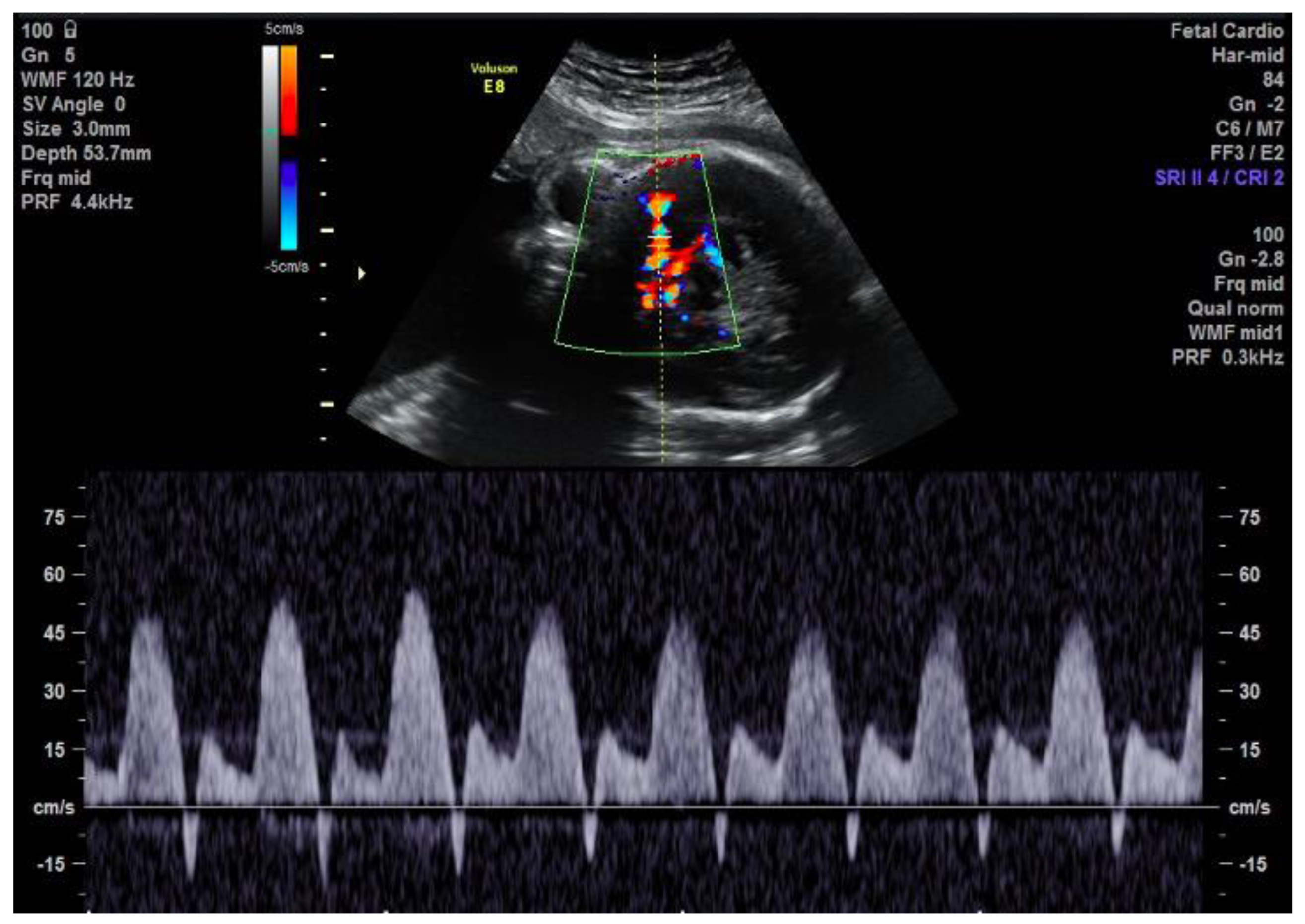
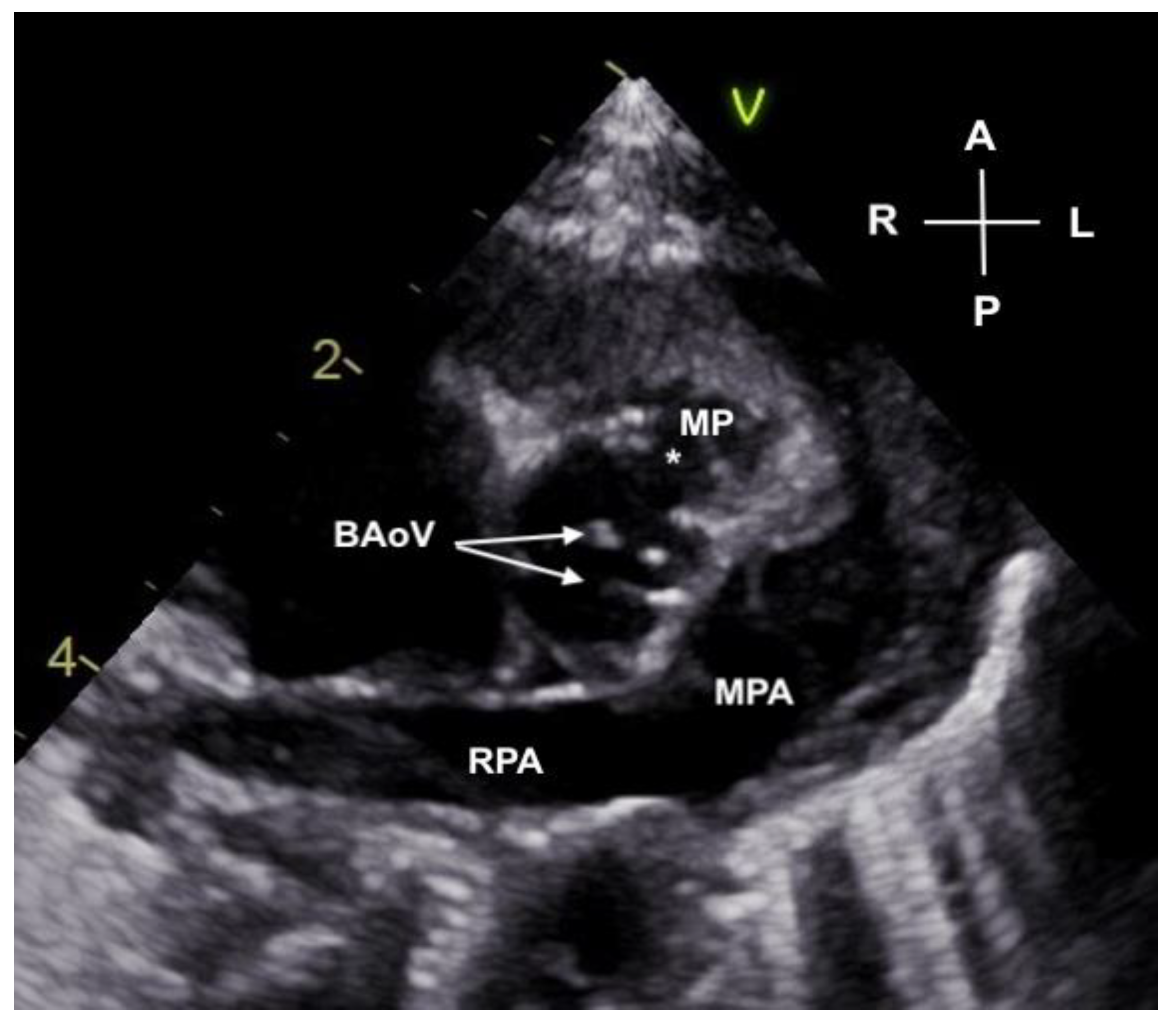
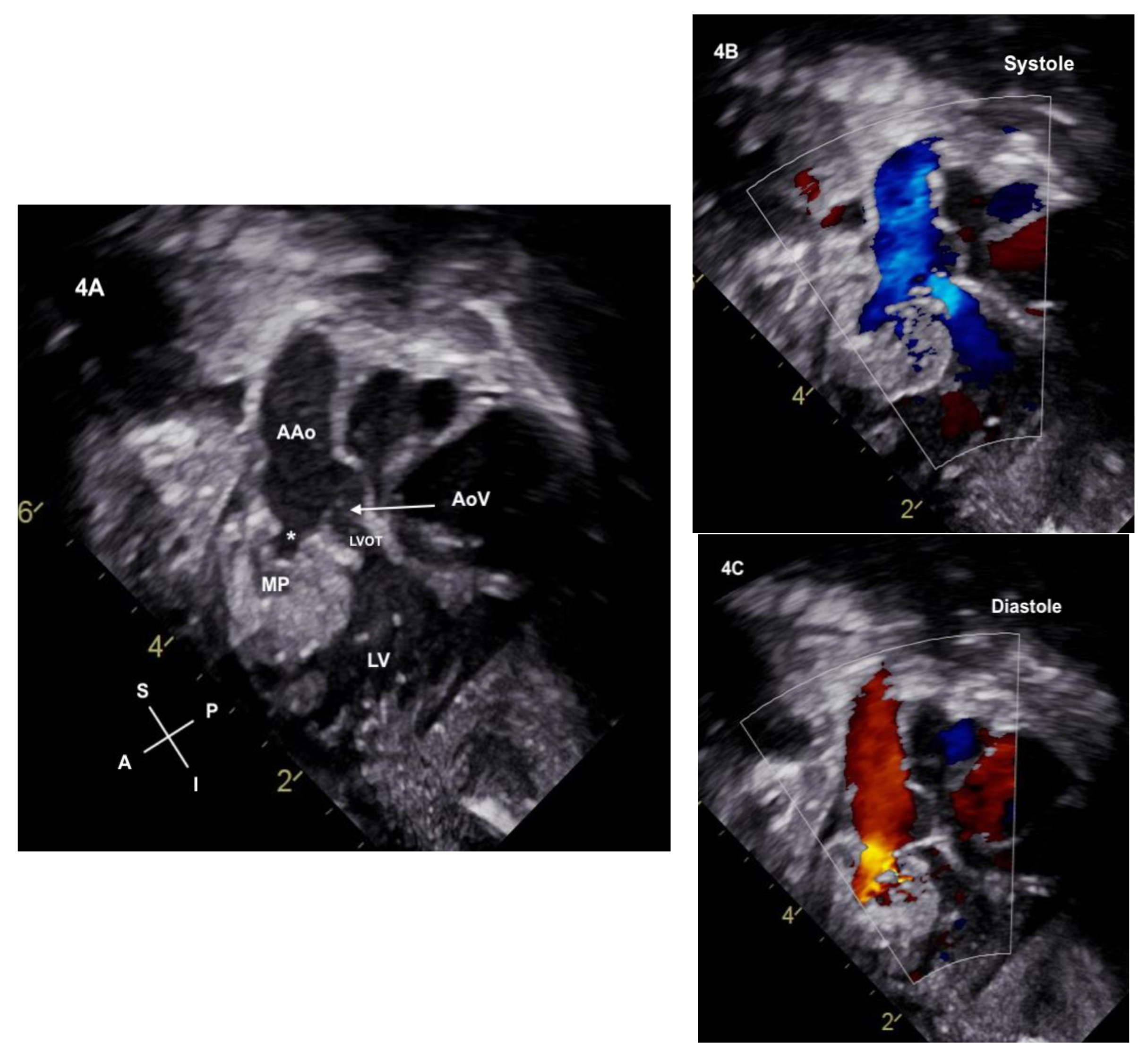
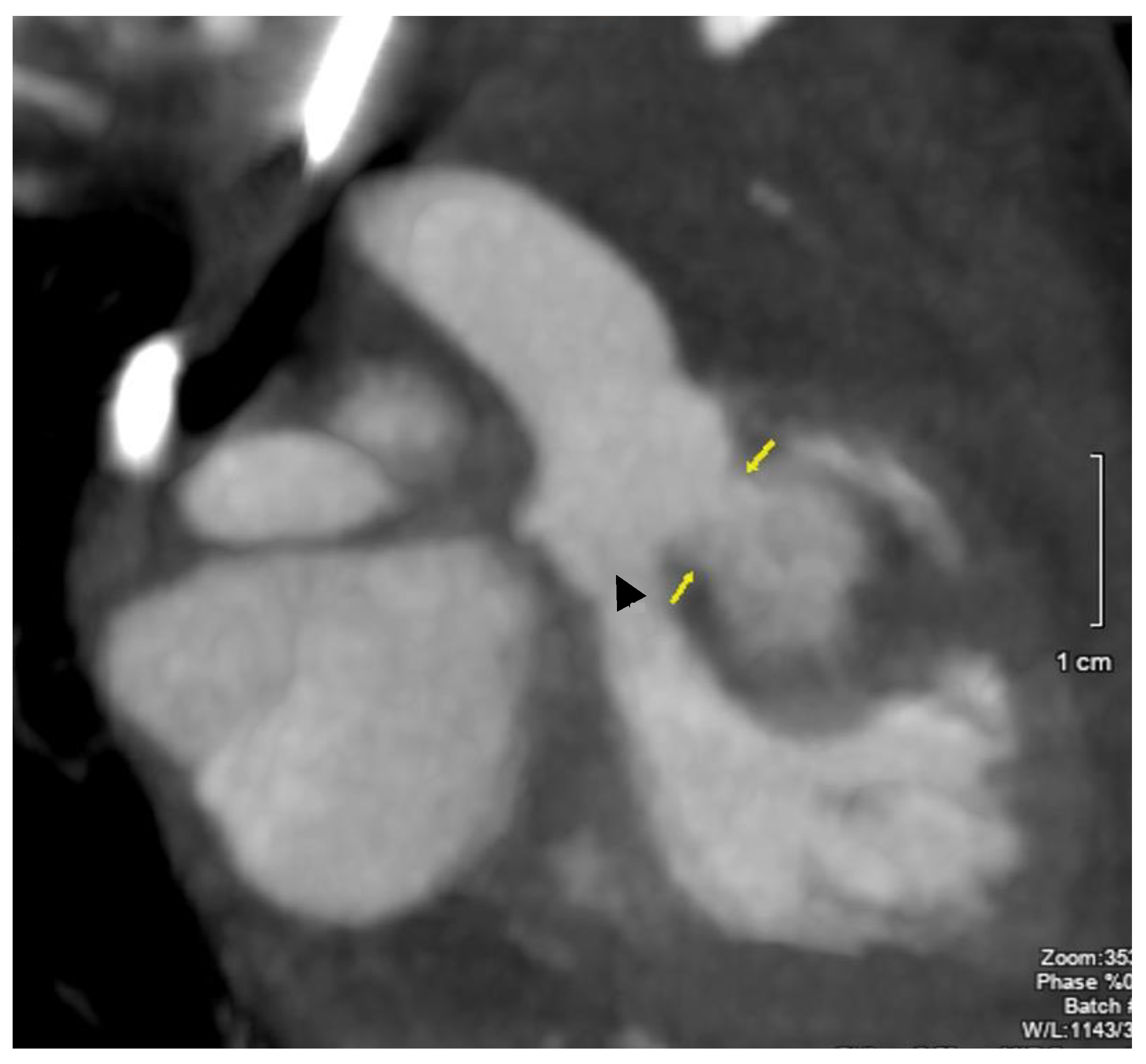
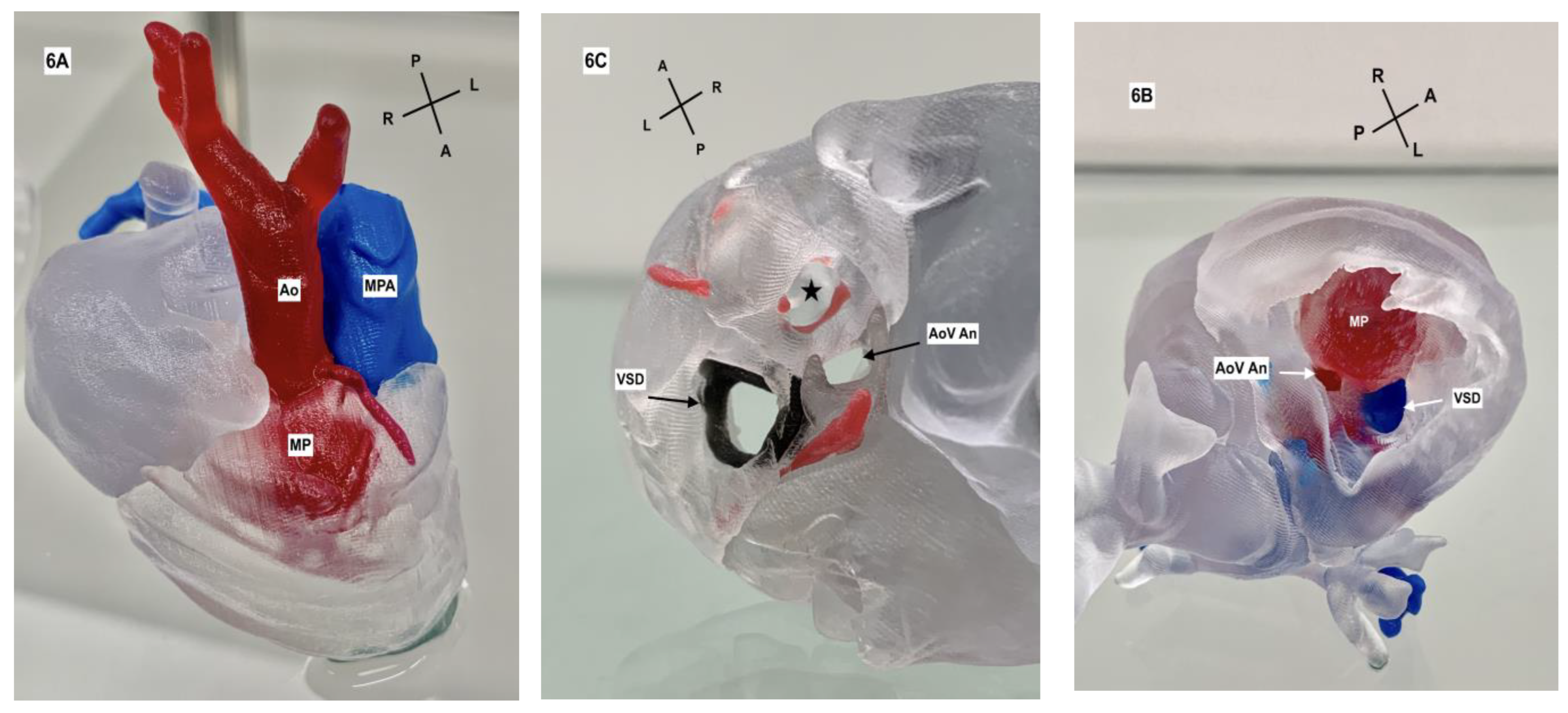
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Nisselrooij, A.E.L.; Moon-Grady, A.J.; Wacker-Gussmann, A.; Tomek, V.; Malčić, I.; Grzyb, A.; Pavlova, A.; Kazamia, K.; Thakur, V.; Sinkovskaya, E.; et al. The aorto-left ventricular tunnel from a fetal perspective: Original case series and literature review. Prenat. Diagn. 2022, 42, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Tretter, J.T.; Steffensen, T.; Westover, T.; Anderson, R.H.; Spicer, D.E. Developmental considerations with regard to so-called absence of the leaflets of the arterial valves. Cardiol. Young. 2017, 27, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Sabati, A.A.; Wong, P.C.; Randolph, L.; Pruetz, J.D. Absent aortic valve associated with double outlet right ventricle and aortopulmonary window: Physiologic implications of a rare malformation in both the fetus and neonate. Congenit. Heart Dis. 2014, 9, E98–E104. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.C.; Recker, F.; Herberg, U.; Oberhoffer, R.; Kurkevych, A.; Axt-Fliedner, R.; Geipel, A.; Gembruch, U.; Berg, C.; Gottschalk, I. Aorto-Left Ventricular Tunnel—Prenatal Diagnosis and Outcome. Ultraschall Med. 2023, 44, e184–e190. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Lin, L.; Ishiodori, T.; Takeuchi, S.; Shiono, J.; Horigome, H. Prenatal diagnosis of congenital absence of aortic valve associated with restrictive foramen ovale: Hemodynamic features and clinical outcome. J. Clin. Ultrasound. 2019, 47, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.K.; Bhan, A.; Sharma, R.; Airan, B.; Kumar, A.S.; Venugopal, P. Sinus of Valsalva aneurysms: 20 years’ experience. J. Card. Surg. 1997, 12, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Yun, T.-J.; Im, Y.-M.; Park, J.-J.; Song, H.; Lee, J.-W.; Seo, D.-M.; Lee, M.-S. Ruptured Sinus of Valsalva Aneurysm: Transaortic repair may cause sinus of Valsalva distortion and aortic regurgitation. J. Thorac. Cardiovasc. Surg. 2008, 135, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Morais, H.; Caceres-Loriga, F.M.; Martins, T.; Vandunem, G.; Cunha, R. Unruptured congenital aneurysm of the sinus of Valsalva in an African population: A six-year experience at an echocardiography laboratory. Cardiovasc. J. Afr. 2009, 20, 192–195. [Google Scholar]
- Weinreich, M.; Yu, P.-J.; Trost, B. Sinus of Valsalva aneurysms: Review of the literature and an update on management. Clin. Cardiol. 2015, 38, 185–189. [Google Scholar] [CrossRef]
- Ainger, L.E.; Pate, J.W. Rupture of a sinus of Valsalva Aneurysm in an infant. Surgical correction. Am. J. Cardiol. 1963, 4, 547–551. [Google Scholar] [CrossRef]
- Bastos, P.; Sousa, A.; Areias, J.C.; Cunha, D.; Gomes, M.R. Congenital aneurysm of the sinus of Valsalva. Surgical repair in an infant. Thorac. Cardiovasc. Surg. 1985, 33, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Breviere, G.; Vaksmann, G.; Francart, C. Rupture of a sinus of Valsalva Aneurysm in a neonate. Eur. J. Pediatr. 1990, 149, 603–604. [Google Scholar] [CrossRef]
- Ghawi, H.; Engelhardt, K.; Dixon, K.; Thankaval, P.; Ramaciotti, C.; Lemler, M.S.; Guleserian, K.J. Sinus of Valsalva Aneurysm in a Patient with Mosaic Trisomy 13: Case Report and Brief Review of the Literature. World Journal for Pediatric and Congenital Surgery 2020, 11, NP1–NP6. [Google Scholar] [CrossRef]
- Nakamura, Y.; Aoki, M.; Hagino, I.; Koshiyama, H.; Fujiwara, T.; Nakajima, H. Case of Congenital Aneurysm of Sinus of Valsalva with Common Arterial Trunk. Ann. Thorac. Surg. 2014, 97, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Overman, D.; Sutton, T.M.; Moga, F.X. Transposition of the Great Arteries with Coexisting Sinus of Valsalva Aneurysm. J. Card. Surg. 2005, 20, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.; Silva, P.V.; Pires, A. Percutaneous management of a ruptured sinus of Valsalva aneurysm in an infant. Cardiol. Young 2021, 31, 1363–1365. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.W.; Martin, G.R.; Galioto, F.M., Jr.; Midgley, F.M. Rupture of congenital sinus of Valsalva aneurysm in a newborn. Am. J. Cardiol. 1991, 68, 1255–1256. [Google Scholar] [CrossRef]
- Shaffer, E.M.; Snider, A.R.; Beekman, R.H.; Behrendt, D.M.; Peschiera, A.W. Valsalva aneurysm complicating bacterial endocarditis in an infant: Diagnosis with two-dimensional and Doppler echocardiography. J. Am. Coll. Card. 1987, 9, 588–591. [Google Scholar] [CrossRef]
- Steflik, D.J.; Churchill, T.L.; Chowdhury, S.M. Sinus of Valsalva Aneurysm Rupture in an Infant. Cardiol. Young 2018, 28, 338–340. [Google Scholar] [CrossRef]
- Koga, R.; Sato, T.; Matsubara, D.; Suzuki, S.; Oka, K.; Seki, M.; Kataoka, K.; Yamagata, T. Case of Hypoplastic Left Heart Syndrome with a Ruptured Sinus of Valsalva Aneurysm in a Fetus. Pediatr. Cardiol. Card. Surg. 2020, 36, 328–333. [Google Scholar] [CrossRef]
- Lubaua, I.; Priedite, I.; Anderson, D. Giant Sinus of Valsalva Aneurysm in a Foetus. Cardiol. Young 2013, 23, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Kniffin, C.L. #614332 CHROMOSOME 2p16.3 DELETION SYNDROME. OMIM, National Center for Biotechnology Information. 7 November 2011. Available online: https://www.omim.org/entry/614332# (accessed on 4 March 2023).
- Feldman, D.N.; Roman, M.J. Aneurysms of the Sinuses of Valsalva. Cardiology 2006, 106, 73–81. [Google Scholar] [CrossRef]
- Bricker, A.O.; Avutu, B.; Mohammed, T.-C.; Williamson, E.E.; Syed, I.S.; Julsrud, P.R.; Schoenhagen, P.; Kirsch, J. Valsalva sinus aneurysms: Findings at CT and MR Imaging. RadioGraphics 2010, 30, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Purnell, R.; Williams, I.; Von Oppell, U.; Wood, A. Giant aneurysms of the sinuses of Valsalva and aortic regurgitation in a patient with Noonan’s syndrome. Eur. J. Cardiothorac. Surg. 2005, 28, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.M.; Coupe, M.O.; Honey, M.; Miller, G.A. Aneurysms of the sinuses of Valsalva in Noonan’s syndrome. Eur. Heart J. 1989, 10, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Unlu, M.; Ozeke, O.; Kara, M.; Yesillik, S. Ruptured sinus of Valsalva aneurysm associated with noncompaction of the ventricular myocardium. Eur. J. Echocardiogr. 2008, 9, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Jalili Shahandashti, F.; Saedi, S.; Pouraliakbar, H.R. Incidental detection of ruptured sinus of Valsalva aneurysm and coronary abnormality in a patient with penetrating chest trauma. Echocardiography 2022, 39, 387–389. [Google Scholar] [CrossRef]
- Muehlschlegel, J.D.; Alomar-Melero, E.; Staples, E.D.; Janelle, G.M. Acute High-Output Failure from an Aortoventricular Fistula Due to a Ruptured Sinus of Valsalva Aneurysm After Blunt Chest Trauma. Anesth. Analg. 2006, 10, 1408–1409. [Google Scholar] [CrossRef]
- Morais, H.; Martins, T. Right sinus of Valsalva aneurysm with rupture into the interventricular septum and into the left ventricle. Heart Views J. 2015, 16, 114–115. [Google Scholar] [CrossRef]
- Choudhary, S.K.; Bhan, A.; Reddy, S.C.R. Aneurysm of sinus of Valsalva dissecting into interventricular septum. Ann. Thorac. Surg. 1998, 65, 735–740. [Google Scholar] [CrossRef]
- Jain, A.; Acuthan, G. Rupture of Sinus of Valsalva Aneurysm into Interventricular Septum: Role of Cardiac CT. Cureus 2019, 11, e5589. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kang, E.-J.; Park, T.-H.; Woo, J.S.; Hong, S.H.; Kim, Y.-D. A case of right sinus of Valsalva rupture with dissection into interventricular septum causing left ventricular outflow tract obstruction. Korean Circ. J. 2013, 43, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wu, Q.Y.; Tang, Y. Ruptured sinus of Valsalva aneurysm: A Beijing experience. Ann. Thorac. Surg. 2002, 74, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Hoevelborn, T.; Doering, J.; Lindemann, S.; Haas, C.S. Newly Discovered Heart Murmur: Noncoronary Sinus of Valsalva Aneurysm with Rupture into the Right Atrium and Right Ventricle. Circulation 2009, 119, e15–e16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigg, H.; Bolin, E.; Zakaria, D.; Bornemeier, R. Ruptured Sinus of Valsalva Aneurysm with Resultant Myocardial Pouch Formation in the Fetal Heart—A Diagnostic Challenge. J. Cardiovasc. Dev. Dis. 2024, 11, 23. https://doi.org/10.3390/jcdd11010023
Bigg H, Bolin E, Zakaria D, Bornemeier R. Ruptured Sinus of Valsalva Aneurysm with Resultant Myocardial Pouch Formation in the Fetal Heart—A Diagnostic Challenge. Journal of Cardiovascular Development and Disease. 2024; 11(1):23. https://doi.org/10.3390/jcdd11010023
Chicago/Turabian StyleBigg, Hugh, Elijah Bolin, Dala Zakaria, and Renee Bornemeier. 2024. "Ruptured Sinus of Valsalva Aneurysm with Resultant Myocardial Pouch Formation in the Fetal Heart—A Diagnostic Challenge" Journal of Cardiovascular Development and Disease 11, no. 1: 23. https://doi.org/10.3390/jcdd11010023
APA StyleBigg, H., Bolin, E., Zakaria, D., & Bornemeier, R. (2024). Ruptured Sinus of Valsalva Aneurysm with Resultant Myocardial Pouch Formation in the Fetal Heart—A Diagnostic Challenge. Journal of Cardiovascular Development and Disease, 11(1), 23. https://doi.org/10.3390/jcdd11010023




