Prognostic Value of Strain by Speckle Tracking Echocardiography in Patients with Arrhythmogenic Right Ventricular Cardiomyopathy
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Electrocardiographic Evaluation
2.3. Echocardiography
3. Statistical Analysis
4. Results
4.1. Patient Cohort
4.2. Baseline Characteristics
4.3. Electrocardiographic Evaluation
4.4. Imaging Evaluation
4.5. Strain Analysis
5. Discussion
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jin, Q.; Lee, K.Y.; Selimi, Z.; Shimura, D.; Wang, E.; Zimmerman, J.F.; Shaw, R.M.; Kucera, J.P.; Parker, K.K.; Saffitz, J.E. Determinants of electrical propagation and propagation block in Arrhythmogenic Cardiomyopathy. J. Mol. Cell. Cardiol. 2024, 186, 71–80. [Google Scholar] [CrossRef]
- Corrado, D.; Link, M.S.; Calkins, H. Arrhythmogenic right ventricular cardiomyopathy. N. Engl. J. Med. 2017, 376, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.; Daubert, J.P. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation 2010, 121, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Wichter, T.; Link, M.S.; Hauer, R.N.; Marchlinski, F.E.; Anastasakis, A.; Bauce, B.; Basso, C.; Brunckhorst, C.; Tsatsopoulou, A. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: An international task force consensus statement. Circulation 2015, 132, 441–453. [Google Scholar] [CrossRef]
- Aneq, M.Å.; Lindström, L.; Fluur, C.; Nylander, E. Long-term follow-up in arrhythmogenic right ventricular cardiomyopathy using tissue Doppler imaging. Scand. Cardiovasc. J. 2008, 42, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Sadeghpour, A.; Hosseini, L.; Rezaeian, N.; Alizadehasl, A.; Maleki, M.; Emkanjoo, Z.; Bakhshandeh, H.; Zadehbagheri, F. Presence and prognostic value of ventricular diastolic function in arrhythmogenic right ventricular cardiomyopathy. Echocardiography 2020, 37, 1766–1773. [Google Scholar] [CrossRef]
- Kamal, N.M.; Salih, A.F.; Ali, B.M. Speckle tracking echocardiography for diagnosis of right ventricular failure in children with totally corrected tetralogy of Fallot in Sulaimani, Iraq. J. Taibah Univ. Med. Sci. 2024, 19, 198–208. [Google Scholar] [CrossRef]
- Badano, L.P.; Muraru, D. Subclinical right ventricular dysfunction by strain analysis: Refining the targets of echocardiographic imaging in systemic sclerosis. Am. Heart Assoc. 2016, 9, e005009. [Google Scholar] [CrossRef]
- Ho, S.Y. Anatomy and myoarchitecture of the left ventricular wall in normal and in disease. Eur. J. Echocardiogr. 2009, 10, iii3–iii7. [Google Scholar] [CrossRef]
- Aljehani, A.; Kew, T.; Baig, S.; Cox, H.; Sommerfeld, L.; Ensam, B.; Kalla, M.; Steeds, R.; Fabritz, L. Characterisation of patients referred to a tertiary-level inherited cardiac condition clinic with suspected arrhythmogenic right ventricular cardiomyopathy (ARVC). BMC Cardiovasc. Disord. 2023, 23, 14. [Google Scholar] [CrossRef]
- Aljehani, A.; Baig, S.; Kew, T.; Kalla, M.; Sommerfeld, L.C.; Murukutla, V.A.; Fabritz, L.; Steeds, R.P. Structural Progression in Patients with Definite and Non-Definite Arrhythmogenic Right Ventricular Cardiomyopathy and Risk of Major Adverse Cardiac Events. Biomedicines 2024, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Rana, B.; Oxborough, D.; Steeds, R.; Monaghan, M.; Stout, M.; Pearce, K.; Harkness, A.; Ring, L.; Paton, M. A practical guideline for performing a comprehensive transthoracic echocardiogram in adults: The British Society of Echocardiography minimum dataset. Echo Res. Pract. 2020, 7, G59–G93. [Google Scholar] [CrossRef] [PubMed]
- Kirkels, F.P.; Rootwelt-Norberg, C.; Bosman, L.P.; Aabel, E.W.; Muller, S.A.; Castrini, A.I.; Taha, K.; van Osta, N.; Lie, Ø.H.; Asselbergs, F.W. The added value of abnormal regional myocardial function for risk prediction in arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J.-Cardiovasc. Imaging 2023, 24, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Claeys, M.; Claessen, G.; Claus, P.; De Bosscher, R.; Dausin, C.; Voigt, J.-U.; Willems, R.; Heidbuchel, H.; La Gerche, A. Right ventricular strain rate during exercise accurately identifies male athletes with right ventricular arrhythmias. Eur. Heart J.-Cardiovasc. Imaging 2020, 21, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Sarvari, S.I.; Haugaa, K.H.; Anfinsen, O.-G.; Leren, T.P.; Smiseth, O.A.; Kongsgaard, E.; Amlie, J.P.; Edvardsen, T. Right ventricular mechanical dispersion is related to malignant arrhythmias: A study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur. Heart J. 2011, 32, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, M.K. Peeking Beyond Strain’s Peak: Regional Strain Patterns and Dispersion in Arrhythmogenic Right Ventricular Cardiomyopathy; American College of Cardiology Foundation: Washington, DC, USA, 2021; Volume 14, pp. 911–914. [Google Scholar]
- Leren, I.S.; Saberniak, J.; Haland, T.F.; Edvardsen, T.; Haugaa, K.H. Combination of ECG and echocardiography for identification of arrhythmic events in early ARVC. JACC Cardiovasc. Imaging 2017, 10, 503–513. [Google Scholar] [CrossRef]
- Lie, Ø.H.; Rootwelt-Norberg, C.; Dejgaard, L.A.; Leren, I.S.; Stokke, M.K.; Edvardsen, T.; Haugaa, K.H. Prediction of life-threatening ventricular arrhythmia in patients with arrhythmogenic cardiomyopathy: A primary prevention cohort study. JACC Cardiovasc. Imaging 2018, 11, 1377–1386. [Google Scholar] [CrossRef]
- Kirkels, F.P.; Lie, Ø.H.; Cramer, M.J.; Chivulescu, M.; Rootwelt-Norberg, C.; Asselbergs, F.W.; Teske, A.J.; Haugaa, K.H. Right ventricular functional abnormalities in arrhythmogenic cardiomyopathy: Association with life-threatening ventricular arrhythmias. Cardiovasc. Imaging 2021, 14, 900–910. [Google Scholar] [CrossRef]
- Saguner, A.M.; Vecchiati, A.; Baldinger, S.H.; Rüeger, S.; Medeiros-Domingo, A.; Mueller-Burri, A.S.; Haegeli, L.M.; Biaggi, P.; Manka, R.; Lüscher, T.F. Different prognostic value of functional right ventricular parameters in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ. Cardiovasc. Imaging 2014, 7, 230–239. [Google Scholar] [CrossRef]
- Roy, C.; Duclos, G.; Nafati, C.; Gardette, M.; Lopez, A.; Pastene, B.; Gaudray, E.; Boussuges, A.; Antonini, F.; Leone, M. Left ventricular longitudinal strain variations assessed by speckle-tracking echocardiography after a passive leg raising maneuver in patients with acute circulatory failure to predict fluid responsiveness: A prospective, observational study. PLoS ONE 2021, 16, e0257737. [Google Scholar] [CrossRef]
- DiLorenzo, M.P.; Bhatt, S.M.; Mercer-Rosa, L. How best to assess right ventricular function by echocardiography. Cardiol. Young 2015, 25, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Poulin, F.; Lim, K.-D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll. Cardiol. 2014, 63 Pt A, 2751–2768. [Google Scholar] [CrossRef] [PubMed]
- Hamada-Harimura, Y.; Seo, Y.; Ishizu, T.; Nishi, I.; Machino-Ohtsuka, T.; Yamamoto, M.; Sugano, A.; Sato, K.; Sai, S.; Obara, K. Incremental prognostic value of right ventricular strain in patients with acute decompensated heart failure. Circ. Cardiovasc. Imaging 2018, 11, e007249. [Google Scholar] [CrossRef] [PubMed]
- Chimed, S.; Stassen, J.; Galloo, X.; Meucci, M.C.; Knuuti, J.; Delgado, V.; van der Bijl, P.; Marsan, N.A.; Bax, J.J. Prognostic Relevance of Left Ventricular Global Longitudinal Strain in Patients with Heart Failure and Reduced Ejection Fraction. Am. J. Cardiol. 2023, 202, 30–40. [Google Scholar] [CrossRef]
- Zhu, D.; Ito, S.; Miranda, W.R.; Nkomo, V.T.; Pislaru, S.V.; Villarraga, H.R.; Pellikka, P.A.; Crusan, D.J.; Oh, J.K. Left ventricular global longitudinal strain is associated with long-term outcomes in moderate aortic stenosis. Circ. Cardiovasc. Imaging 2020, 13, e009958. [Google Scholar] [CrossRef] [PubMed]
- Tower-Rader, A.; Betancor, J.; Popovic, Z.B.; Sato, K.; Thamilarasan, M.; Smedira, N.G.; Lever, H.M.; Desai, M.Y. Incremental prognostic utility of left ventricular global longitudinal strain in hypertrophic obstructive cardiomyopathy patients and preserved left ventricular ejection fraction. J. Am. Heart Assoc. 2017, 6, e006514. [Google Scholar] [CrossRef]
- Segura-Rodríguez, D.; Bermúdez-Jiménez, F.J.; González-Camacho, L.; Moreno Escobar, E.; García-Orta, R.; Alcalá-López, J.E.; Bautista Pavés, A.; Oyonarte-Ramírez, J.M.; López-Fernández, S.; Álvarez, M. Layer-specific global longitudinal strain predicts arrhythmic risk in arrhythmogenic cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 748003. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef]
- Nagy, V.K.; Széplaki, G.; Apor, A.; Kutyifa, V.; Kovács, A.; Kosztin, A.; Becker, D.; Boros, A.M.; Gellér, L.; Merkely, B. Role of right ventricular global longitudinal strain in predicting early and long-term mortality in cardiac resynchronization therapy patients. PLoS ONE 2015, 10, e0143907. [Google Scholar] [CrossRef]
- Gao, Y.; Li, H.; He, L.; Zhang, Y.; Sun, W.; Li, M.; Gao, L.; Lin, Y.; Ji, M.; Lv, Q. Superior prognostic value of right ventricular free wall compared to global longitudinal strain in patients with repaired tetralogy of Fallot. Front. Cardiovasc. Med. 2022, 9, 996398. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
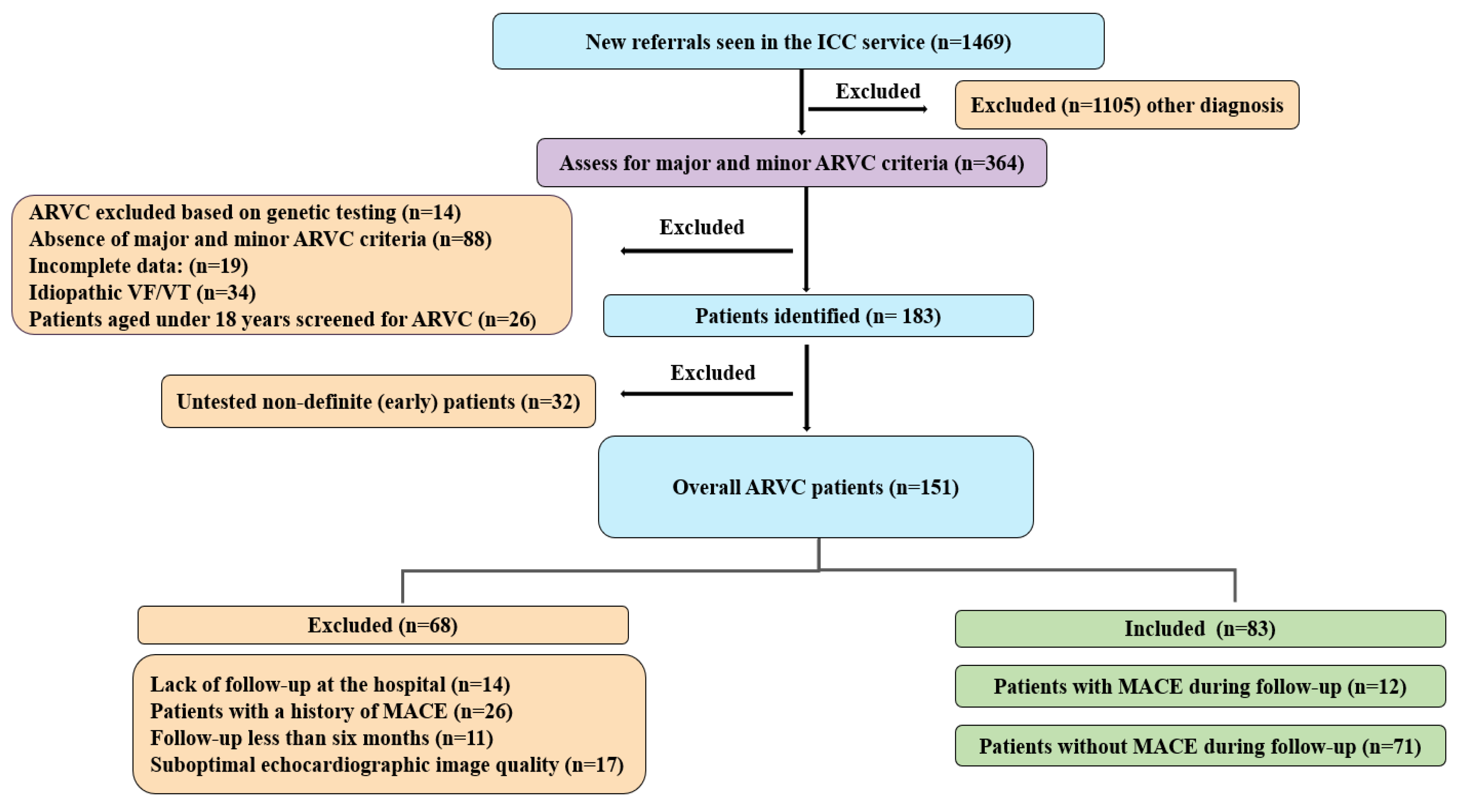

| Demographic | Overall = 83 | MACE During Follow-Up = 12 | No MACE During Follow-Up = 71 | p Value |
|---|---|---|---|---|
| Age (years), median (IQR) | 37 (23, 53) | 43 (32, 52) | 37 (23, 53) | 0.641 |
| Male sex, (%) | 42 (51%) | 7 (58%) | 35 (49%) | 0.756 |
| Body surface area (m2), median (IQR) | 1.9 (1.7, 2.0) | 1.9 (1.7, 2.0) | 1.9 (1.7, 2.0) | 0.466 |
| Stage of ARVC | ||||
| Definite, (%) | 26 (31%) | 9 (75%) | 17 (24%) | 0.001 |
| Early, (%) | 57 (69%) | 3 (25%) | 54 (76%) | |
| a Pathogenic variant (%) | 58 (71%) | 7 (58%) | 51 (73%) | 0.320 |
| PKP2, (%) | 32 (39%) | 4 (33%) | 28 (40%) | 0.757 |
| DSP, (%) | 25 (30%) | 2 (17%) | 23 (33%) | 0.328 |
| DSG2, (%) | 1 (1%) | 1 (8%) | 0 (0%) | 0.146 |
| Variant of unknown significance (VUS), (%) | 1 (1%) | 1 (8%) | 0 (0%) | 0.146 |
| No pathogenic mutation identified, (%) | 23 (28%) | 4 (33%) | 19 (27%) | 0.731 |
| Symptoms | ||||
| Palpitation, (%) | 26 (31%) | 9 (75%) | 17 (24%) | 0.001 |
| Syncope, (%) | 10 (12%) | 7 (58%) | 3 (4%) | <0.001 |
| b History of sport | ||||
| History of Competitive sport, (%) | 15 (20%) | 4 (36%) | 11 (17%) | 0.302 |
| History of Non-competitive sport, (%) | 14 (19%) | 1 (9%) | 13 (21%) | |
| No history of sport, (%) | 45 (61%) | 6 (55%) | 39 (62%) | |
| Medication | ||||
| Statins, (%) | 4 (5%) | 3 (25%) | 1 (1%) | 0.009 |
| Anticoagulant, (%) | 5 (6%) | 3 (25%) | 2 (3%) | 0.020 |
| Antiarrhythmic drugs, (%) | 5 (6%) | 3 (25%) | 2 (3%) | 0.020 |
| Beta blocker, (%) | 11 (13%) | 6 (50%) | 5 (7%) | <0.001 |
| Follow-up period (years), median (IQR) | 3 (1, 5) | 1 (1, 5) | 3 (2, 5) | 0.123 |
| Overall = 83 | MACE During Follow-Up = 12 | No MACE During Follow-Up = 71 | p Value | |
|---|---|---|---|---|
| Echo Data | ||||
| RVOT PLAX (cm), mean ± SD | 3.0 ± 0.7 | 3.8 ± 0.6 | 2.8 ± 0.6 | <0.001 |
| RVEDA (cm2), median, (IQR) | 19 (16, 24) | 27 (20, 35) | 18 (15, 21) | <0.001 |
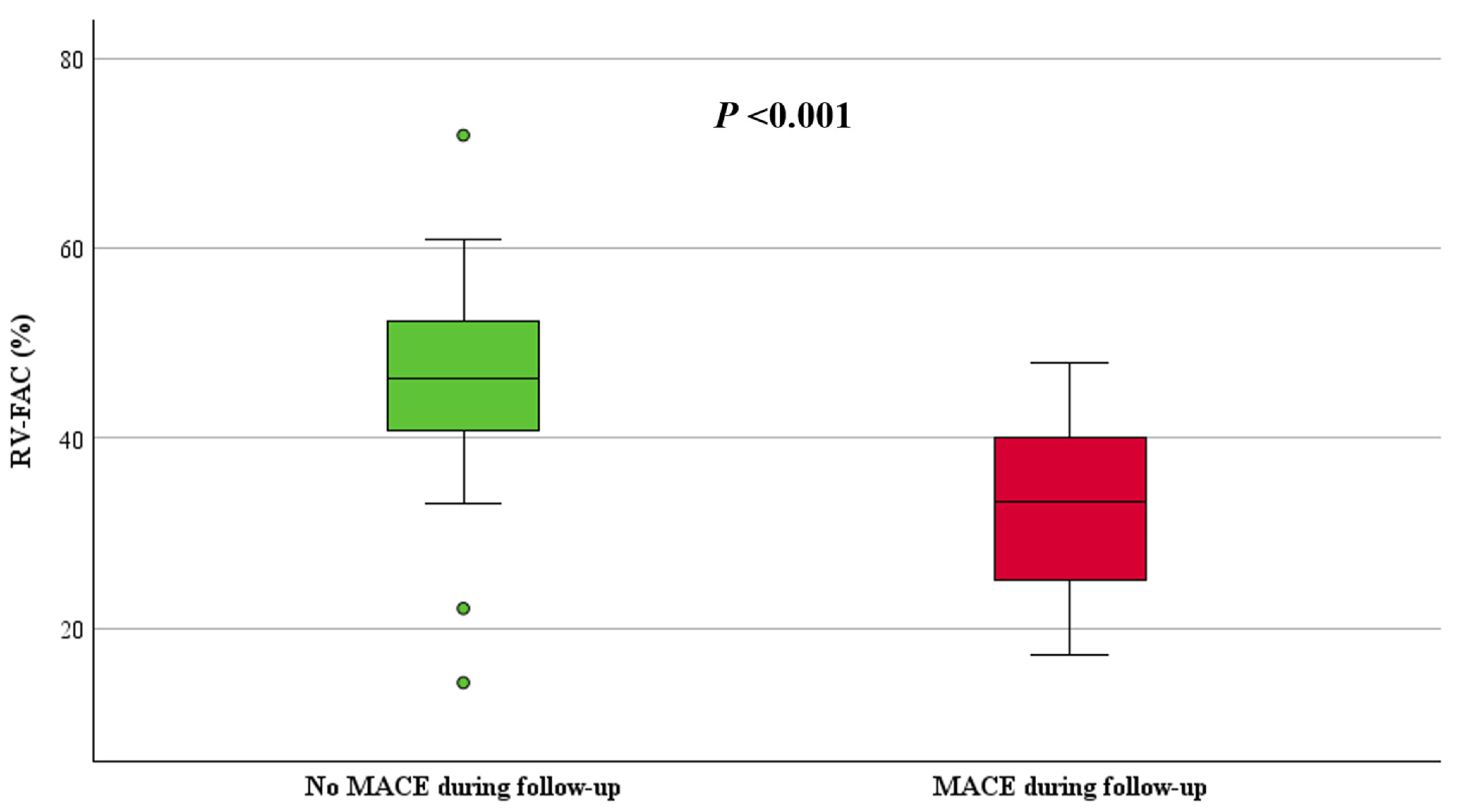
| RV-FAC (%), median, (IQR) | 45 (39, 51) | 33 (25, 40) | 46 (41, 53) | <0.001 |
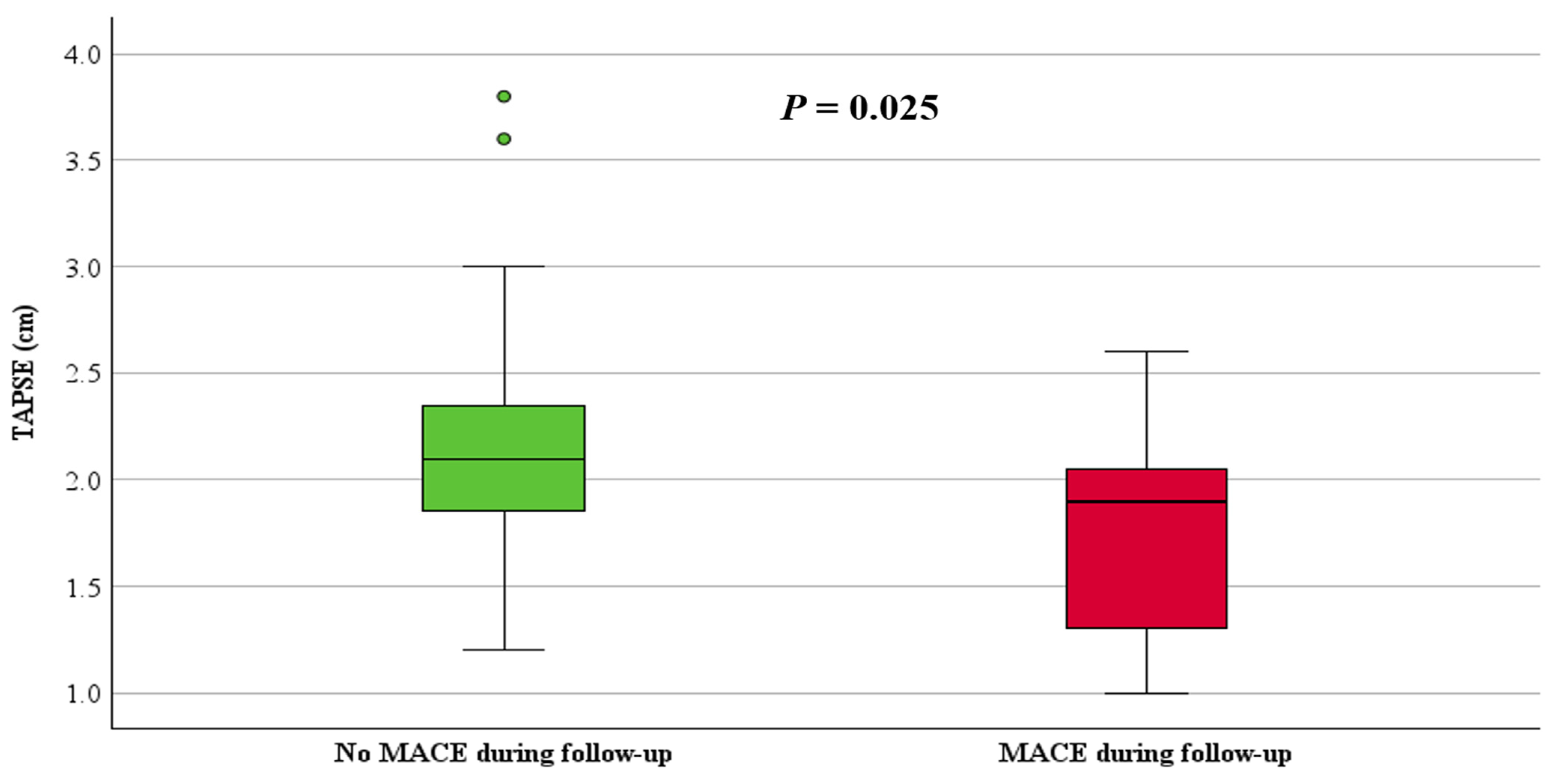
| TAPSE (cm), median, (IQR) | 2.0 (1.8, 2.3) | 1.9 (1.3, 2.1) | 2.1 (1.8, 2.4) | 0.025 |
| LVEDV (ml), mean ± SD | 98 ± 30 | 99 ± 42 | 98 ± 28 | 0.926 |
| LV-EF (%), median, (IQR) | 61 (57, 66) | 57 (48, 62) | 62 (58, 67) | 0.027 |
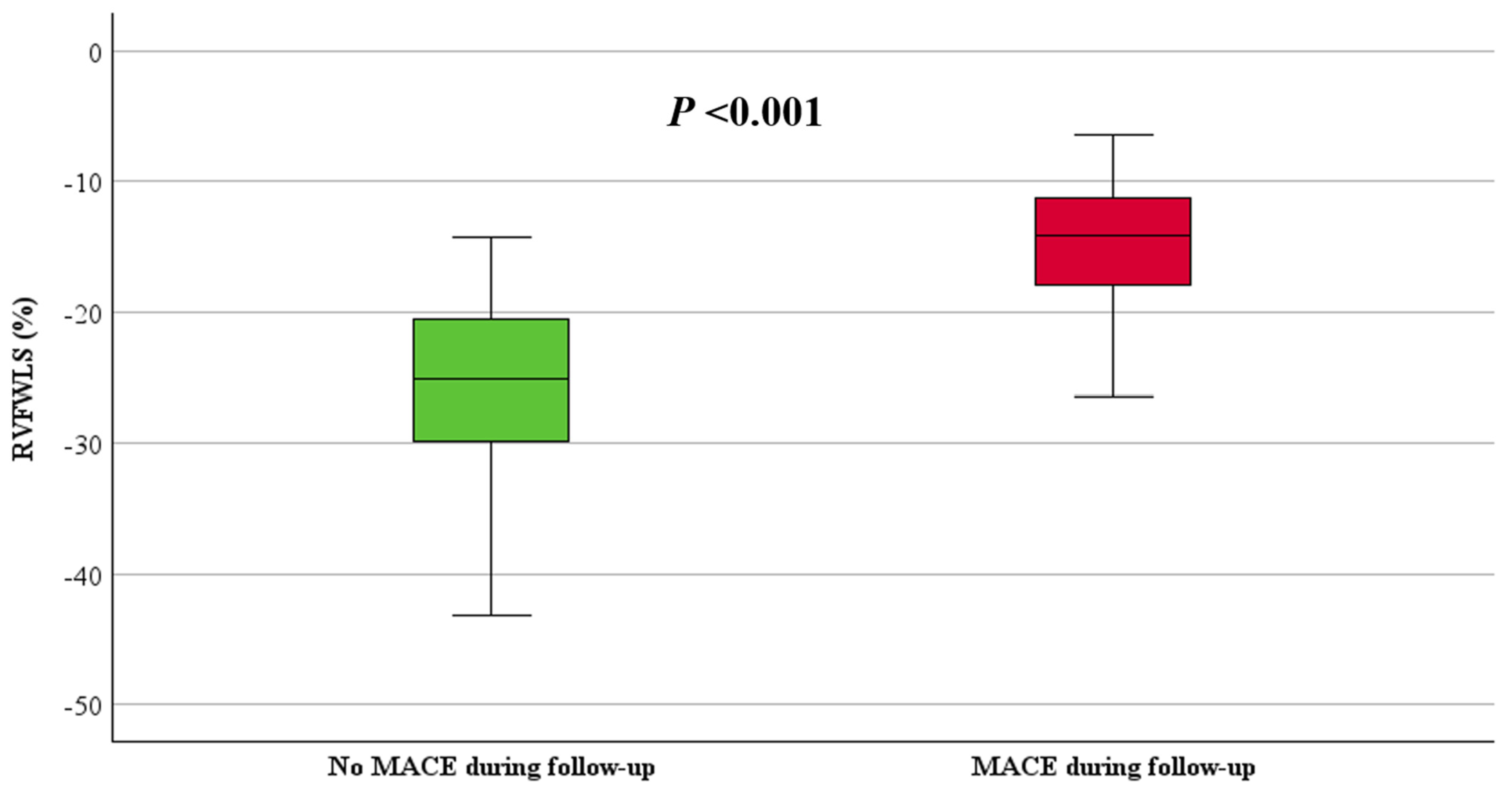
| STE-RVFWLS (%), mean ± SD | −24 ± 8 | −15 ± 5 | −25 ± 7 | <0.001 |
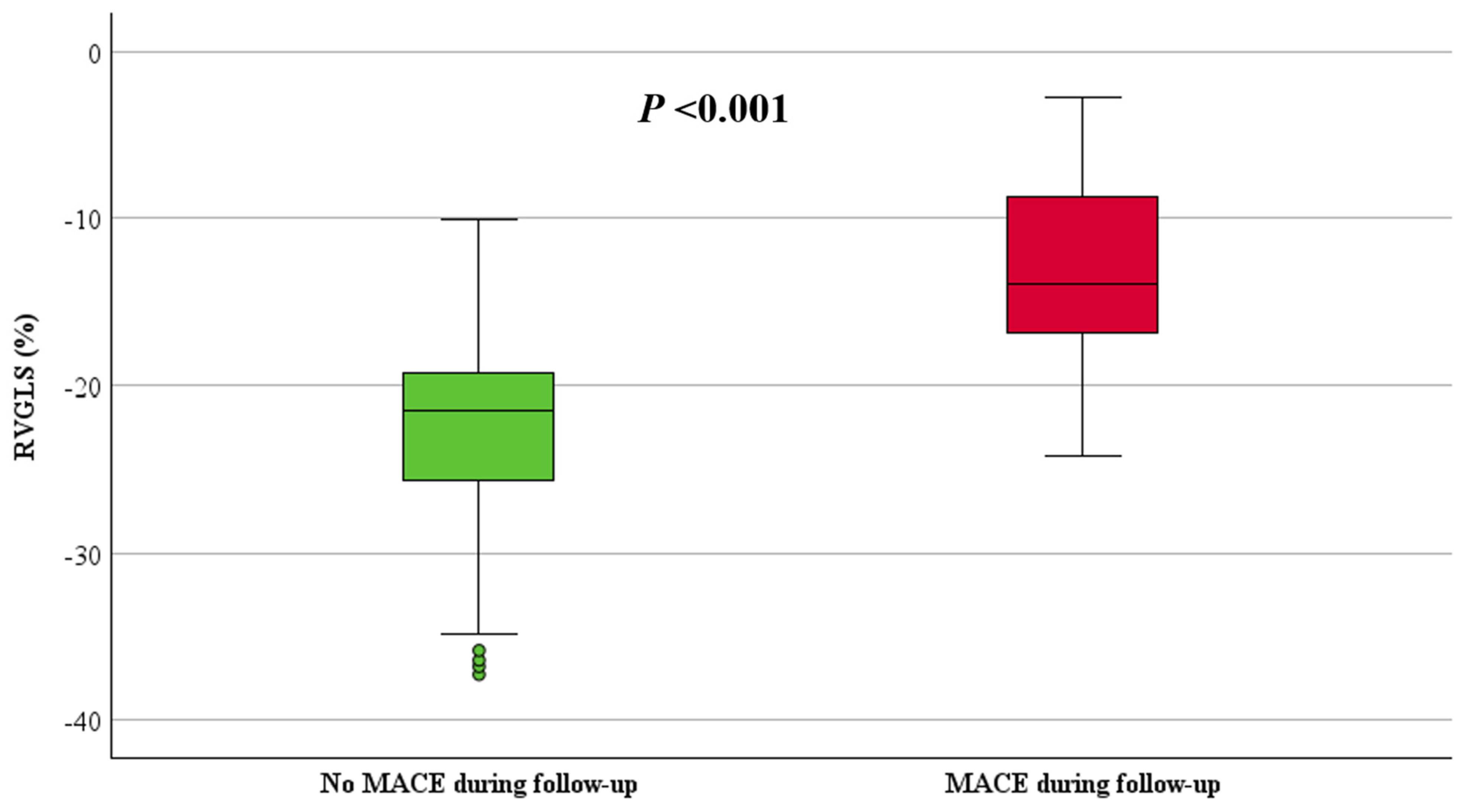
| STE-RVGLS (%), mean ± SD | −21 ± 7 | −13 ± 6 | −23 ± 6 | <0.001 |
| STE-LVGLS (%), mean ± SD | −22 ± 5 | −18 ± 4 | −23 ± 5 | 0.002 |
| a LGE present, (%) | 16 (34%) | 6 (86%) | 10 (25%) | 0.004 |
| RV-LGE, (%) | 11 (23%) | 4 (57%) | 7 (18%) | 0.042 |
| LV-LGE, (%) | 10 (21%) | 4 (57%) | 6 (15%) | 0.029 |
| Univariable Model | ||
|---|---|---|
| HR (95% CI) | p-Value | |
| RV-FAC (%) | 0.9 (0.9, 1.0) | 0.004 |
| TAPSE (cm) | 0.1 (0.0, 0.7) | 0.015 |
| STE-RVFWLS (%) | 1.3 (1.1, 1.4) | <0.001 |
| STE-RVGLS (%) | 1.2 (1.1, 1.3) | <0.001 |
| STE-LVGLS (%) | 1.5 (1.2, 1.9) | 0.002 |
| Multivariable Model 1 | Multivariable Model 2 | Multivariable Model 3 | Multivariable Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| RV-FAC | TAPSE | STE-LVGLS | ||||||
| HR (95% CI) | p-Value | HR | p-Value | HR | p-Value | HR | p-Value | |
| (95% CI) | (95% CI) | (95% CI) | ||||||
| RV-FAC (%), per unit measure | 1.0 (0.9, 1.1) | 0.926 | - | - | - | - | - | - |
| TAPSE (cm), per unit measure | - | - | 0.5 (0.1, 2.5) | 0.367 | - | - | - | - |
| STE-RVFWLS (%), per unit measure | 1.4 (1.0, 2.0) | 0.031 | 1.4 (1.1, 2.0) | 0.020 | 1.6 (1.1, 2.4) | 0.010 | 1.4 (1.0, 2.0) | 0.026 |
| STE-RVGLS (%) per unit measure | 0.9 (0.7,1.2) | 0.379 | 0.9 (0.6, 1.1) | 0.243 | 0.7 (0.5, 1.0) | 0.060 | 0.9 (0.7, 1.2) | 0.377 |
| STE-LVGLS (%) per unit measure | - | - | - | - | 1.3 (1.0, 1.7) | 0.034 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljehani, A.; Win, K.Z.; Baig, S.; Kalla, M.; Ensam, B.; Fabritz, L.; Steeds, R.P. Prognostic Value of Strain by Speckle Tracking Echocardiography in Patients with Arrhythmogenic Right Ventricular Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2024, 11, 388. https://doi.org/10.3390/jcdd11120388
Aljehani A, Win KZ, Baig S, Kalla M, Ensam B, Fabritz L, Steeds RP. Prognostic Value of Strain by Speckle Tracking Echocardiography in Patients with Arrhythmogenic Right Ventricular Cardiomyopathy. Journal of Cardiovascular Development and Disease. 2024; 11(12):388. https://doi.org/10.3390/jcdd11120388
Chicago/Turabian StyleAljehani, Areej, Kyaw Zaw Win, Shanat Baig, Manish Kalla, Bode Ensam, Larissa Fabritz, and Richard P. Steeds. 2024. "Prognostic Value of Strain by Speckle Tracking Echocardiography in Patients with Arrhythmogenic Right Ventricular Cardiomyopathy" Journal of Cardiovascular Development and Disease 11, no. 12: 388. https://doi.org/10.3390/jcdd11120388
APA StyleAljehani, A., Win, K. Z., Baig, S., Kalla, M., Ensam, B., Fabritz, L., & Steeds, R. P. (2024). Prognostic Value of Strain by Speckle Tracking Echocardiography in Patients with Arrhythmogenic Right Ventricular Cardiomyopathy. Journal of Cardiovascular Development and Disease, 11(12), 388. https://doi.org/10.3390/jcdd11120388







