Thrombus Imaging Characteristics to Predict Early Recanalization in Anterior Circulation Large Vessel Occlusion Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Ethical Approval
2.3. Patient and Thrombus Imaging Characteristics
2.3.1. Regional Database
2.3.2. MR CLEAN Database
2.4. Statistical Analysis
2.4.1. Group Comparison
2.4.2. Prediction Model
3. Results
3.1. Patient and Thrombus Imaging Characteristics
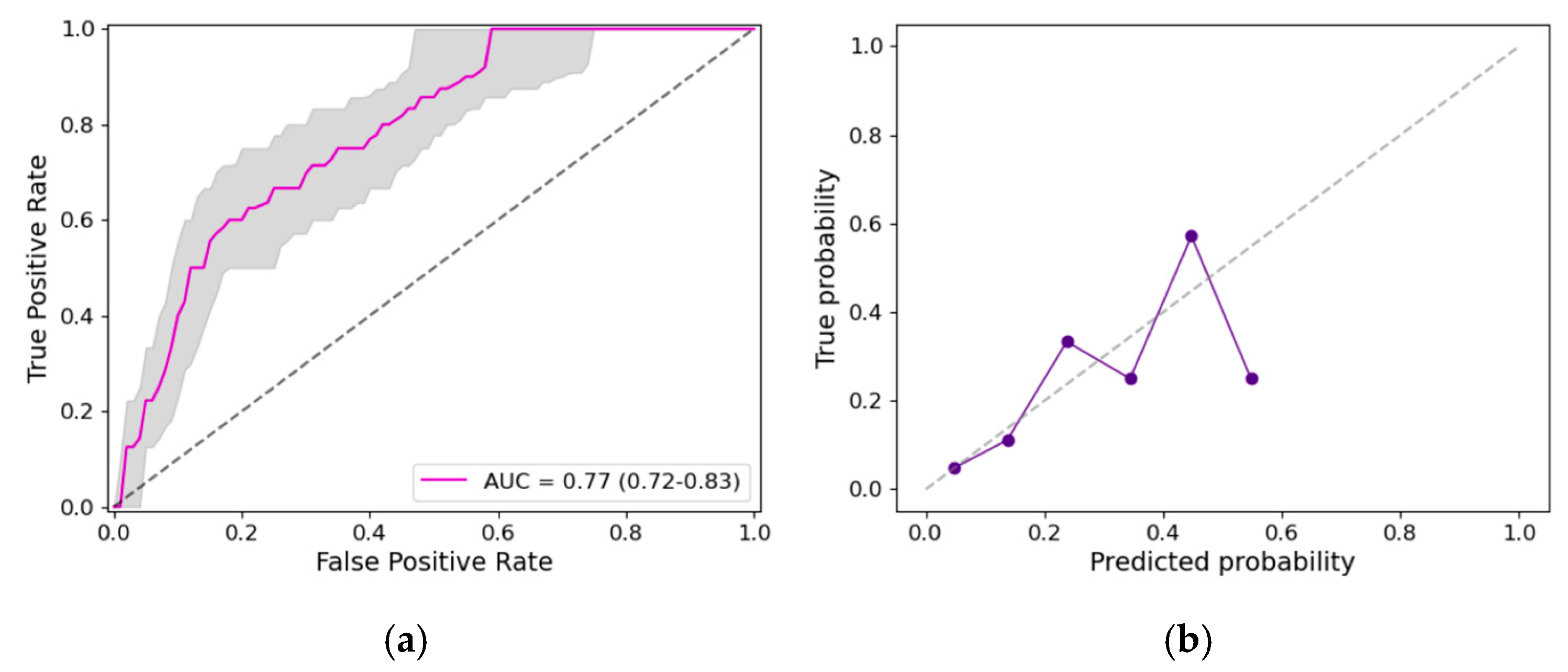
3.2. Prediction Model
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACA | anterior cerebral artery |
| CI | confidence interval |
| CSC | comprehensive stroke center |
| CT | computed tomography |
| CTA | computed tomography angiography |
| DT | distance from intracranial carotid artery terminus to the thrombus |
| ER-LVO | early recanalized large vessel occlusion |
| EVT | endovascular treatment |
| HU | Hounsfield units |
| IQR | interquartile range |
| ICA | internal carotid artery |
| IVT | intravenous treatment with alteplase |
| IVT to CSC door | time from intravenous treatment with alteplase initiation to arrival at the comprehensive stroke center |
| mm | millimeters |
| MCA | middle cerebral artery |
| mRS | modified Rankin scale |
| NCCT | non-contrast computed tomography |
| NER-LVO | non-early recanalized large vessel occlusion |
| NIHSS | National Institute of Health Stroke Scale |
| Onset to PSC door | time from stroke onset to arrival at the primary stroke center |
| Onset to PSC imaging | time from stroke onset to imaging at the primary stroke center |
| PSC | primary stroke center |
| PSC door to CSC door | time from arrival at the primary stroke center to arrival at the comprehensive stroke center |
| PSC door to IVT | time from arrival at the primary stroke center to initiation of intravenous treatment with alteplase sICH, symptomatic intracranial hemorrhage |
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke a Guideline for Healthcare Professionals from the American Heart Association/American Stroke A. Stroke 2019, 50, E344–E418. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, P.; Schirmer, C.M. Early Recanalization with Intravenous Thrombolysis before Mechanical Thrombectomy: Considerations to Explore with Tenecteplase. J. NeuroInterv. Surg. 2022, 15, 513–514. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.; Pult, F.; Meisterernst, J.; Heldner, M.R.; Mono, M.L.; Kurmann, R.; Buehlmann, M.; Fischer, U.; Mattle, H.P.; Arnold, M.; et al. Impact of Intravenous Thrombolysis on Recanalization Rates in Patients with Stroke Treated with Bridging Therapy. Eur. J. Neurol. 2017, 24, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Adeoye, O. Intravenous Thrombolysis before Endovascular Thrombectomy for Acute Ischemic Stroke. JAMA J. Am. Med. Assoc. 2021, 325, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Simpson, J.R.; Silver, B.; Silver, B. Safety of Thrombolysis in Acute Ischemic Stroke: A Review of Complications, Risk Factors, and Newer Technologies. Neurohospitalist 2011, 1, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Menon, B.K.; Al-Ajlan, F.S.; Horn, M.; Najm, M.; Al-Sultan, A.; Puig, J.; Dowlatshahi, D.; Calleja Sanz, A.I.; Sohn, S.-I.; et al. Thrombus Migration and Fragmentation After Intravenous Alteplase Treatment: The INTERRSeCT Study. Stroke 2021, 52, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Chapot, R.; Agid, R.; Hassan, A.; Jadhav, A.P.; Liebeskind, D.S.; Lobotesis, K.; Meila, D.; Meyer, L.; Raphaeli, G.; et al. Thrombectomy for Distal, Medium Vessel Occlusions: A Consensus Statement on Present Knowledge and Promising Directions. Stroke 2020, 51, 2872–2884. [Google Scholar] [CrossRef] [PubMed]
- Arrarte Terreros, N.; Bruggeman, A.A.E.; Swijnenburg, I.S.J.; Van Meenen, L.C.C.; Groot, A.E.; Coutinho, J.M.; Roos, Y.B.W.E.M.; Emmer, B.J.; Beenen, L.F.M.; Van Bavel, E.; et al. Early Recanalization in Large-Vessel Occlusion Stroke Patients Transferred for Endovascular Treatment. J. NeuroInterv. Surg. 2021, 14, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, B.; Alonso de Leciñana, M.; Ximénez-Carrillo, A.; Martínez-Sánchez, P.; Cruz-Culebras, A.; Zapata-Wainberg, G.; Ruiz-Ares, G.; Frutos, R.; Fandiño, E.; Caniego, J.L.; et al. Futile Interhospital Transfer for Endovascular Treatment in Acute Ischemic Stroke: The Madrid Stroke Network Experience. Stroke 2015, 46, 2156–2161. [Google Scholar] [CrossRef]
- Seners, P.; Turc, G.; Naggara, O.; Henon, H.; Piotin, M.; Arquizan, C.; Cho, T.H.; Narata, A.P.; Lapergue, B.; Richard, S.; et al. Post-Thrombolysis Recanalization in Stroke Referrals for Thrombectomy: Incidence, Predictors, and Prediction Scores. Stroke 2018, 49, 2975–2982. [Google Scholar] [CrossRef]
- Lucas-Noll, J.; Clua-Espuny, J.L.; Lleixà-Fortuño, M.; Gavaldà-Espelta, E.; Queralt-Tomas, L.; Panisello-Tafalla, A.; Carles-Lavila, M. The Costs Associated with Stroke Care Continuum: A Systematic Review. Health Econ. Rev. 2023, 13, 32. [Google Scholar] [CrossRef]
- Schlemm, L.; Endres, M.; Nolte, C.H. Cost Effectiveness of Interhospital Transfer for Mechanical Thrombectomy of Acute Large Vessel Occlusion Stroke: Role of Predicted Recanalization Rates. Circ. Cardiovasc. Qual. Outcomes 2021, 14, E007444. [Google Scholar] [CrossRef] [PubMed]
- Van Meenen, L.C.C.; Arrarte Terreros, N.; Groot, A.E.; Kappelhof, M.; Beenen, L.F.M.; Marquering, H.A.; Emmer, B.J.; Roos, Y.B.W.E.M.; Majoie, C.B.L.M.; Coutinho, J.M. Value of Repeated Imaging in Patients with a Stroke Who Are Transferred for Endovascular Treatment. J. NeuroInterv. Surg. 2021, 14, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.K.; Al-Ajlan, F.S.; Najm, M.; Puig, J.; Castellanos, M.; Dowlatshahi, D.; Calleja, A.; Sohn, S.I.; Ahn, S.H.; Poppe, A.; et al. Association of Clinical, Imaging, and Thrombus Characteristics with Recanalization of Visible Intracranial Occlusion in Patients with Acute Ischemic Stroke. JAMA J. Am. Med. Assoc. 2018, 320, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, A.B.; Gocmen, R.; Arsava, E.M.; Topcuoglu, M.A. The Effect of Clot Volume and Permeability on Response to Intravenous Tissue Plasminogen Activator in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 104541. [Google Scholar] [CrossRef]
- Seners, P.; Delepierre, J.; Turc, G.; Henon, H.; Piotin, M.; Arquizan, C.; Cho, T.H.; Lapergue, B.; Cottier, J.P.; Richard, S.; et al. Thrombus Length Predicts Lack of Post-Thrombolysis Early Recanalization in Minor Stroke with Large Vessel Occlusion. Stroke 2019, 50, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Niesten, J.; van der Schaaf, I.; van der Graaf, Y.; Kappelle, L.; Biessels, G.; Horsch, A.; Dankbaar, J.; Luitse, M.; van Seeters, T.; Smit, E.; et al. Predictive Value of Thrombus Attenuation on Thin-Slice Non-Contrast CT for Persistent Occlusion after Intravenous Thrombolysis. Cerebrovasc. Dis. 2014, 37, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Jolugbo, P.; Ariëns, R.A.S. Thrombus Composition and Efficacy of Thrombolysis and Thrombectomy in Acute Ischemic Stroke. Stroke 2021, 52, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, H.; Girdhar, S.; Sreedharan, S.E.; Enakshy Rajan, J.; Kannath, S.K.; Vinoda Thulaseedharan, J.; Sukumaran, S.; Sylaja, P.N. Does Thrombus Imaging Characteristics Predict the Degree of Recanalisation after Endovascular Thrombectomy in Acute Ischaemic Stroke? J. Stroke Cerebrovasc. Dis. 2022, 31, 106621. [Google Scholar] [CrossRef]
- Bushnell, C.D.; Johnston, D.C.C.; Goldstein, L.B. Retrospective Assessment of Initial Stroke Severity Comparison of the NIH Stroke Scale and the Canadian Neurological Scale. Stroke 2001, 32, 656–660. [Google Scholar] [CrossRef]
- Klein, S.; Staring, M.; Murphy, K.; Viergever, M.A.; Pluim, J.P.W. Elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Trans. Med. Imaging 2010, 29, 196–205. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-Guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Von Kummer, R.; Broderick, J.P.; Campbell, B.C.V.; Demchuk, A.; Goyal, M.; Hill, M.D.; Treurniet, K.M.; Majoie, C.B.L.M.; Marquering, H.A.; Mazya, M.V.; et al. The Heidelberg Bleeding Classification: Classification of Bleeding Events after Ischemic Stroke and Reperfusion Therapy. Stroke 2015, 46, 2981–2986. [Google Scholar] [CrossRef]
- Jansen, I.G.H.; Mulder, M.J.H.L.; Goldhoorn, R.J.B. Endovascular Treatment for Acute Ischaemic Stroke in Routine Clinical Practice: Prospective, Observational Cohort Study (MR CLEAN Registry). BMJ 2018, 360, k949. [Google Scholar] [CrossRef]
- Arrarte Terreros, N.; Bruggeman, A.A.E.; Kappelhof, M.; Tolhuisen, M.L.; Brouwer, J.; Hoving, J.W.; Konduri, P.R.; Van Kranendonk, K.R.; Dutra, B.G.; Alves, H.C.B.R.; et al. Thrombus Imaging Characteristics within Acute Ischemic Stroke: Similarities and Interdependence. J. Neurointerv. Surg. 2022, 15, e60–e68. [Google Scholar] [CrossRef]
- Muchada, M.; Rodriguez-Luna, D.; Pagola, J.; Flores, A.; Sanjuan, E.; Meler, P.; Boned, S.; Alvarez-Sabin, J.; Ribo, M.; Molina, C.A.; et al. Impact of Time to Treatment on Tissue-Type Plasminogen Activator-Induced Recanalization in Acute Ischemic Stroke. Stroke 2014, 45, 2734–2738. [Google Scholar] [CrossRef]
- Qazi, E.M.; Sohn, S.I.; Mishra, S.; Almekhlafi, M.A.; Eesa, M.; D’Esterre, C.D.; Qazi, A.A.; Puig, J.; Goyal, M.; Demchuk, A.M.; et al. Thrombus Characteristics Are Related to Collaterals and Angioarchitecture in Acute Stroke. Can. J. Neurol. Sci. 2015, 42, 381–388. [Google Scholar] [CrossRef]
- Cahalane, R.; Boodt, N.; Akyildiz, A.C.; Giezen, J.A.; Mondeel, M.; van der Lugt, A.; Marquering, H.; Gijsen, F. A Review on the Association of Thrombus Composition with Mechanical and Radiological Imaging Characteristics in Acute Ischemic Stroke. J. Biomech. 2021, 129, 110816. [Google Scholar] [CrossRef]
- Requena, M.; Vanden Bavière, H.; Verma, S.; Gerrits, C.; Kokhuis, T.; Tomasello, A.; Molina, C.A.; Ribo, M. Cost-Utility of Direct Transfer to Angiography Suite (DTAS) Bypassing Conventional Imaging for Patients with Acute Ischemic Stroke in Spain: Results from the ANGIOCAT Trial. J. NeuroInterv. Surg. 2023, 16, 138–142. [Google Scholar] [CrossRef]
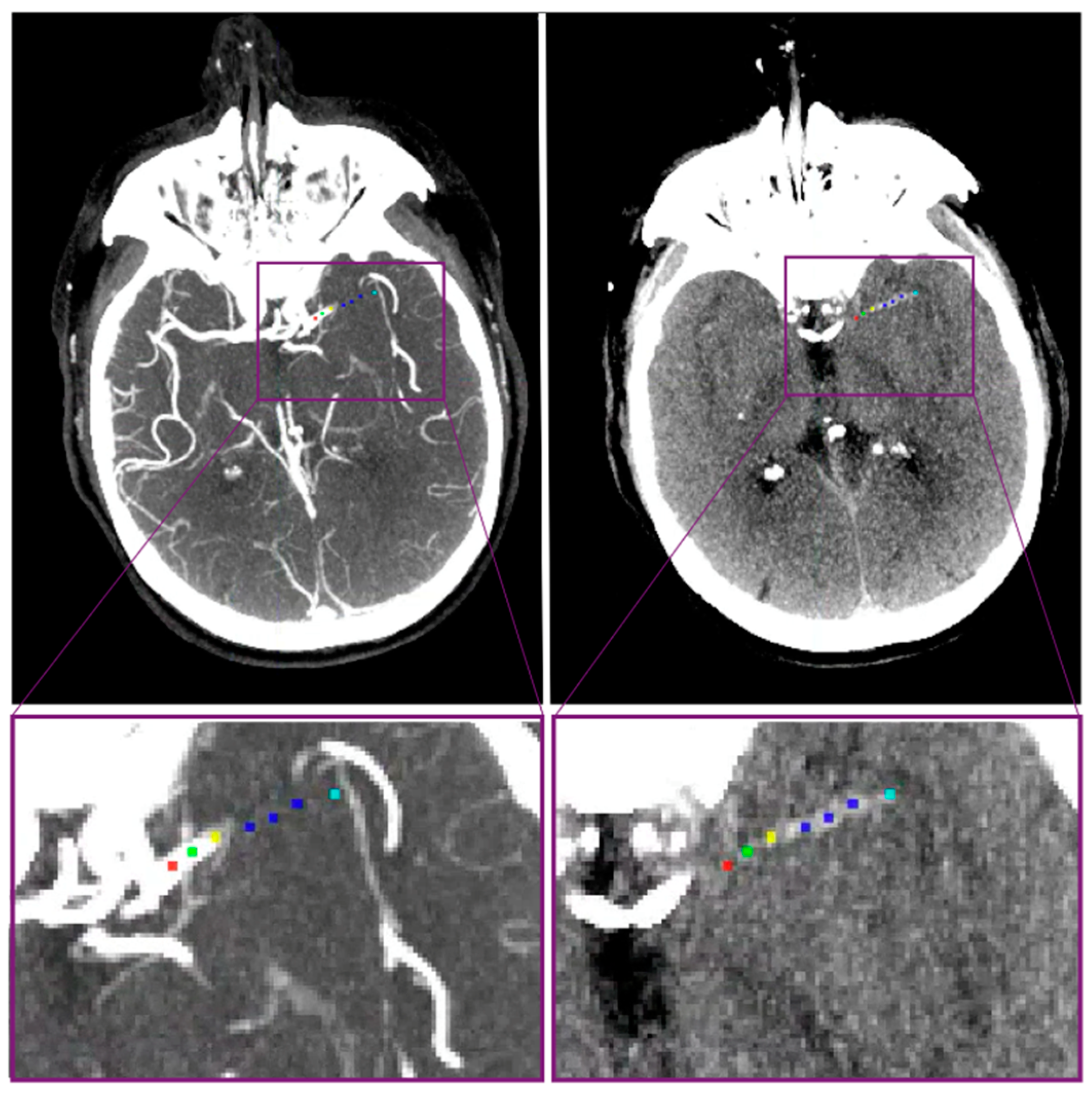
| Baseline Clinical Characteristics * | ER-LVO, n = 81 | NER-LVO, n = 322 | p-Value |
|---|---|---|---|
| Age (years)—median (IQR) | 75 (63–85) | 71 (62–80) | 0.05 |
| Sex, female—no./total (%) | 45/81 (56) | 166/322 (52) | 0.52 |
| Medical history—no./total (%) | |||
| Previous stroke | 17/80 (21) | 54/317 (17) | 0.38 |
| Diabetes mellitus | 16/80 (20) | 54/319 (17) | 0.52 |
| Hypertension | 28/80 (35) | 142/310 (46) | 0.08 |
| Atrial fibrillation | 14/80 (18) | 79/317 (25) | 0.16 |
| Pre-stroke mRS 0–2—no./total (%) | 59/81 (92) | 289/314 (92) | 0.97 |
| Systolic blood pressure α (mmHg)—median (IQR) | 149 (130–166) | 150 (133–164) | 0.61 |
| Diastolic blood pressure β (mmHg)—median (IQR) | 82 (76–90) | 80 (70–90) | 0.31 |
| NIHSSCSC γ– median (IQR) | 5 (2–10) | 16 (12–20) | <0.01 |
| IVT—no./total (%) | 75/81 (93) | 251/322 (78) | <0.01 |
| Workflow related timing variables—median (IQR) | ER-LVO, n = 81 | NER-LVO, n = 322 | p-value |
| Onset †-to-PSC-door δ (min) | 50 (35–96) | 52 (39–97) | 0.52 |
| Onset-to-PSC-imaging ε (min) | 67 (52–118) | 67 (47–110) | 0.72 |
| PSC door-to-IVT ζ (min) | 25 (18–33) | 24 (18–33) | 0.79 |
| PSC door to CSC door η (min) | 157 (122–240) | 101 (81–128) | <0.01 |
| IVT-to-CSC door θ (min) | 126 (92–178) | 76 (56–97) | <0.01 |
| Thrombus imaging characteristics ‡ | ER-LVO, n = 81 | NER-LVO, n = 322 | p-value |
| Occlusion location—no./total (%) | <0.01 | ||
| ICA | 1/81 (1) | 74/320 (23) | |
| M1 | 53/81 (65) | 201/320 (63) | |
| M2 | 26/81 (32) | 43/320 (13) | |
| A1/A2 | 1/81 (1) | 2/320 (1) | |
| DT ι (mm)—median (IQR) | 23 (14–32) | 10 (0–21) | <0.01 |
| Thrombus length κ (mm)—median (IQR) | 16 (11–21) | 18 (12–30) | 0.02 |
| Thrombus perviousness λ (HU)—median (IQR) | 12 (4–21) | 5 (−2–12) | <0.01 |
| Thrombus density λ (HU)—median (IQR) | 43 (36–52) | 50 (44–56) | <0.01 |
| Patient functional outcome | ER-LVO, n = 81 | NER-LVO, n = 322 | p-value |
| mRS at 90 days | <0.01 | ||
| 0 | 8/50 (16) | 15/293 (5) | |
| 1 | 12/50 (24) | 40/293 (14) | |
| 2 | 6/50 (12) | 57/293 (19) | |
| 3 | 3/50 (6) | 46/293 (16) | |
| 4 | 4/50 (8) | 33/293 (11) | |
| 5 | 5/50 (10) | 15/293 (5) | |
| 6 | 12/50 (24) | 87/293 (30) | |
| Good functional outcome (mRS 0–2) at 90 days | 26/50 (52) | 112/293 (38) | 0.06 |
| sICH | 0/81 (0) | 13/322 (4) | 0.07 |
| Prediction model | Before Backward Elimination, n = 348 | ||
| Characteristics Associated with Early Recanalization | Odds Ratio (95% CI) | p-Value | |
| Age (per year) | 1.01 (0.98–1.04) | 0.29 | |
| Hypertension | 0.45 (0.20–1.01) | 0.05 | |
| IVT administration | 3.77 (1.04–13.64) | 0.04 | |
| DT (per mm) | 1.03 (1.01–1.06) | <0.01 | |
| Thrombus length (per mm) | 1.00 (0.97–1.03) | 0.98 | |
| Thrombus perviousness (per HU) | 1.01 (0.98–1.04) | 0.40 | |
| Thrombus density (per HU) | 0.94 (0.90–0.98) | <0.01 | |
| After backward elimination, n = 360 | |||
| Characteristics associated with early recanalization | Odds ratio (95% CI) | p-value | |
| IVT | 4.3 (1.2–15.4) | 0.03 | |
| DT (per mm) | 1.03 (1.01–1.05) | <0.01 | |
| Thrombus density (per HU) | 0.94 (0.90–0.97) | <0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrarte Terreros, N.; Stolp, J.; Bruggeman, A.A.E.; Swijnenburg, I.S.J.; Lopes, R.R.; van Meenen, L.C.C.; Groot, A.E.D.; Kappelhof, M.; Coutinho, J.M.; Roos, Y.B.W.E.M.; et al. Thrombus Imaging Characteristics to Predict Early Recanalization in Anterior Circulation Large Vessel Occlusion Stroke. J. Cardiovasc. Dev. Dis. 2024, 11, 107. https://doi.org/10.3390/jcdd11040107
Arrarte Terreros N, Stolp J, Bruggeman AAE, Swijnenburg ISJ, Lopes RR, van Meenen LCC, Groot AED, Kappelhof M, Coutinho JM, Roos YBWEM, et al. Thrombus Imaging Characteristics to Predict Early Recanalization in Anterior Circulation Large Vessel Occlusion Stroke. Journal of Cardiovascular Development and Disease. 2024; 11(4):107. https://doi.org/10.3390/jcdd11040107
Chicago/Turabian StyleArrarte Terreros, Nerea, Jeffrey Stolp, Agnetha A. E. Bruggeman, Isabella S. J. Swijnenburg, Ricardo R. Lopes, Laura C. C. van Meenen, Adrien E. D. Groot, Manon Kappelhof, Jonathan M. Coutinho, Yvo B. W. E. M. Roos, and et al. 2024. "Thrombus Imaging Characteristics to Predict Early Recanalization in Anterior Circulation Large Vessel Occlusion Stroke" Journal of Cardiovascular Development and Disease 11, no. 4: 107. https://doi.org/10.3390/jcdd11040107
APA StyleArrarte Terreros, N., Stolp, J., Bruggeman, A. A. E., Swijnenburg, I. S. J., Lopes, R. R., van Meenen, L. C. C., Groot, A. E. D., Kappelhof, M., Coutinho, J. M., Roos, Y. B. W. E. M., Emmer, B. J., Beenen, L. F. M., Dippel, D. W. J., van Zwam, W. H., van Bavel, E., Marquering, H. A., & Majoie, C. B. L. M., on behalf of the MR CLEAN Registry investigators. (2024). Thrombus Imaging Characteristics to Predict Early Recanalization in Anterior Circulation Large Vessel Occlusion Stroke. Journal of Cardiovascular Development and Disease, 11(4), 107. https://doi.org/10.3390/jcdd11040107







