Ductus Arteriosus in Fetal and Perinatal Life
Abstract
:1. Introduction
2. Ductus Arteriosus in Fetal Life
2.1. Embryology, Morphology, and Histology
2.2. Ductus Arteriosus Physiology in Fetal Life
Inizio Modulo
2.3. Echographic Imaging
2.4. Fetal Ductus Arteriosus Pathology
3. Ductus Arteriosus in the Transitional Period
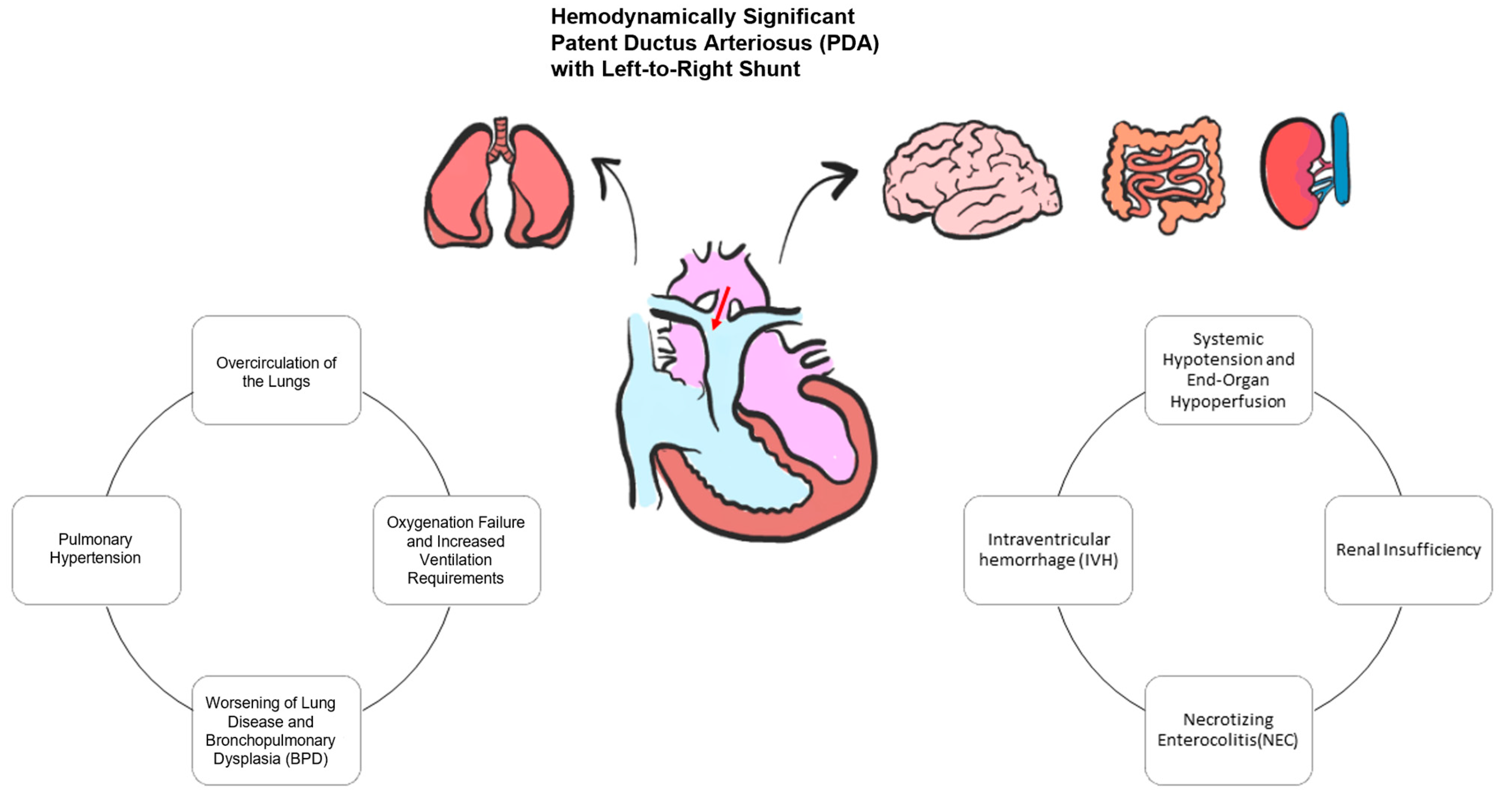
Ductus Arteriosus during Transition in Premature Preterm Neonates
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obladen, M. History of the Ductus Arteriosus: 1. Anatomy and Spontaneous Closure. Neonatology 2011, 99, 83–89. [Google Scholar] [CrossRef]
- Gittenberger-de Groot, A.C. Persistent Ductus Arteriosus: Most Probably a Primary Congenital Malformation. Br. Heart J. 1977, 39, 610–618. [Google Scholar] [CrossRef]
- Jager, B.V.; Wollenman, O.J. An Anatomical Study of the Closure of the Ductus Arteriosus. Am. J. Pathol. 1942, 18, 595–613. [Google Scholar]
- Clyman, R.I. Mechanisms Regulating the Ductus Arteriosus. Neonatology 2006, 89, 330–335. [Google Scholar] [CrossRef]
- Coceani, F.; Olley, P.M. The Response of the Ductus Arteriosus to Prostaglandins. Can. J. Physiol. Pharmacol. 1973, 51, 220–225. [Google Scholar] [CrossRef]
- Crockett, S.L.; Berger, C.D.; Shelton, E.L.; Reese, J. Molecular and Mechanical Factors Contributing to Ductus Arteriosus Patency and Closure. Congenit. Heart Dis. 2019, 14, 15–20. [Google Scholar] [CrossRef]
- Alvarez, S.G.V.; McBrien, A. Ductus Arteriosus and Fetal Echocardiography: Implications for Practice. Semin. Fetal Neonatal Med. 2018, 23, 285–291. [Google Scholar] [CrossRef]
- Schneider, D.J.; Moore, J.W. Patent Ductus Arteriosus. Circulation 2006, 114, 1873–1882. [Google Scholar] [CrossRef]
- May, L.A.; Masand, P.M.; Qureshi, A.M.; Jadhav, S.P. The Ductus Arteriosus: A Review of Embryology to Intervention. Pediatr. Radiol. 2023, 53, 509–522. [Google Scholar] [CrossRef]
- Rudolph, A.M. Congenital Cardiovascular Malformations and the Fetal Circulation. Arch. Dis. Child.—Fetal Neonatal Ed. 2010, 95, F132–F136. [Google Scholar] [CrossRef]
- Krichenko, A.; Benson, L.N.; Burrows, P.; Möes, C.A.F.; McLaughlin, P.; Freedom, R.M. Angiographic Classification of the Isolated, Persistently Patent Ductus Arteriosus and Implications for Percutaneous Catheter Occlusion. Am. J. Cardiol. 1989, 63, 877–880. [Google Scholar] [CrossRef]
- Sathanandam, S.; Balduf, K.; Chilakala, S.; Washington, K.; Allen, K.; Knott-Craig, C.; Rush Waller, B.; Philip, R. Role of Transcatheter Patent Ductus Arteriosus Closure in Extremely Low Birth Weight Infants. Catheter. Cardiovasc. Interv. 2019, 93, 89–96. [Google Scholar] [CrossRef]
- Benson, C.B.; Brown, D.L.; Doubilet, P.M.; DiSalvo, D.N.; Laing, F.C.; Frates, M.C. Increasing Curvature of the Normal Fetal Ductus Arteriosus with Advancing Gestational Age. Ultrasound Obstet. Gynecol. 1995, 5, 95–97. [Google Scholar] [CrossRef]
- Bergwerff, M.; DeRuiter, M.C.; Gittenberger-de Groot, A.C. Comparative Anatomy and Ontogeny of the Ductus Arteriosus, a Vascular Outsider. Anat. Embryol. 1999, 200, 559–571. [Google Scholar] [CrossRef]
- Sobh, D.M.; Batouty, N.M.; Abdelwahab, R.M.; El-Badrawy, A.; Tawfik, A.M. Ductus Arteriosus Location in Relation to Aortic Arch Position, Branching Pattern, and Viscero-Atrial Situs. Clin. Radiol. 2019, 74, 732.e1–732.e8. [Google Scholar] [CrossRef]
- Gao, Y.; Raj, J.U. Regulation of the Pulmonary Circulation in the Fetus and Newborn. Physiol. Rev. 2010, 90, 1291–1335. [Google Scholar] [CrossRef]
- Yarboro, M.T.; Boatwright, N.; Sekulich, D.C.; Hooper, C.W.; Wong, T.; Poole, S.D.; Berger, C.D.; Brown, A.J.; Jetter, C.S.; Sucre, J.M.S.; et al. A Novel Role for PGE2-EP4 in the Developmental Programming of the Mouse Ductus Arteriosus: Consequences for Vessel Maturation and Function. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H687–H701. [Google Scholar] [CrossRef]
- Sarzani, R.; Allevi, M.; Di Pentima, C.; Schiavi, P.; Spannella, F.; Giulietti, F. Role of Cardiac Natriuretic Peptides in Heart Structure and Function. Int. J. Mol. Sci. 2022, 23, 14415. [Google Scholar] [CrossRef]
- Nagaraj, C.; Li, Y.; Tang, B.; Bordag, N.; Guntur, D.; Enyedi, P.; Olschewski, H.; Olschewski, A. Potassium Channels in the Transition from Fetal to the Neonatal Pulmonary Circulation. Int. J. Mol. Sci. 2022, 23, 4681. [Google Scholar] [CrossRef]
- Ovalı, F. Molecular and Mechanical Mechanisms Regulating Ductus Arteriosus Closure in Preterm Infants. Front. Pediatr. 2020, 8, 516. [Google Scholar] [CrossRef]
- Yokoyama, U.; Oka, S.; Saito, J. Molecular Mechanisms Regulating Extracellular Matrix-Mediated Remodeling in the Ductus Arteriosus. Semin. Perinatol. 2023, 47, 151716. [Google Scholar] [CrossRef]
- Lewis, T.R.; Shelton, E.L.; Van Driest, S.L.; Kannankeril, P.J.; Reese, J. Genetics of the Patent Ductus Arteriosus (PDA) and Pharmacogenetics of PDA Treatment. Semin Fetal Neonatal Med. 2018, 23, 232–238. [Google Scholar] [CrossRef]
- Dagle, J.M.; Lepp, N.T.; Cooper, M.E.; Schaa, K.L.; Kelsey, K.J.; Orr, K.L.; Caprau, D.; Zimmerman, C.R.; Steffen, K.M.; Johnson, K.J.; et al. Determination of Genetic Predisposition to Patent Ductus Arteriosus in Preterm Infants. Pediatrics 2009, 123, 1116–1123. [Google Scholar] [CrossRef]
- Patel, P.M.; Momany, A.M.; Schaa, K.L.; Romitti, P.A.; Druschel, C.; Cooper, M.E.; Marazita, M.L.; Murray, J.C.; Dagle, J.M. Genetic Modifiers of Patent Ductus Arteriosus in Term Infants. J. Pediatr. 2016, 176, 57–61.e1. [Google Scholar] [CrossRef]
- Yarboro, M.T.; Gopal, S.H.; Su, R.L.; Morgan, T.M.; Reese, J. Mouse Models of Patent Ductus Arteriosus (PDA) and Their Relevance for Human PDA. Dev. Dyn. 2022, 251, 424–443. [Google Scholar] [CrossRef]
- Kiserud, T.; Acharya, G. The Fetal Circulation. Prenat. Diagn. 2004, 24, 1049–1059. [Google Scholar] [CrossRef]
- Mielke, G.; Benda, N. Reference Ranges for Two-Dimensional Echocardiographic Examination of the Fetal Ductus Arteriosus. Ultrasound Obstet. Gynecol. 2000, 15, 219–225. [Google Scholar] [CrossRef]
- Ishida, H.; Kawazu, Y.; Kayatani, F.; Inamura, N. Prognostic Factors of Premature Closure of the Ductus Arteriosus in Utero: A Systematic Literature Review. Cardiol. Young 2017, 27, 634–638. [Google Scholar] [CrossRef]
- Zielinsky, P.; Piccoli, A.L.; Manica, J.L.L.; Nicoloso, L.H.S. New Insights on Fetal Ductal Constriction: Role of Maternal Ingestion of Polyphenol-Rich Foods. Expert Rev. Cardiovasc. Ther. 2010, 8, 291–298. [Google Scholar] [CrossRef]
- Mielke, G.; Steil, E.; Breuer, J.; Goelz, R. Circulatory Changes Following Intrauterine Closure of the Ductus Arteriosus in the Human Fetus and Newborn. Prenat. Diagn. 1998, 18, 139–145. [Google Scholar] [CrossRef]
- Li, T.; Han, J.; Han, Y.; Liu, X.; Gu, X.; Zhang, Y.; Sun, L.; Zhao, Y.; Gao, S.; Hao, X.; et al. Evaluation of Changes of Cardiac Morphology and Function in Fetuses with Ductus Arteriosus Constriction by Speckle-Tracking Echocardiography. Front. Pediatr. 2023, 11, 1085352. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Cao, H.; Hong, L.; Liu, J.; Song, X.; Shi, J.; Zhang, Y.; Cui, L.; Zhang, L.; Xie, M. Cardiac Function Assessment in Fetuses with Ductus Arteriosus Constriction: A Two-Dimensional Echocardiography and FetalHQ Study. Front. Cardiovasc. Med. 2022, 9, 868675. [Google Scholar] [CrossRef]
- Kondo, T.; Kitazawa, R.; Noda-Maeda, N.; Kitazawa, S. Fetal Hydrops Associated with Spontaneous Premature Closure of Ductus Arteriosus. Pathol. Int. 2006, 56, 554–557. [Google Scholar] [CrossRef]
- Rudolph, A.M.; Auld, P.A.; Golinko, R.J.; Paul, M.H. Pulmonary Vascular Adjustments in the Neonatal Period. Pediatrics 1961, 28, 28–34. [Google Scholar] [CrossRef]
- Deshpande, P.; Baczynski, M.; McNamara, P.J.; Jain, A. Patent Ductus Arteriosus: The Physiology of Transition. Semin. Fetal Neonatal Med. 2018, 23, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.B.; te Pas, A.B.; Lang, J.; van Vonderen, J.J.; Roehr, C.C.; Kluckow, M.; Gill, A.W.; Wallace, E.M.; Polglase, G.R. Cardiovascular Transition at Birth: A Physiological Sequence. Pediatr. Res. 2015, 77, 608–614. [Google Scholar] [CrossRef]
- Hsu, H.-W.; Lin, T.-Y.; Liu, Y.-C.; Yeh, J.-L.; Hsu, J.-H. Molecular Mechanisms Underlying Remodeling of Ductus Arteriosus: Looking beyond the Prostaglandin Pathway. Int. J. Mol. Sci. 2021, 22, 3238. [Google Scholar] [CrossRef] [PubMed]
- Haworth, S.G. Pulmonary Endothelium in the Perinatal Period. Pharmacol. Rep. 2006, 58, 153–164. [Google Scholar]
- Wojciak-Stothard, B.; Haworth, S.G. Perinatal Changes in Pulmonary Vascular Endothelial Function. Pharmacol. Ther. 2006, 109, 78–91. [Google Scholar] [CrossRef]
- Popat, H.; Kluckow, M. Noninvasive Assessment of the Early Transitional Circulation in Healthy Term Infants. Neonatology 2012, 101, 166–171. [Google Scholar] [CrossRef]
- Singh, Y.; Tissot, C. Echocardiographic Evaluation of Transitional Circulation for the Neonatologists. Front. Pediatr. 2018, 6, 140. [Google Scholar] [CrossRef]
- Jain, A.; Mohamed, A.; El-Khuffash, A.; Connelly, K.A.; Dallaire, F.; Jankov, R.P.; McNamara, P.J.; Mertens, L. A Comprehensive Echocardiographic Protocol for Assessing Neonatal Right Ventricular Dimensions and Function in the Transitional Period: Normative Data and Z Scores. J. Am. Soc. Echocardiogr. 2014, 27, 1293–1304. [Google Scholar] [CrossRef]
- EL-Khuffash, A.; James, A.T.; Corcoran, J.D.; Dicker, P.; Franklin, O.; Elsayed, Y.N.; Ting, J.Y.; Sehgal, A.; Malikiwi, A.; Harabor, A.; et al. A Patent Ductus Arteriosus Severity Score Predicts Chronic Lung Disease or Death before Discharge. J. Pediatr. 2015, 167, 1354–1361.e2. [Google Scholar] [CrossRef]
- Schena, F.; Francescato, G.; Cappelleri, A.; Picciolli, I.; Mayer, A.; Mosca, F.; Fumagalli, M. Association between Hemodynamically Significant Patent Ductus Arteriosus and Bronchopulmonary Dysplasia. J. Pediatr. 2015, 166, 1488–1492. [Google Scholar] [CrossRef]
- Liu, H.; Manganiello, V.; Waleh, N.; Clyman, R.I. Expression, Activity and Function of Phosphodiesterases in the Mature and Immature Ductus Arteriosus. Pediatr. Res. 2008, 64, 477–481. [Google Scholar] [CrossRef]
- Watterberg, K.L.; Scott, S.M.; Backstrom, C.; Gifford, K.L.; Cook, K.L. Links Between Early Adrenal Function and Respiratory Outcome in Preterm Infants: Airway Inflammation and Patent Ductus Arteriosus. Pediatrics 2000, 105, 320–324. [Google Scholar] [CrossRef]
- Alyamac Dizdar, E.; Ozdemir, R.; Nur Sari, F.; Yurttutan, S.; Gokmen, T.; Erdeve, O.; Emre Canpolat, F.; Uras, N.; Suna Oguz, S.; Dilmen, U. Low Platelet Count Is Associated with Ductus Arteriosus Patency in Preterm Newborns. Early Hum. Dev. 2012, 88, 813–816. [Google Scholar] [CrossRef]
- Sallmon, H.; Weber, S.C.; Hüning, B.; Stein, A.; Horn, P.A.; Metze, B.C.; Dame, C.; Bührer, C.; Felderhoff-Müser, U.; Hansmann, G.; et al. Thrombocytopenia in the First 24 Hours After Birth and Incidence of Patent Ductus Arteriosus. Pediatrics 2012, 130, e623–e630. [Google Scholar] [CrossRef]
- Read, A.D.; Bentley, R.E.T.; Martin, A.Y.; Mewburn, J.D.; Alizadeh, E.; Wu, D.; Lima, P.D.A.; Dunham-Snary, K.J.; Thébaud, B.; Sharp, W.; et al. Electron Leak From the Mitochondrial Electron Transport Chain Complex I at Site IQ Is Crucial for Oxygen Sensing in Rabbit and Human Ductus Arteriosus. J. Am. Heart Assoc. 2023, 12, e029131. [Google Scholar] [CrossRef]
- Kajimoto, H.; Hashimoto, K.; Bonnet, S.N.; Haromy, A.; Harry, G.; Moudgil, R.; Nakanishi, T.; Rebeyka, I.; Thébaud, B.; Michelakis, E.D.; et al. Oxygen Activates the Rho/Rho-Kinase Pathway and Induces RhoB and ROCK-1 Expression in Human and Rabbit Ductus Arteriosus by Increasing Mitochondria-Derived Reactive Oxygen Species. Circulation 2007, 115, 1777–1788. [Google Scholar] [CrossRef]
- Satoda, M.; Zhao, F.; Diaz, G.A.; Burn, J.; Goodship, J.; Davidson, H.R.; Pierpont, M.E.; Gelb, B.D. Mutations in TFAP2B Cause Char Syndrome, a Familial Form of Patent Ductus Arteriosus. Nat. Genet. 2000, 25, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R.E.T.; Hindmarch, C.C.T.; Archer, S.L. Using Omics to Breathe New Life into Our Understanding of the Ductus Arteriosus Oxygen Response. Semin. Perinatol. 2023, 47, 151715. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Ruoss, J.L.; Stanford, A.H.; Lakshminrusimha, S.; McNamara, P.J. Hemodynamic Consequences of Respiratory Interventions in Preterm Infants. J. Perinatol. 2022, 42, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Finch, A.M.; Lumbers, E.R.; Boyce, A.C.; Gibson, K.J.; Eiby, Y.A.; Lingwood, B.E. Expression of Adrenoceptor Subtypes in Preterm Piglet Heart Is Different to Term Heart. PLoS ONE 2014, 9, e92167. [Google Scholar] [CrossRef]
- Noori, S.; Seri, I. Hemodynamic Antecedents of Peri/Intraventricular Hemorrhage in Very Preterm Neonates. Semin. Fetal Neonatal Med. 2015, 20, 232–237. [Google Scholar] [CrossRef]
- Kluckow, M.; Jeffery, M.; Gill, A.; Evans, N. A Randomised Placebo-Controlled Trial of Early Treatment of the Patent Ductus Arteriosus. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F99–F104. [Google Scholar] [CrossRef]
- Hundscheid, T.; Onland, W.; Kooi, E.M.W.; Vijlbrief, D.C.; De Vries, W.B.; Dijkman, K.P.; Van Kaam, A.H.; Villamor, E.; Kroon, A.A.; Visser, R.; et al. Expectant Management or Early Ibuprofen for Patent Ductus Arteriosus. N. Engl. J. Med. 2023, 388, 980–990. [Google Scholar] [CrossRef]
- Rozé, J.-C.; Cambonie, G.; Marchand-Martin, L.; Gournay, V.; Durrmeyer, X.; Durox, M.; Storme, L.; Porcher, R.; Ancel, P.-Y.; Hemodynamic EPIPAGE 2 Study Group. Association Between Early Screening for Patent Ductus Arteriosus and In-Hospital Mortality Among Extremely Preterm Infants. JAMA 2015, 313, 2441–2448. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugnaloni, F.; Doni, D.; Lucente, M.; Fiocchi, S.; Capolupo, I. Ductus Arteriosus in Fetal and Perinatal Life. J. Cardiovasc. Dev. Dis. 2024, 11, 113. https://doi.org/10.3390/jcdd11040113
Pugnaloni F, Doni D, Lucente M, Fiocchi S, Capolupo I. Ductus Arteriosus in Fetal and Perinatal Life. Journal of Cardiovascular Development and Disease. 2024; 11(4):113. https://doi.org/10.3390/jcdd11040113
Chicago/Turabian StylePugnaloni, Flaminia, Daniela Doni, Mariella Lucente, Stefano Fiocchi, and Irma Capolupo. 2024. "Ductus Arteriosus in Fetal and Perinatal Life" Journal of Cardiovascular Development and Disease 11, no. 4: 113. https://doi.org/10.3390/jcdd11040113
APA StylePugnaloni, F., Doni, D., Lucente, M., Fiocchi, S., & Capolupo, I. (2024). Ductus Arteriosus in Fetal and Perinatal Life. Journal of Cardiovascular Development and Disease, 11(4), 113. https://doi.org/10.3390/jcdd11040113





