Minimally Invasive and Full Sternotomy Aortic Valve Replacements Lead to Comparable Long-Term Outcomes in Elderly Higher-Risk Patients: A Propensity-Matched Comparison †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
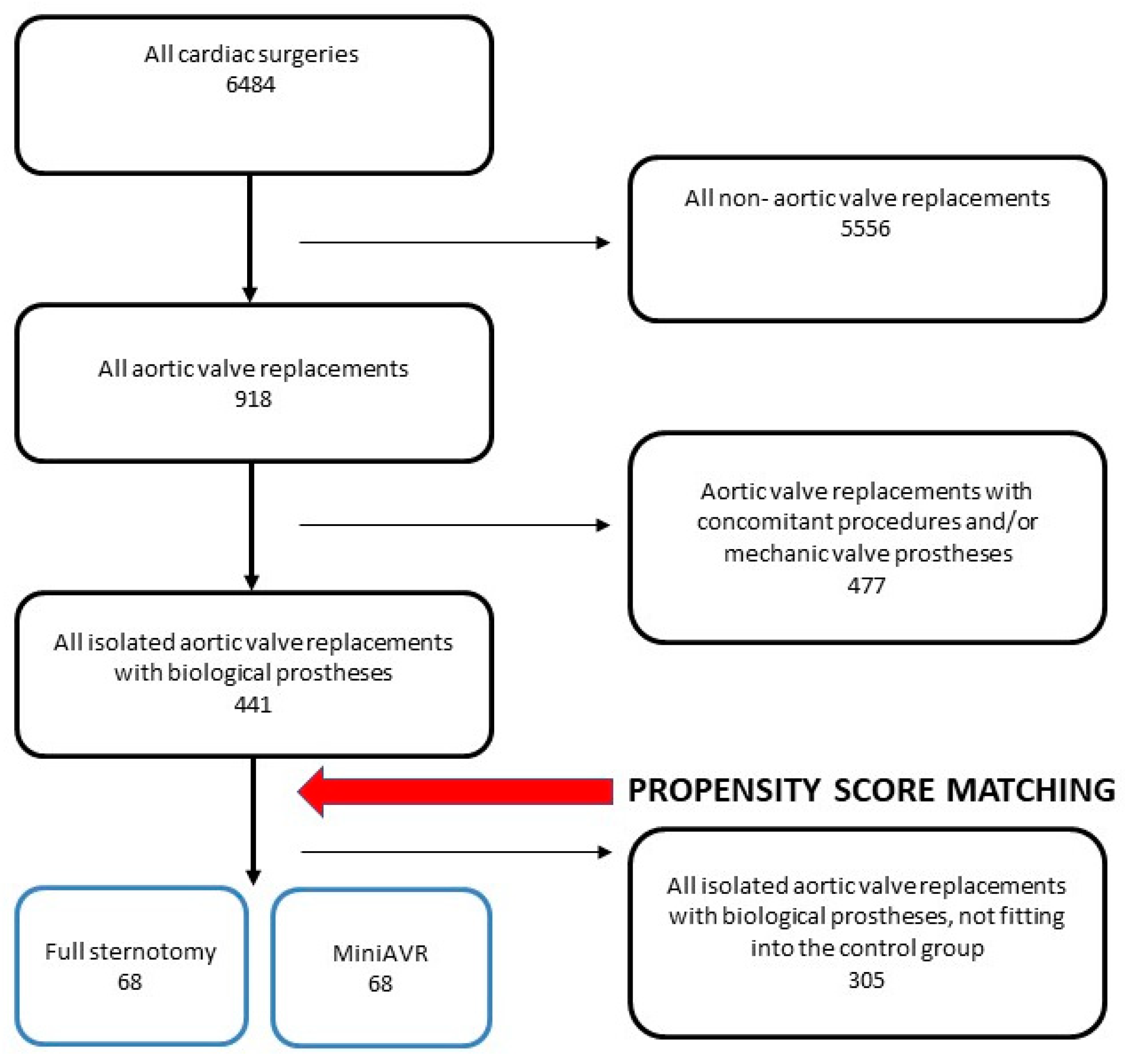
2.2. Study Design and Patients’ Characteristics
2.3. Patient Management
2.4. Surgical Technique
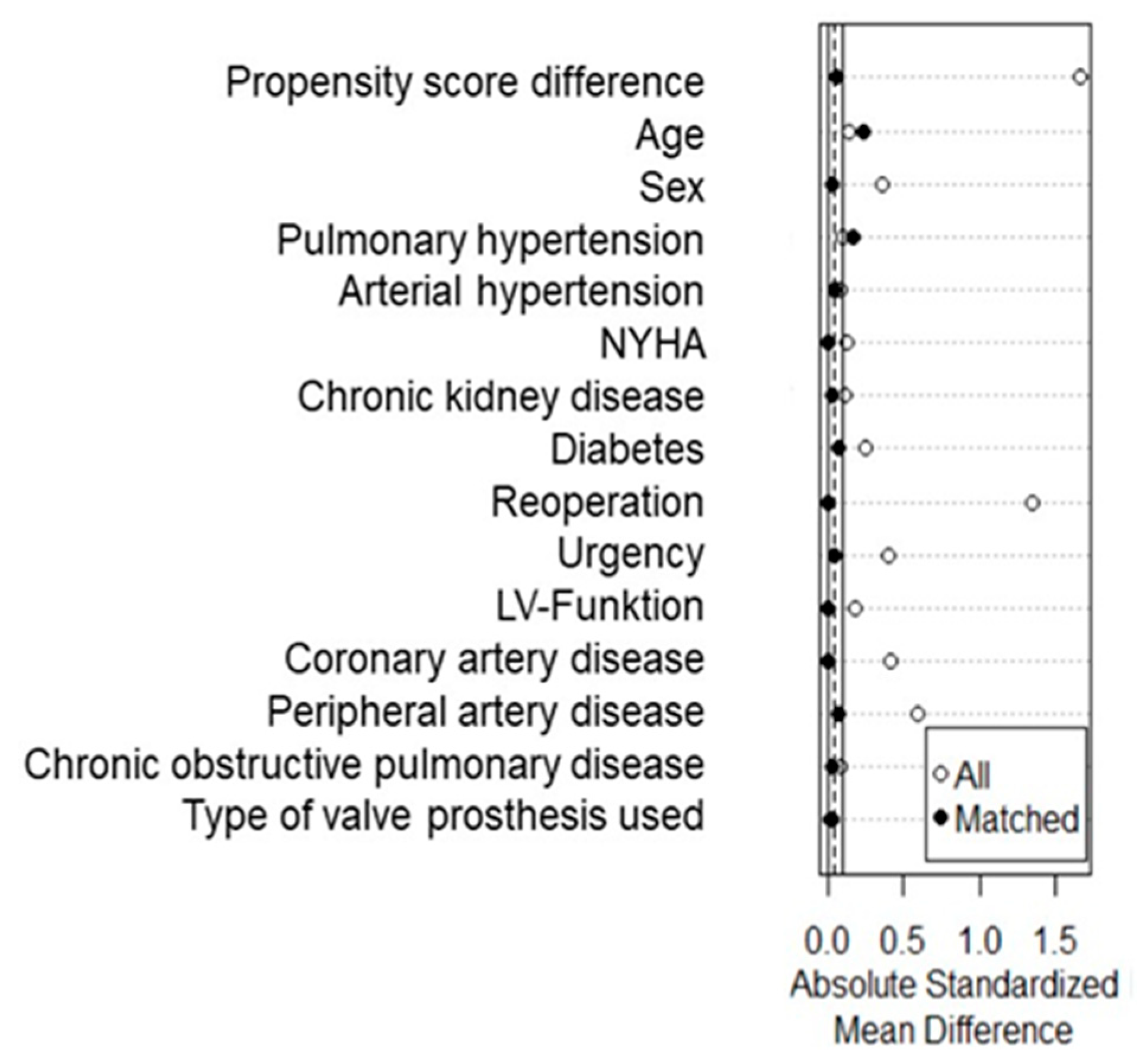
2.5. Statistical Analysis
3. Results
- OVERALL COHORT
3.1. Patients’ Characteristics
3.2. Operative Data
3.3. Early Postoperative and Follow-Up Results
- PROPENSITY SCORE-MATCHED COHORT
3.4. Patients’ Characteristics
Operative Data
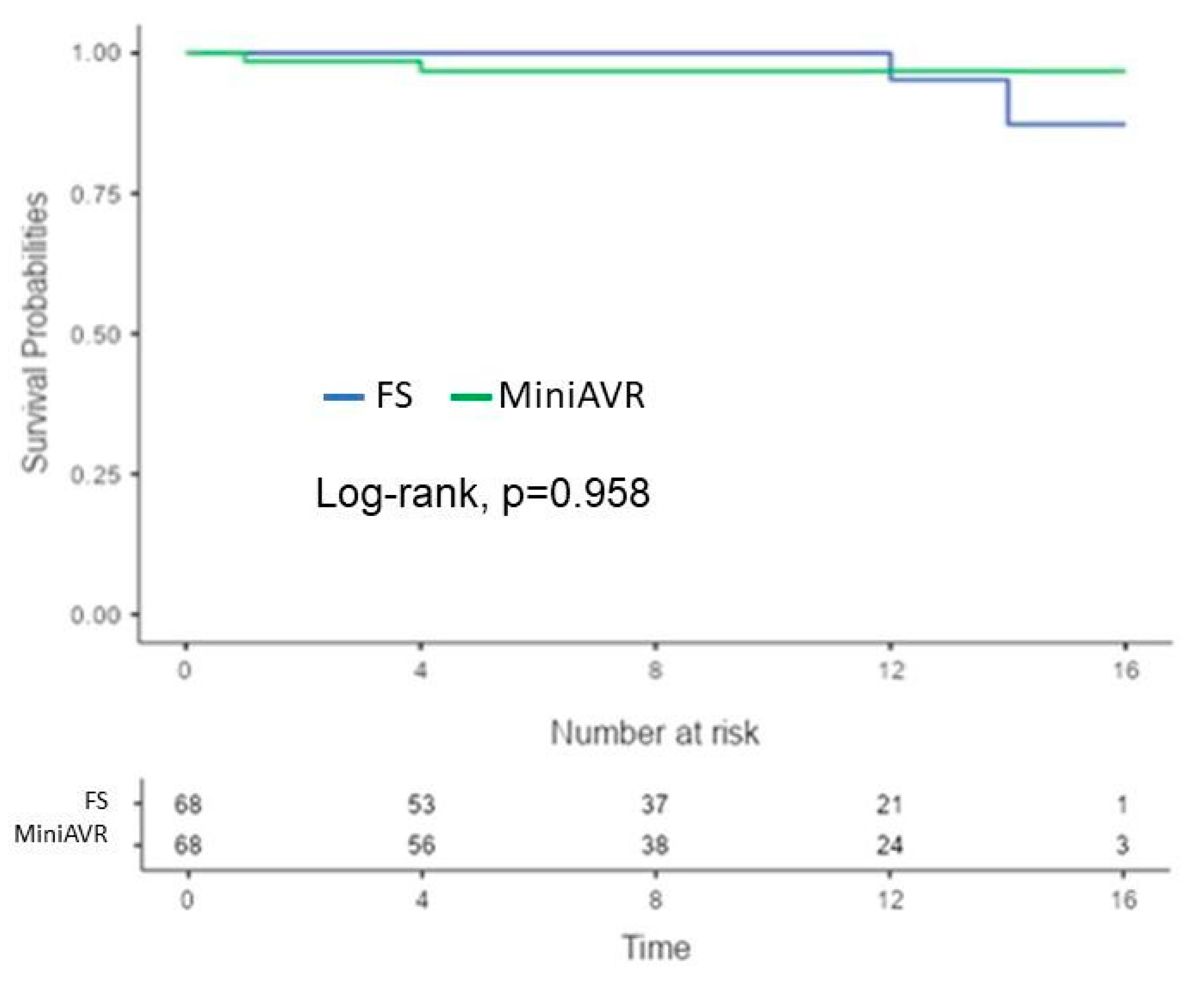
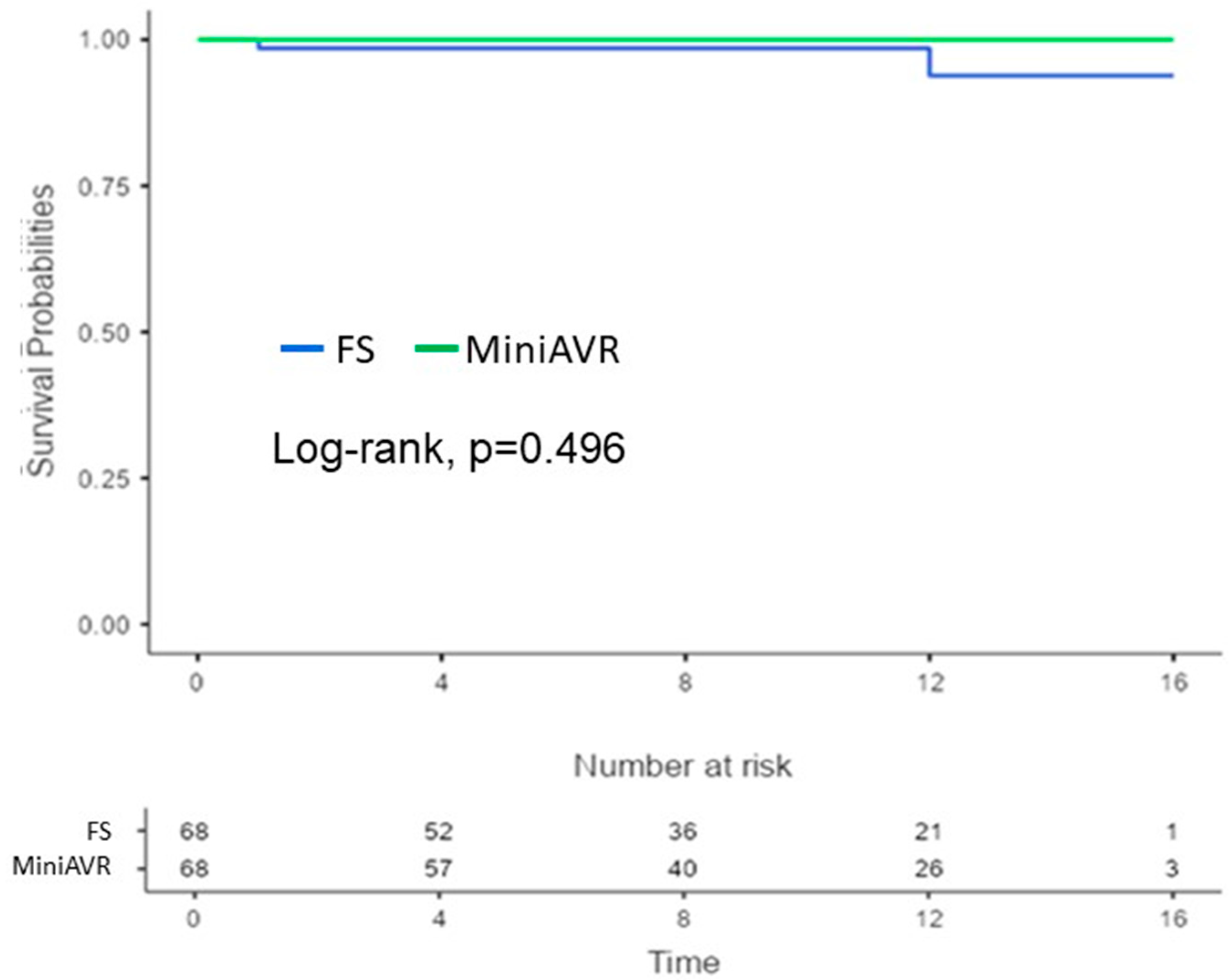
3.5. Early Postoperative and Follow-Up Results
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thaden, J.J.; Nkomo, V.T.; Enriquez-Sarano, M. The Global Burden of Aortic Stenosis. Prog. Cardiovasc. Dis. 2014, 56, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, A.; Thaden, J.J.; Nkomo, V.T. Management of Patients With Aortic Valve Stenosis. Mayo Clin. Proc. 2018, 93, 488–508. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.L.; McKellar, S.H.; Sundt, T.M.; Schaff, H.V. Ministernotomy versus conventional sternotomy for aortic valve replacement: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2009, 137, 670–679.e5. [Google Scholar] [CrossRef] [PubMed]
- Neely, R.C.; Boskovski, M.T.; Gosev, I.; Kaneko, T.; McGurk, S.; Leacche, M.; Cohn, L.H. Minimally invasive aortic valve replacement versus aortic valve replacement through full sternotomy: The Brigham and Women’s Hospital experience. Ann. Cardiothorac. Surg. 2015, 4, 38–48. [Google Scholar] [CrossRef]
- Aliahmed, H.M.A.; Karalius, R.; Valaika, A.; Grebelis, A.; Semėnienė, P.; Čypienė, R. Efficacy of Aortic Valve Replacement through Full Sternotomy and Minimal Invasion (Ministernotomy). Medicina 2018, 54, 26. [Google Scholar] [CrossRef] [PubMed]
- Hlavicka, J.; Janda, D.; Budera, P.; Tousek, P.; Maly, M.; Fojt, R.; Linkova, H.; Holubec, T.; Kacer, P. Partial upper sternotomy for aortic valve replacement provides similar mid-term outcomes as the full sternotomy. J. Thorac. Dis. 2022, 14, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.; Falk, V.; Metz, S.; Diegeler, A.; Battellini, R.; Autschbach, R.; Mohr, F.W. Pain and quality of life after minimally invasive versus conventional cardiac surgery. Ann. Thorac. Surg. 1999, 67, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Fudulu, D.; Lewis, H.; Benedetto, U.; Caputo, M.; Angelini, G.; Vohra, H.A. Minimally invasive aortic valve replacement in high risk patient groups. J. Thorac. Dis. 2017, 9, 1672–1696. [Google Scholar] [CrossRef] [PubMed]
- Sharony, R.; Grossi, E.A.; Saunders, P.C.; Schwartz, C.F.; Ribakove, G.H.; Culliford, A.T.; Ursomanno, P.; Baumann, F.G.; Galloway, A.C.; Colvin, S.B. Minimally invasive aortic valve surgery in the elderly: A case-control study. Circ. 2003, 108, II-43–II-47. [Google Scholar] [CrossRef] [PubMed]
- Glauber, M.; Ferrarini, M.; Miceli, A. Minimally invasive aortic valve surgery: State of the art and future directions. Ann. Cardiothorac. Surg. 2015, 4, 26–32. [Google Scholar]
- Kaneko, T.; Loberman, D.; Gosev, I.; Rassam, F.; McGurk, S.; Leacche, M.; Cohn, L. Reoperative aortic valve replacement in the octogenarians—Minimally invasive technique in the era of transcatheter valve replacement. J. Thorac. Cardiovasc. Surg. 2014, 147, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Gilmanov, D.; Solinas, M.; Farneti, P.A.; Cerillo, A.G.; Kallushi, E.; Santarelli, F.; Glauber, M. Minimally invasive aortic valve replacement: 12-year single center experience. Ann. Cardiothorac. Surg. 2015, 4, 160–169. [Google Scholar] [CrossRef]
- Lim, J.Y.; Deo, S.V.; Altarabsheh, S.E.; Jung, S.H.; Erwin, P.J.; Markowitz, A.H.; Park, S.J. Conventional versus minimally invasive aortic valve replacement: Pooled analysis of propensity-matched data. J. Card. Surg. 2015, 30, 125–134. [Google Scholar] [CrossRef]
- Mikus, E.; Calvi, S.; Campo, G.; Pavasini, R.; Paris, M.; Raviola, E.; Panzavolta, M.; Tripodi, A.; Ferrari, R.; Del Giglio, M. Full Sternotomy, Hemisternotomy, and Minithoracotomy for Aortic Valve Surgery: Is There a Difference? Ann. Thorac. Surg. 2018, 106, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Del Giglio, M.; Mikus, E.; Nerla, R.; Micari, A.; Calvi, S.; Tripodi, A.; Campo, G.; Maietti, E.; Castriota, F.; Cremonesi, A. Right anterior mini-thoracotomy vs. conventional sternotomy for aortic valve replacement: A propensity-matched comparison. J. Thorac. Dis. 2018, 10, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Shehada, S.-E.; Öztürk, Ö.; Wottke, M.; Lange, R. Propensity score analysis of outcomes following minimal access versus conventional aortic valve replacement. Eur. J. Cardio-Thoracic Surg. 2016, 49, 464–470. [Google Scholar] [CrossRef]
- Merk, D.R.; Lehmann, S.; Holzhey, D.M.; Dohmen, P.; Candolfi, P.; Misfeld, M.; Mohr, F.W.; Borger, M.A. Minimal invasive aortic valve replacement surgery is associated with improved survival: A propensity-matched comparison. Eur. J. Cardio-Thoracic Surg. 2015, 47, 11–17. [Google Scholar] [CrossRef]
- Borger, M.A.; Moustafine, V.; Conradi, L.; Knosalla, C.; Richter, M.; Merk, D.R.; Doenst, T.; Hammerschmidt, R.; Treede, H.; Dohmen, P.; et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann. Thorac. Surg. 2015, 99, 17–25. [Google Scholar] [CrossRef]
- Gilmanov, D.; Bevilacqua, S.; Murzi, M.; Cerillo, A.G.; Gasbarri, T.; Kallushi, E.; Miceli, A.; Glauber, M. Minimally invasive and conventional aortic valve replacement: A propensity score analysis. Ann. Thorac. Surg. 2013, 96, 837–843. [Google Scholar] [CrossRef]
- Welp, H.A.; Herlemann, I.; Martens, S.; Deschka, H. Outcomes of aortic valve replacement via partial upper sternotomy versus conventional aortic valve replacement in obese patients. Interact. CardioVascular Thorac. Surg. 2018, 27, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Hirji, S.A.; Funamoto, M.; Lee, J.; Romirez, F.; Kolkailah, A.A.; McGurk, S.; Pelletier, M.P.; Aranki, S.; Shekar, P.S.; Kaneko, T. Minimally invasive versus full sternotomy aortic valve replacement in low-risk patients: Which will stand against transcatheter aortic valve replacement? Surgery 2018, 164, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Prendergast, B.D. Risk of infective endocarditis after left-sided surgical valve replacement. Eur. Heart J. 2018, 39, 2676–2678. [Google Scholar] [CrossRef] [PubMed]
- Glaser, N.; Jackson, V.; Holzmann, M.J.; Franco-Cereceda, A.; Sartipy, U. Prosthetic Valve Endocarditis After Surgical Aortic Valve Replacement. Circulation 2017, 136, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.M.; Edwards, F.H.; Zhao, Y.; O’Brien, S.; Booth, M.E.; Dokholyan, R.S.; Douglas, P.S.; Peterson, E.D.; on behalf of the DEcIDE AVR (Developing Evidence to Inform Decisions about Effectiveness–Aortic Valve Replacement) Research Team. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: Results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Circulation 2013, 127, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Muntané-Carol, G.; Urena, M.; Munoz-Garcia, A.; Padrón, R.; Gutiérrez, E.; Regueiro, A.; Serra, V.; Capretti, G.; Himbert, D.; Moris, C.; et al. Late Cerebrovascular Events Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2020, 13, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, N.; Laakso, T.; Biancari, F.; Raivio, P.; Jalava, M.P.; Jaakkola, J.; Dahlbacka, S.; Kinnunen, E.-M.; Juvonen, T.; Husso, A.; et al. Prosthetic valve endocarditis after transcatheter or surgical aortic valve replacement with a bioprosthesis: Results from the FinnValve Registry. EuroIntervention 2019, 15, e500–e507. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Ashraf, S.; Villablanca, P.A.; Telila, T.A.; Takagi, H.; Grines, C.L.; Afonso, L.; Briasoulis, A. Meta-Analysis Comparing the Incidence of Infective Endocarditis Following Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement. Am. J. Cardiol. 2019, 123, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Grubitzsch, H.; Tarar, W.; Claus, B.; Gabbieri, D.; Falk, V.; Christ, T. Risks and Challenges of Surgery for Aortic Prosthetic Valve Endocarditis. Heart Lung Circ. 2018, 27, 333–343, Erratum in Heart Lung Circ. 2020, 29, e84. [Google Scholar] [CrossRef] [PubMed]
- Alexis, S.L.; Malik, A.H.; George, I.; Hahn, R.T.; Khalique, O.K.; Seetharam, K.; Bhatt, D.L.; Tang, G.H.L. Infective Endocarditis After Surgical and Transcatheter Aortic Valve Replacement: A State of the Art Review. J. Am. Heart Assoc. 2020, 9, e017347. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.K.; Williams, M.R.; Smith, C.R.; Svensson, L.G.; Webb, J.G.; Makkar, R.R.; Fontana, G.P.; Dewey, T.M.; Thourani, V.H.; Pichard, A.D.; et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N. Engl. J. Med. 2012, 366, 1686–1695. [Google Scholar] [CrossRef]
- Dvir, D.; Webb, J.; Brecker, S.; Bleiziffer, S.; Hildick-Smith, D.; Colombo, A.; Descoutures, F.; Hengstenberg, C.; Moat, N.E.; Bekeredjian, R.; et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: Results from the global valve-in-valve registry. Circulation 2012, 126, 2335–2344. [Google Scholar] [CrossRef]


| Unmatched Groups | Propensity Score-Matched Groups | |||||
|---|---|---|---|---|---|---|
| Variables a | FS (n = 137) | MiniAVR (n = 304) | p-Value | FS (n = 68) | MiniAVR (n = 68) | p-Value |
| Age | 74.1 ± 7.5 | 74.8 ± 7.3 | 0.581 | 74.5 ± 7.0 | 76.15 ± 7.0 | 0.212 |
| BMI | 27.25 ± 4.5 | 27.1 ± 4.4 | 0.869 | 27.6 ± 4.0 | 28.0 ± 4.0 | 0.625 |
| ES II | 4.4 ± 5.5 (0.7–42) | 3.3 ± 3.6 (0.7–36) | 0.006 | 3.5 ± 3.4 (1–27) | 3.6 ± 5.0 (1–36) | 0.670 |
| Male | 80 (58.4) | 142 (46.7) | 0.024 | 37 (57.4) | 38 (55.9) | 1.000 |
| NYHA > 2 | 72 (54.5) | 184 (60.7) | 0.245 | 35 (51.5) | 35(51.5) | 1.000 |
| PH | 0.303 | 0.091 | ||||
| Moderate | 89 (65) | 217 (71.4) | 39 (57.4) | 51 (75.0) | ||
| Severe | 9 (6.6) | 21 (6.9) | 6 (8.8) | 3 (4.4) | ||
| HR | 0.424 | 0.843 | ||||
| SR | 109 (79.6) | 252 (82.9) | 50 (73.5) | 52 (76.5) | ||
| AF | 28 (20.4) | 52 (17.1) | 18 (26.5) | 16 (23.5) | ||
| Redo | 28 (20.4) | 5 (1.6) | <0.001 | 3 (4.4) | 3 (4.4) | 1.000 |
| LV EF | 0.077 | 0.849 | ||||
| >55% | 81 (60) | 218 (72.2) | 41 (60.3) | 45 (66.2) | ||
| 45–55% | 23 (17) | 40 (13.2) | 15 (22.1) | 11 (16.2) | ||
| 30–45% | 27 (20) | 39 (12.9) | 11 (16.2) | 11 (16.2) | ||
| <30% | 4 (3) | 5 (1.7) | 1 (1.5) | 1 (1.5) | ||
| CHD | 50 (36.5) | 57 (18,8) | <0.001 | 14 (20.6) | 14 (20.6) | 1.000 |
| AH | 109 (89.3) | 247 (86.7) | 0.516 | 60 (88.2) | 61 (89.7) | 1.000 |
| DM | 45 (34.1) | 71 (23.7) | 0.034 | 18 (26.5) | 16 (23.5) | 0.843 |
| COPD | 36 (29.8) | 70 (25.1) | 0.388 | 20 (29.4) | 19 (27.9) | 1.000 |
| CKD | 53 (38.7) | 92 (30.3) | 0.100 | 23 (33.8) | 22 (32.4) | 1.000 |
| Stroke | 16 (11.9) | 11 (3.7) | 0.002 | 7 (10.4) | 4 (5.9) | 0.122 |
| PAD | 16 (11.9) | 15 (5.2) | 0.013 | 4 (5.9) | 5 (7.4) | 1.000 |
| AI > 2 | 33 (24.1) | 54 (17.8) | 0.154 | 14 (20.6) | 12 (17.6) | 0.828 |
| Unmatched Groups | Propensity Score-Matched Groups | |||||
|---|---|---|---|---|---|---|
| Variables a | FS (n = 137) | MiniAVR (n = 304) | p-Value | FS (n = 68) | MiniAVR (n = 68) | p-Value |
| Aortic valve anatomy | 0.037 | 0.974 | ||||
| Tricuspid | 115 (83.9) | 240 (78.9) | 56(82.4) | 55 (80.9) | ||
| Bicuspid | 18 (13.1) | 62 (20.4) | 11(16.2) | 12 (17.6) | ||
| Prosthesis | 4 (2.9) | 2 (0.7) | 1(1.5) | 1 (1.5) | ||
| Indication | <0.001 | 0.321 | ||||
| Stenosis | 99 (72.3) | 251 (82.6) | 49(72.1) | 56 (82.4) | ||
| Insufficiency | 18 (13.1) | 11 (3.6) | 8(11.8) | 4 (5.9) | ||
| Combination | 20 (14.6) | 42 (13.8) | 11(16.2) | 8 (11.8) | ||
| Urgency | <0.001 | 0.976 | ||||
| Elective | 97 (70.8) | 259 (85.2) | 55(80.9) | 54 (79.4) | ||
| Urgent | 31 (22.%) | 44 (14.5) | 12(17.6) | 13 (19.1) | ||
| Emergency | 9 (6.6) | 1 (0.3) | 1(1.5) | 1 (1.5) | ||
| Model of the valve | 0.624 | 0.315 | ||||
| CE Perimount | 115 (83.9) | 253 (83.2) | 57(83.8) | 53 (77.9) | ||
| CE Perimount Magna | 1 (0.7) | 6 (2.1) | 0 (0) | 2 (2.9) | ||
| Medtronic Mosaic | 21 (15.3) | 45 (14.8) | 11 (16.2) | 13 (19.1) | ||
| CPB time | 93 (47–307) | 101 (46–246) | 0.075 | 90 (47–194) | 100 (46–246) | 0.039 |
| Cross-clamp time | 59 (33–190) | 70 (29–131) | <0.001 | 57 (33–156) | 69 (32–118) | 0.006 |
| Second bypass | 2 (1.5) | 12 (3.9) | 0.243 | 1 (1.5) | 4 (5.9) | 0.366 |
| Unmatched Groups | Propensity Score-Matched Groups | |||||
|---|---|---|---|---|---|---|
| Variables a | FS (n = 137) | MiniAVR (n = 304) | p-Value | FS (n = 68) | MiniAVR (n = 68) | p-Value |
| Wound healing disorder | 13 (9.5) | 27 (8.9) | 0.859 | 6 (8.8) | 6 (8.8) | 1.000 |
| Re-exploration | 14 (10.2) | 29 (9.5) | 0.863 | 6 (8.8) | 3 (4.4) | 0.493 |
| Stroke | 0.053 | 0.506 | ||||
| TIA | 1(1.5) | 0 | 1 (1.5) | 0 (0) | ||
| Major insult | 3 (2.2) | 1 (0.3) | 2 (2.9) | 1 (1.5) | ||
| CVVHD | 13 (9.5) | 24 (7.9) | 0.581 | 6 (8.8) | 3 (4.4) | 0.493 |
| AV block | 13 (9.5) | 21 (6.9) | 0.341 | 5 (7.4) | 5 (7.4) | 1.000 |
| Permanent pacemaker | 5 (3.6) | 8 (2.6) | 0.553 | 1 (1.5) | 1 (1.5) | 1.000 |
| Low cardiac output | 0.378 | 0.261 | ||||
| Medication | 6 (4.4) | 7 (2.3) | 1 (1.5) | 0 (0) | ||
| IABP | 0 (0) | 3 (1.0) | 0 (0) | 2 (2.9) | ||
| ECMO | 1 (0.7) | 1 (0.3) | 1 (1.5) | 0 (0) | ||
| Early mortality | 0.635 | 1.000 | ||||
| Intrahospital | 0 (0) | 2 (0.7) | 0 (0) | 0 (0) | ||
| 30-day | 7 (5.1) | 15 (4.9) | 2 (2.9) | 2 (2.9) | ||
| ICU stay (days) | 2 (0–25) | 2 (0–51) | 0.706 | 1 (1–17) | 2 (1–44) | 0.293 |
| Ventilation time (h) | 12 (5–842) | 10 (3–1153) | 0.012 | 12 (5–192) | 10 (3–812) | 0.204 |
| EF at discharge (%) | 54.6 ± 8.0 | 59.0 ± 8.8 | 0.157 | 54.0 ± 9.0 | 53.0 ± 9.0 | 0.872 |
| Follow-up | ||||||
| Embolic event | 2 (1.5) | 18 (5.9) | 0.046 | 1 (1.5) | 2 (2.9) | 0.596 |
| Endocarditis | 3 (2.2) | 9 (3.0) | 0.762 | 2 (2.9) | 0 (0) | 0.496 |
| Reoperation | 3 (2.2) | 16 (5.3) | 0.205 | 2 (2.9) | 2 (2.9) | 1.000 |
| Complication + Reoperation | 5 (3.6) | 25 (8.2) | 0.101 | 4 (5.9) | 4 (5.9) | 0.958 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlavicka, J.; Gettwart, L.; Landgraf, J.; Salem, R.; Hecker, F.; Salihi, E.; Van Linden, A.; Walther, T.; Holubec, T. Minimally Invasive and Full Sternotomy Aortic Valve Replacements Lead to Comparable Long-Term Outcomes in Elderly Higher-Risk Patients: A Propensity-Matched Comparison. J. Cardiovasc. Dev. Dis. 2024, 11, 112. https://doi.org/10.3390/jcdd11040112
Hlavicka J, Gettwart L, Landgraf J, Salem R, Hecker F, Salihi E, Van Linden A, Walther T, Holubec T. Minimally Invasive and Full Sternotomy Aortic Valve Replacements Lead to Comparable Long-Term Outcomes in Elderly Higher-Risk Patients: A Propensity-Matched Comparison. Journal of Cardiovascular Development and Disease. 2024; 11(4):112. https://doi.org/10.3390/jcdd11040112
Chicago/Turabian StyleHlavicka, Jan, Larissa Gettwart, Julian Landgraf, Razan Salem, Florian Hecker, Enis Salihi, Arnaud Van Linden, Thomas Walther, and Tomas Holubec. 2024. "Minimally Invasive and Full Sternotomy Aortic Valve Replacements Lead to Comparable Long-Term Outcomes in Elderly Higher-Risk Patients: A Propensity-Matched Comparison" Journal of Cardiovascular Development and Disease 11, no. 4: 112. https://doi.org/10.3390/jcdd11040112
APA StyleHlavicka, J., Gettwart, L., Landgraf, J., Salem, R., Hecker, F., Salihi, E., Van Linden, A., Walther, T., & Holubec, T. (2024). Minimally Invasive and Full Sternotomy Aortic Valve Replacements Lead to Comparable Long-Term Outcomes in Elderly Higher-Risk Patients: A Propensity-Matched Comparison. Journal of Cardiovascular Development and Disease, 11(4), 112. https://doi.org/10.3390/jcdd11040112








