Impact of Obstructive Sleep Apnea and Sympathetic Nervous System on Cardiac Health: A Comprehensive Review
Abstract
:1. Introduction
2. Materials and Methods
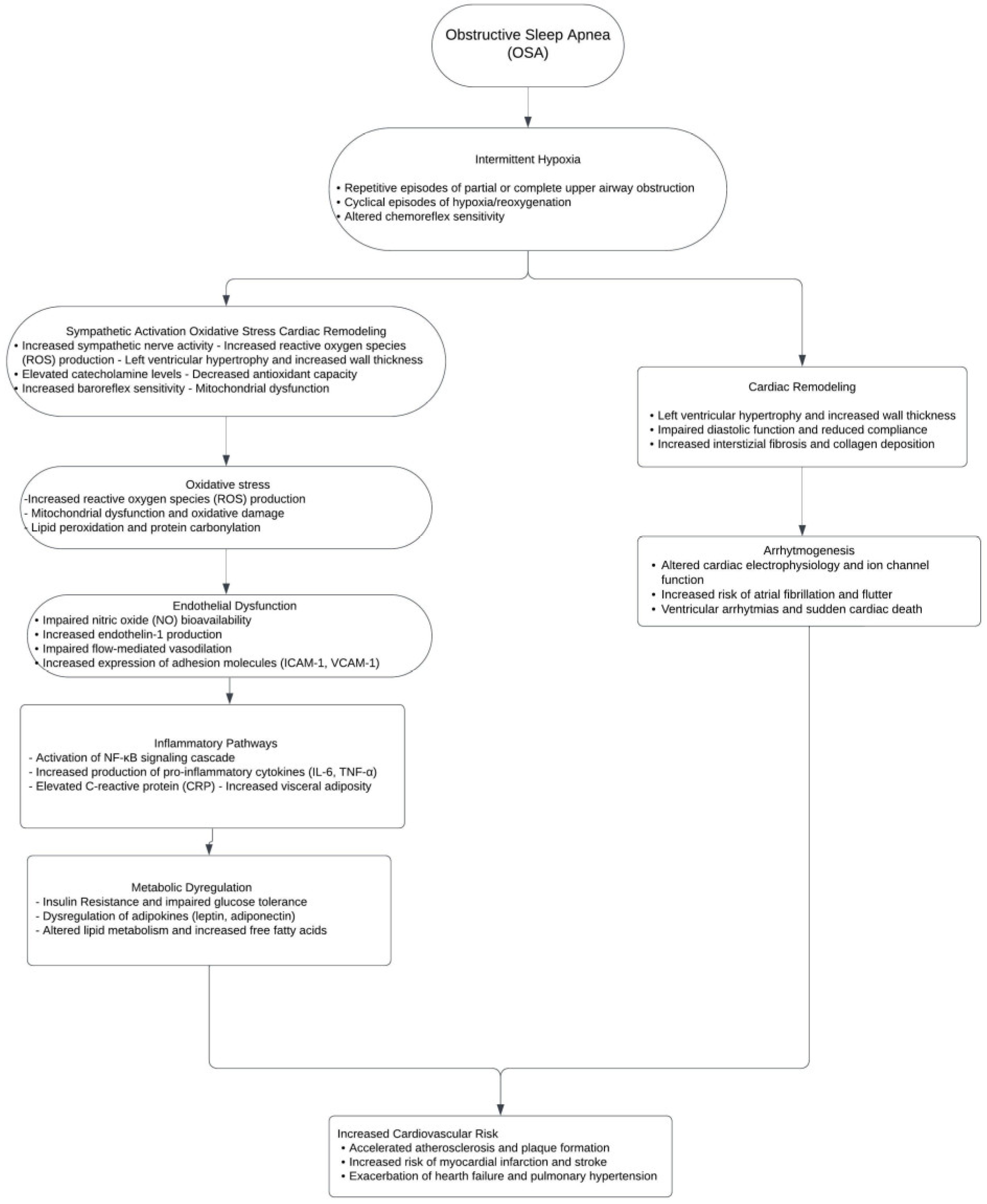
3. What Is the Pathophysiology of Obstructive Sleep Apnea and Sympathetic Nervous System Activation in OSA?
4. How Do Chemoreflex, Baroreflex, Inflammation, and Oxidative Stress Contribute to Sympathetic Overactivity in OSA?
5. What Is the Impact of Sympathetic Overactivity on Cardiovascular Health?
6. What Are the Relevant Diagnostic Approaches to Assess Sympathetic Activity in the Case of OSA?
7. What Therapeutic Interventions Can Influence the Sympathetic Nervous System?
8. What Role Do Pharmacological Treatments Play in Managing OSA and Sympathetic Nervous System?
9. What Are the Future Directions and Research Priorities?
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, K.; Baril, A.A.; Gagnon, J.F.; Fortin, M.; Décary, A.; Lafond, C.; Desautels, A.; Montplaisir, J.; Gosselin, N. Cognitive impairment in obstructive sleep apnea. Pathol. Biol. 2014, 62, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Tregear, S.; Reston, J.; Schoelles, K.; Phillips, B. Obstructive sleep apnea and risk of motor vehicle crash: Systematic review and meta-analysis. J. Clin. Sleep Med. 2009, 5, 573–581. [Google Scholar] [CrossRef]
- Knauert, M.; Naik, S.; Gillespie, M.B.; Kryger, M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J. Otorhinolaryngol. Head Neck Surg. 2015, 1, 17–27. [Google Scholar] [CrossRef]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep apnea and cardiovascular disease: An American Heart Association/american College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008, 118, 1080–1111, Erratum in Circulation 2009, 31, e380. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Lavie, P.; Herer, P.; Hoffstein, V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: Population study. BMJ 2000, 320, 479–482. [Google Scholar] [CrossRef]
- Gami, A.S.; Hodge, D.O.; Herges, R.M.; Olson, E.J.; Nykodym, J.; Kara, T.; Somers, V.K. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 2007, 49, 565–571. [Google Scholar] [CrossRef]
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.L.; Sahadevan, J.; Redline, S. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Shahar, E.; Whitney, C.W.; Redline, S.; Lee, E.T.; Newman, A.B.; Nieto, F.J.; O’Connor, G.T.; Boland, L.L.; Schwartz, J.E.; Samet, J.M. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia—Revisited—The bad ugly and good: Implications to the heart and brain. Sleep Med. Rev. 2015, 20, 27–45. [Google Scholar] [CrossRef]
- Kohler, M.; Stradling, J.R. Mechanisms of vascular damage in obstructive sleep apnea. Nat. Rev. Cardiol. 2010, 7, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006, 7, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, N.; Rabbitts, J.A. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin. Proc. 2009, 84, 822–830. [Google Scholar] [CrossRef]
- Fisher, J.P.; Young, C.N.; Fadel, P.J. Central sympathetic overactivity: Maladies and mechanisms. Auton. Neurosci. 2009, 148, 5–15. [Google Scholar] [CrossRef]
- Grassi, G.; Mark, A.; Esler, M. The sympathetic nervous system alterations in human hypertension. Circ. Res. 2015, 116, 976–990. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Karayannis, G.; Giamouzis, G.; Skoularigis, J.; Louridas, G.; Butler, J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J. Am. Coll. Cardiol. 2009, 54, 1747–1762. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Somers, V.K. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol. Scand. 2003, 177, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Lee, R.W.; Cistulli, P.A. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: Impact of ethnicity. Respirology 2012, 17, 213–222. [Google Scholar] [CrossRef] [PubMed]
- White, D.P. Pathogenesis of obstructive and central sleep apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L. Obstructive sleep apnoea syndrome--an oxidative stress disorder. Sleep Med. Rev. 2003, 7, 35–51. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Semenza, G.L. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 2012, 92, 967–1003. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive sleep apnea: An emerging risk factor for atherosclerosis. Chest 2011, 140, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Peracaula, M.; Torres, D.; Poyatos, P.; Luque, N.; Rojas, E.; Obrador, A.; Orriols, R.; Tura-Ceide, O. Endothelial Dysfunction and Cardiovascular Risk in Obstructive Sleep Apnea: A Review Article. Life 2022, 12, 537. [Google Scholar] [CrossRef]
- Malhotra, A.; White, D.P. Obstructive sleep apnoea. Lancet 2002, 360, 237–245. [Google Scholar] [CrossRef]
- Seneviratne, U.; Puvanendran, K. Excessive daytime sleepiness in obstructive sleep apnea: Prevalence, severity, and predictors. Sleep Med. 2004, 5, 339–343. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef]
- Martynowicz, H.; Wichniak, A.; Wieckiewicz, M. Sleep disorders and cardiovascular risk: Focusing on sleep fragmentation. Dent. Med. Probl. 2024. ahead of print. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Somers, V.K. The sympathetic nervous system and obstructive sleep apnea: Implications for hypertension. J. Hypertens. 1997, 15 Pt 2, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.T.; Hedner, J.; Elam, M.; Ejnell, H.; Sellgren, J.; Wallin, B.G. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 1993, 103, 1763–1768. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Kumar, G.K. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir. Physiol. Neurobiol. 2010, 174, 156–161. [Google Scholar] [CrossRef]
- Cooper, V.L.; Bowker, C.M.; Pearson, S.B.; Elliott, M.W.; Hainsworth, R. Effects of simulated obstructive sleep apnoea on the human carotid baroreceptor-vascular resistance reflex. J. Physiol. 2004, 557 Pt 3, 1055–1065. [Google Scholar] [CrossRef]
- Kara, T.; Narkiewicz, K.; Somers, V.K. Chemoreflexes—Physiology and clinical implications. Acta Physiol. Scand. 2003, 177, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.R.; Parati, G.; Insalaco, G.; Marrone, O.; Castiglioni, P.; Romano, S.; Di Rienzo, M.; Mancia, G.; Bonsignore, G. Continuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2002, 166, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Monahan, K.D.; Leuenberger, U.A.; Ray, C.A. Effect of repetitive hypoxic apnoeas on baroreflex function in humans. J. Physiol. 2006, 574 Pt 2, 605–613. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005, 112, 2660–2667. [Google Scholar] [CrossRef]
- Semenza, G.L.; Prabhakar, N.R. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid. Redox Signal. 2007, 9, 1391–1396. [Google Scholar] [CrossRef]
- Shamsuzzaman, A.S.; Winnicki, M.; Lanfranchi, P.; Wolk, R.; Kara, T.; Accurso, V.; Somers, V.K. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 2002, 105, 2462–2464. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-M.; Zhang, Z.-H.; Johnson, R.F.; Yu, Y.; Beltz, T.G.; Johnson, A.K.; Weiss, R.M.; Felder, R.B. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ. Res. 2006, 99, 758–766. [Google Scholar] [CrossRef]
- Lavalle, S.; Masiello, E.; Iannella, G.; Magliulo, G.; Pace, A.; Lechien, J.R.; Calvo-Henriquez, C.; Cocuzza, S.; Parisi, F.M.; Favier, V.; et al. Unraveling the Complexities of Oxidative Stress and Inflammation Biomarkers in Obstructive Sleep Apnea Syndrome: A Comprehensive Review. Life 2024, 14, 425. [Google Scholar] [CrossRef]
- Kanclerska, J.; Wieckiewicz, M.; Nowacki, D.; Szymanska-Chabowska, A.; Poreba, R.; Mazur, G.; Martynowicz, H. Sleep architecture and vitamin D in hypertensives with obstructive sleep apnea: A polysomnographic study. Dent. Med. Probl. 2024, 61, 43–52. [Google Scholar] [CrossRef]
- Abboud, M. Vitamin D Supplementation and Sleep: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2022, 14, 1076. [Google Scholar] [CrossRef]
- Mirzaei-Azandaryani, Z.; Abdolalipour, S.; Mirghafourvand, M. The effect of vitamin D on sleep quality: A systematic review and meta-analysis. Nutr. Health 2022, 28, 515–526. [Google Scholar] [CrossRef]
- Loh, H.H.; Lim, Q.H.; Kang, W.H.; Yee, A.; Yong, M.C.; Sukor, N. Obstructive sleep apnea and vitamin D: An updated systematic review and meta-analysis. Hormones 2023, 22, 563–580. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Quarti-Trevano, F.; Dell’Oro, R.; Arenare, F.; Spaziani, D.; Mancia, G. Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. Hypertension 2009, 53, 205–209. [Google Scholar] [CrossRef]
- loras, J.S.; Wilkinson, J. Sympathetic activation by obstructive sleep apnea: A challenging ‘off-label’ meta-analysis. J. Hypertens. 2022, 40, 30–32. [Google Scholar] [CrossRef]
- Kato, M.; Roberts-Thomson, P.; Phillips, B.G.; Haynes, W.G.; Winnicki, M.; Accurso, V.; Somers, V.K. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 2000, 102, 2607–2610. [Google Scholar] [CrossRef] [PubMed]
- Treptow, E.; Pepin, J.L.; Bailly, S.; Levy, P.; Bosc, C.; Destors, M.; Woehrle, H.; Tamisier, R. Reduction in sympathetic tone in patients with obstructive sleep apnoea: Is fixed CPAP more effective than APAP? A randomised, parallel trial protocol. BMJ Open 2019, 9, e024253. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Kato, M.; Phillips, B.G.; Pesek, C.A.; Davison, D.E.; Somers, V.K. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 1999, 100, 2332–2335. [Google Scholar] [CrossRef]
- Venkataraman, S.; Vungarala, S.; Covassin, N.; Somers, V.K. Sleep Apnea, Hypertension and the Sympathetic Nervous System in the Adult Population. J. Clin. Med. 2020, 9, 591. [Google Scholar] [CrossRef]
- Rossi, V.A.; Stradling, J.R.; Kohler, M. Effects of obstructive sleep apnoea on heart rhythm. Eur. Respir. J. 2013, 41, 1439–1451. [Google Scholar] [CrossRef]
- López-Gálvez, R.; Rivera-Caravaca, J.M.; Mandaglio-Collados, D.; Orenes-Piñero, E.; Lahoz, Á.; Hernández-Romero, D.; Martínez, C.M.; Carpes, M.; Arribas, J.M.; Cánovas, S.; et al. Molecular mechanisms of postoperative atrial fibrillation in patients with obstructive sleep apnea. FASEB J. 2023, 37, e22941. [Google Scholar] [CrossRef] [PubMed]
- Kanagala, R.; Murali, N.S.; Friedman, P.A.; Ammash, N.M.; Gersh, B.J.; Ballman, K.V.; Shamsuzzaman, A.S.M.; Somers, V.K. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003, 107, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Fein, A.S.; Shvilkin, A.; Shah, D.; Haffajee, C.I.; Das, S.; Kumar, K.; Kramer, D.B.; Zimetbaum, P.J.; Buxton, A.E.; Josephson, M.E.; et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J. Am. Coll. Cardiol. 2013, 62, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.D.; Floras, J.S. Sleep apnea and heart failure: Part I: Obstructive sleep apnea. Circulation 2003, 107, 1671–1678. [Google Scholar] [CrossRef]
- Arias, M.A.; García-Río, F.; Alonso-Fernández, A.; Mediano, O.; Martínez, I.; Villamor, J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: Effects of nasal continuous positive airway pressure in men. Circulation 2005, 112, 375–383. [Google Scholar] [CrossRef]
- Park, J.U.; Urtnasan, E.; Kim, S.H.; Lee, K.J. A Prediction Model of Incident Cardiovascular Disease in Patients with Sleep-Disordered Breathing. Diagnostics 2021, 11, 2212. [Google Scholar] [CrossRef]
- Hayashi, M.; Fujimoto, K.; Urushibata, K.; Uchikawa, S.-I.; Imamura, H.; Kubo, K. Nocturnal oxygen desaturation correlates with the severity of coronary atherosclerosis in coronary artery disease. Chest 2003, 124, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Floras, J.S.; Usui, K.; Plante, J.; Tkacova, R.; Kubo, T.; Ando, S.-I.; Bradley, T.D. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N. Engl. J. Med. 2003, 348, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Colish, J.; Walker, J.R.; Elmayergi, N.; Almutairi, S.; Alharbi, F.; Lytwyn, M.; Francis, A.; Bohonis, S.; Zeglinski, M.; Kirkpatrick, I.D.C.; et al. Obstructive sleep apnea: Effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest 2012, 141, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Durgan, D.J.; Bryan, R.M., Jr. Cerebrovascular consequences of obstructive sleep apnea. J. Am. Heart Assoc. 2012, 1, e000091. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Bortolotto, L.A.; Lorenzi, M.C.; Figueiredo, A.C.; Krieger, E.M.; Lorenzi-Filho, G. Early signs of atherosclerosis in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-Israel, S.; Stone, K.L. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011, 306, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Buratti, L.; Viticchi, G.; Falsetti, L.; Cagnetti, C.; Luzzi, S.; Bartolini, M.; Provinciali, L.; Silvestrini, M. Vascular impairment in Alzheimer’s disease: The role of obstructive sleep apnea. J. Alzheimers Dis. 2014, 38, 445–453. [Google Scholar] [CrossRef]
- Prilipko, O.; Huynh, N.; Schwartz, S.; Tantrakul, V.; Kim, J.H.; Peralta, A.R.; Kushida, C.; Paiva, T.; Guilleminault, C. Task positive and default mode networks during a parametric working memory task in obstructive sleep apnea patients and healthy controls. Sleep 2011, 34, 293–301. [Google Scholar] [CrossRef]
- Prilipko, O.; Huynh, N.; Thomason, M.E.; Kushida, C.A.; Guilleminault, C. An fMRI study of cerebrovascular reactivity and perfusion in obstructive sleep apnea patients before and after CPAP treatment. Sleep Med. 2014, 15, 892–898. [Google Scholar] [CrossRef]
- Vallbo, A.B.; Hagbarth, K.-E.; Wallin, B.G.; Filingeri, D.; Zhang, H.; Arens, E.A.; Strzalkowski, N.D.J.; Peters, R.M.; Inglis, J.T.; Bent, L.R.; et al. Microneurography: How the technique developed and its role in the investigation of the sympathetic nervous system. J. Appl. Physiol. 2004, 96, 1262–1269. [Google Scholar] [CrossRef]
- Grassi, G.; Esler, M. How to assess sympathetic activity in humans. J. Hypertens. 1999, 17, 719–734. [Google Scholar] [CrossRef]
- Quarti-Trevano, F.; Biffi, A.; Bonzani, M.; Seravalle, G.; Corrao, G.; Mancia, G.; Grassi, G. Neuroadrenergic activation in obstructive sleep apnea syndrome: A systematic review and meta-analysis. J. Hypertens. 2021, 39, 2281–2289, Erratum in J. Hypertens. 2022, 40, 196. [Google Scholar] [CrossRef]
- Loredo, J.S.; Ziegler, M.G.; Ancoli-Israel, S.; Clausen, J.L.; Dimsdale, J.E. Relationship of arousals from sleep to sympathetic nervous system activity and BP in obstructive sleep apnea. Chest 1999, 116, 655–659. [Google Scholar] [CrossRef]
- Narkiewicz, K.; van de Borne, P.J.H.; Cooley, R.L.; Dyken, M.E.; Somers, V.K. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 1998, 98, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, M.; Knoll, G.; Leenen, F.H.; Leech, J.; Aaron, S.D.; Hiremath, S. Effects of CPAP on Blood Pressure and Sympathetic Activity in Patients with Diabetes Mellitus, Chronic Kidney Disease, and Resistant Hypertension. CJC Open 2020, 2, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Iannella, G.; Magliulo, G.; Iacono, C.A.M.L.; Visconti, I.C.; Lechien, J.R.; Perrone, T.; Cammaroto, G.; Meccariello, G.; Maniaci, A.; Cocuzza, S.; et al. Quality of Life and Excessive Daytime Sleepiness in Adults with Obstructive Sleep Apnea Who Are Treated with Multilevel Surgery or Adherent to Continuous Positive Airway Pressure. J. Clin. Med. 2022, 11, 2375. [Google Scholar] [CrossRef] [PubMed]
- Sica, E.; De Bernardi, F.; Nosetti, L.; Martini, S.; Cosentino, M.; Castelnuovo, P.; Marino, F. Catecholamines and children obstructive sleep apnea: A systematic review. Sleep Med. 2021, 87, 227–232. [Google Scholar] [CrossRef]
- Goldstein, D.S.; McCarty, R.; Polinsky, R.J.; Kopin, I.J. Relationship between plasma norepinephrine and sympathetic neural activity. Hypertension 1983, 5, 552–559. [Google Scholar] [CrossRef]
- Greenlund, I.M.; Carter, J.R. Sympathetic neural responses to sleep disorders and insufficiencies. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H337–H349. [Google Scholar] [CrossRef]
- Redline, S.; Azarbarzin, A.; Peker, Y. Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.G.; Mills, P.J.; Loredo, J.S.; Ancoli-Israel, S.; Dimsdale, J.E. Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest 2001, 120, 887–893. [Google Scholar] [CrossRef]
- Lombardi, C.; Pengo, M.F.; Parati, G.; Sarkar, P.; Mukherjee, S.; Chai-Coetzer, C.L.; McEvoy, R.D. Systemic hypertension in obstructive sleep apnea. J. Thorac. Dis. 2018, 10 (Suppl. 34), S4231–S4243. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Malik, M.; Camm, A.J. Heart rate variability. Clin. Cardiol. 1990, 13, 570–576. [Google Scholar] [CrossRef] [PubMed]
- La Via, L.; Vasile, F.; Perna, F.; Zawadka, M. Prediction of fluid responsiveness in critical care: Current evidence and future perspective. Trends Anaesth. Crit. Care 2024, 54, 101316. [Google Scholar] [CrossRef]
- Pagani, M.; Lombardi, F.; Guzzetti, S.; Rimoldi, O.; Furlan, R.; Pizzinelli, P.; Sandrone, G.; Malfatto, G.; Dell’Orto, S.; Piccaluga, E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 1986, 59, 178–193. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Montano, N.; Cogliati, C.; van de Borne, P.J.H.; Dyken, M.E.; Somers, V.K. Altered cardiovascular variability in obstructive sleep apnea. Circulation 1998, 98, 1071–1077. [Google Scholar] [CrossRef]
- Sanfilippo, F.; La Via, L.; Flower, L.; Madhivathanan, P.; Astuto, M. The value of subcostal echocardiographic assessment, and directions for future research. Can. J. Anaesth. 2022, 69, 676–677. [Google Scholar] [CrossRef]
- Parati, G.; Di Rienzo, M.; Mancia, G. How to measure baroreflex sensitivity: From the cardiovascular laboratory to daily life. J. Hypertens. 2000, 18, 7–19. [Google Scholar] [CrossRef]
- Eckberg, D.L.; Sleight, P. Human Baroreflexes in Health and Disease; Oxford Academic: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- Chapleau, M.W.; Sabharwal, R. Methods of assessing vagus nerve activity and reflexes. Heart Fail. Rev. 2011, 16, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Peng, Y.-J.; Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Investig. 2020, 130, 5042–5051. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.T.; A Hedner, J.; Sellgren, J.; Elam, M.; Wallin, B.G. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1996, 154, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Raczak, G. Baroreflex sensitivity: Measurement and clinical implications. Ann. Noninvasive Electrocardiol. 2008, 13, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.R.; Parati, G.; Insalaco, G.; Castiglioni, P.; Marrone, O.; Romano, S.; Salvaggio, A.; Mancia, G.; Bonsignore, G.; Di Rienzo, M. Baroreflex control of heart rate during sleep in severe obstructive sleep apnoea: Effects of acute CPAP. Eur. Respir. J. 2006, 27, 128–135. [Google Scholar] [CrossRef]
- Kohler, M.; Pepperell, J.C.T.; Casadei, B.; Craig, S.; Crosthwaite, N.; Stradling, J.R.; Davies, R.J.O. CPAP and measures of cardiovascular risk in males with OSAS. Eur. Respir. J. 2008, 32, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Pun, M.; Beaudin, A.E.; Raneri, J.K.; Anderson, T.J.; Hanly, P.J.; Poulin, M.J. Impact of nocturnal oxygen and CPAP on the ventilatory response to hypoxia in OSA patients free of overt cardiovascular disease. Exp. Neurol. 2021, 346, 113852. [Google Scholar] [CrossRef]
- Sullivan, C.E.; Issa, F.G.; Berthon-Jones, M.; Eves, L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981, 1, 862–865. [Google Scholar] [CrossRef]
- He, W.; Tang, Y.; Meng, G.; Wang, D.; Wong, J.; Mitscher, G.A.; Adams, D.; Everett, T.H.; Chen, P.-S.; Manchanda, S. Skin sympathetic nerve activity in patients with obstructive sleep apnea. Heart Rhythm. 2020, 17, 1936–1943. [Google Scholar] [CrossRef]
- Waradekar, N.V.; I Sinoway, L.; Zwillich, C.W.; Leuenberger, U.A. Influence of treatment on muscle sympathetic nerve activity in sleep apnea. Am. J. Respir. Crit. Care Med. 1996, 153 Pt 1, 1333–1338. [Google Scholar] [CrossRef]
- Khoo, M.C.K.; Belozeroff, V.; Berry, R.B.; Sassoon, C.S.H. Cardiac autonomic control in obstructive sleep apnea: Effects of long-term CPAP therapy. Am. J. Respir. Crit. Care Med. 2001, 164, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, H.U.; Bin, Y.S.; Sutherland, K.; Ucak, S.; de Chazal, P.; A Cistulli, P. The effect of obstructive sleep apnea therapy on cardiovascular autonomic function: A systematic review and meta-analysis. Sleep 2022, 45, zsac210. [Google Scholar] [CrossRef]
- Pepperell, J.C.; Ramdassingh-Dow, S.; Crosthwaite, N.; Mullins, R.; Jenkinson, C.; Stradling, J.R.; Davies, R.J. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: A randomised parallel trial. Lancet 2002, 359, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Bansal, A.; Yanamaladoddi, V.R.; Sarvepalli, S.S.; Vemula, S.L.; Aramadaka, S.; Mannam, R. Atrial Fibrillation in Obstructive Sleep Apnea Patients: Mechanisms, Risk Factors, and Management Strategies. Cureus 2023, 15, e36282. [Google Scholar] [CrossRef]
- Ferguson, K.A.; Cartwright, R.; Rogers, R.; Schmidt-Nowara, W. Oral appliances for snoring and obstructive sleep apnea: A review. Sleep 2006, 29, 244–262. [Google Scholar] [CrossRef]
- Sutherland, K.; Vanderveken, O.M.; Tsuda, H.; Marklund, M.; Gagnadoux, F.; Kushida, C.A.; Cistulli, P.A. Oral appliance treatment for obstructive sleep apnea: An update. J. Clin. Sleep Med. 2014, 10, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Gotsopoulos, H.; Kelly, J.J.; Cistulli, P.A. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: A randomized, controlled trial. Sleep 2004, 27, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Manetta, I.P.; Ettlin, D.; Sanz, P.M.; Rocha, I.; Meira, E.; Cruz, M. Mandibular advancement devices in obstructive sleep apnea: An updated review. Sleep Sci. 2022, 15, 398–405. [Google Scholar] [CrossRef]
- Itzhaki, S.; Dorchin, H.; Clark, G.; Lavie, L.; Lavie, P.; Pillar, G. The effects of 1-year treatment with a herbst mandibular advancement splint on obstructive sleep apnea, oxidative stress, and endothelial function. Chest 2007, 131, 740–749. [Google Scholar] [CrossRef]
- Tsioufis, C.; Kasiakogias, A.; Thomopoulos, C.; Manolis, A.; Stefanadis, C. Managing hypertension in obstructive sleep apnea: The interplay of continuous positive airway pressure, medication and chronotherapy. J. Hypertens. 2010, 28, 875–882. [Google Scholar] [CrossRef]
- Khan, A.; Patel, N.K.; O’hearn, D.J.; Khan, S. Resistant hypertension and obstructive sleep apnea. Int. J. Hypertens. 2013, 2013, 193010. [Google Scholar] [CrossRef]
- Heitmann, J.; Ehlenz, K.; Penzel, T.; Becker, H.; Grote, L.; Voigt, K.; Peter, J.H.; Vogelmeier, C. Sympathetic activity is reduced by nCPAP in hypertensive obstructive sleep apnoea patients. Eur. Respir. J. 2004, 23, 255–262. [Google Scholar] [CrossRef]
- Floras, J.S. Sleep Apnea and Cardiovascular Disease: An Enigmatic Risk Factor. Circ. Res. 2018, 122, 1741–1764. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, J.; Greulich, T.; Reinke, C.; Koehler, U.; Vogelmeier, C.; Becker, H.; Schmidt, A.; Canisius, S. Comparison of the effects of nebivolol and valsartan on BP reduction and sleep apnoea activity in patients with essential hypertension and OSA. Curr. Med. Res. Opin. 2010, 26, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Kraiczi, H.; Hedner, J.; Peker, Y.; Grote, L. Comparison of atenolol, amlodipine, enalapril, hydrochlorothiazide, and losartan for antihypertensive treatment in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2000, 161, 1423–1428. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Colombo, M.; Bolla, G.; Cattaneo, B.M.; Cavagnini, F.; Mancia, G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 1998, 97, 2037–2042. [Google Scholar] [CrossRef]
- Eikelis, N.; Esler, M. The neurobiology of human obesity. Exp. Physiol. 2005, 90, 673–682. [Google Scholar] [CrossRef]
- Trombetta, I.C.; Somers, V.K.; Maki-Nunes, C.; Drager, L.F.; Toschi-Dias, E.; Alves, M.J.N.N.; Fraga, R.F.; Rondon, M.U.P.B.; Bechara, M.G.; Lorenzi-Filho, G.; et al. Consequences of comorbid sleep apnea in the metabolic syndrome--implications for cardiovascular risk. Sleep 2010, 33, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Straznicky, N.E.; Grima, M.T.; Eikelis, N.; Nestel, P.J.; Dawood, T.; Schlaich, M.P.; Chopra, R.; Masuo, K.; Esler, M.D.; Sari, C.I.; et al. The effects of weight loss versus weight loss maintenance on sympathetic nervous system activity and metabolic syndrome components. J. Clin. Endocrinol. Metab. 2011, 96, E503–E508. [Google Scholar] [CrossRef]
- Maki-Nunes, C.; Toschi-Dias, E.; Cepeda, F.X.; Rondon, M.U.P.B.; Alves, M.N.N.; Fraga, R.F.; Braga, A.M.F.W.; Aguilar, A.M.; Amaro, A.C.; Drager, L.F.; et al. Diet and exercise improve chemoreflex sensitivity in patients with metabolic syndrome and obstructive sleep apnea. Obesity 2015, 23, 1582–1590. [Google Scholar] [CrossRef]
- Noda, A.; Nakata, S.; Koike, Y.; Miyata, S.; Kitaichi, K.; Nishizawa, T.; Nagata, K.; Yasuma, F.; Murohara, T.; Yokota, M. Continuous positive airway pressure improves daytime baroreflex sensitivity and nitric oxide production in patients with moderate to severe obstructive sleep apnea syndrome. Hypertens. Res. 2007, 30, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000, 284, 3015–3021. [Google Scholar] [CrossRef] [PubMed]
- de Zambotti, M.; Cellini, N.; Goldstone, A.; Colrain, I.M.; Baker, F.C. Wearable Sleep Technology in Clinical and Research Settings. Med. Sci. Sports Exerc. 2019, 51, 1538–1557. [Google Scholar] [CrossRef]
- Fontes, M.A.P.; Marzano, L.A.S.; Silva, C.C.; e Silva, A.C.S. Renal sympathetic denervation for resistant hypertension: Where do we stand after more than a decade. J. Bras. Nefrol. 2020, 42, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Thorp, A.A.; Schlaich, M.P. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J. Diabetes Res. 2015, 2015, 341583. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.-H.; Fong, D.Y.T.; Lui, M.M.S.; Lam, D.C.L.; Ip, M.S.M. Cardiovascular outcomes in obstructive sleep apnoea and implications of clinical phenotyping on effect of CPAP treatment. Thorax 2023, 78, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Montesi, S.B.; Edwards, B.A.; Malhotra, A.; Bakker, J.P. The effect of continuous positive airway pressure treatment on blood pressure: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Sleep Med. 2012, 8, 587–596. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Leischik, R.; Dworrak, B.; Strauss, M.; Przybylek, B.; Dworrak, T.; Schöne, D.; Horlitz, M.; Mügge, A. Plasticity of Health. German J. Med. 2016, 1, 1–17. [Google Scholar] [CrossRef]
| Study | Year | Country | Sample Size | Study Design | Main Results | Study Limitations |
|---|---|---|---|---|---|---|
| Martynowicz et al. [32] | 2024 | Poland | - | Examined relationship between sleep fragmentation and cardiovascular risk | Sleep fragmentation parameters like AB, SFI, and ArI may be useful to quantify sleep fragmentation and determine cardiovascular risk | Study design not adequate |
| Kanclerska et al. [45] | 2024 | Poland | 133 patients | Investigated relationship between AH and OSA by examining sleep architecture, vitamin D, and electrolyte levels | No significant differences in vitamin D levels found between hypertensive and normotensive OSA patients, contrasting previous studies | the study did not comprehensively examine the potential influence of calcium, magnesium, vitamin D and uric acid concentrations on the sleep architecture of patients with comorbid arterial hypertension and obstructive sleep apnea |
| Abboud et al. [47] | 2022 | United Arab Emirates | 19 studies | Systematic review and meta-analysis | Vitamin D supplementation showed promise in improving sleep quality but had less clear effects on sleep quantity and disorders | Limitations of included studies not discussed in the review |
| Mirzaei-Azandaryani et al. [48] | 2022 | Iran | 18 studies | Meta-analysis | Vitamin D administration significantly improved sleep quality | Limitations of included studies not discussed in the review |
| Loh et al. [49] | 2023 | Malaysia | 18 studies | Systematic review and meta-analysis | OSA patients had higher prevalence of vitamin D deficiency and significantly lower serum vitamin D levels vs. non-OSA controls, especially in moderate-severe OSA | Limitations of included studies not discussed in the review |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniaci, A.; Lavalle, S.; Parisi, F.M.; Barbanti, M.; Cocuzza, S.; Iannella, G.; Magliulo, G.; Pace, A.; Lentini, M.; Masiello, E.; et al. Impact of Obstructive Sleep Apnea and Sympathetic Nervous System on Cardiac Health: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2024, 11, 204. https://doi.org/10.3390/jcdd11070204
Maniaci A, Lavalle S, Parisi FM, Barbanti M, Cocuzza S, Iannella G, Magliulo G, Pace A, Lentini M, Masiello E, et al. Impact of Obstructive Sleep Apnea and Sympathetic Nervous System on Cardiac Health: A Comprehensive Review. Journal of Cardiovascular Development and Disease. 2024; 11(7):204. https://doi.org/10.3390/jcdd11070204
Chicago/Turabian StyleManiaci, Antonino, Salvatore Lavalle, Federica Maria Parisi, Marco Barbanti, Salvatore Cocuzza, Giannicola Iannella, Giuseppe Magliulo, Annalisa Pace, Mario Lentini, Edoardo Masiello, and et al. 2024. "Impact of Obstructive Sleep Apnea and Sympathetic Nervous System on Cardiac Health: A Comprehensive Review" Journal of Cardiovascular Development and Disease 11, no. 7: 204. https://doi.org/10.3390/jcdd11070204







