Noninvasive Mapping System for the Stereotactic Radioablation Treatment of Ventricular Tachycardia: A Case Description
Abstract
:1. Introduction
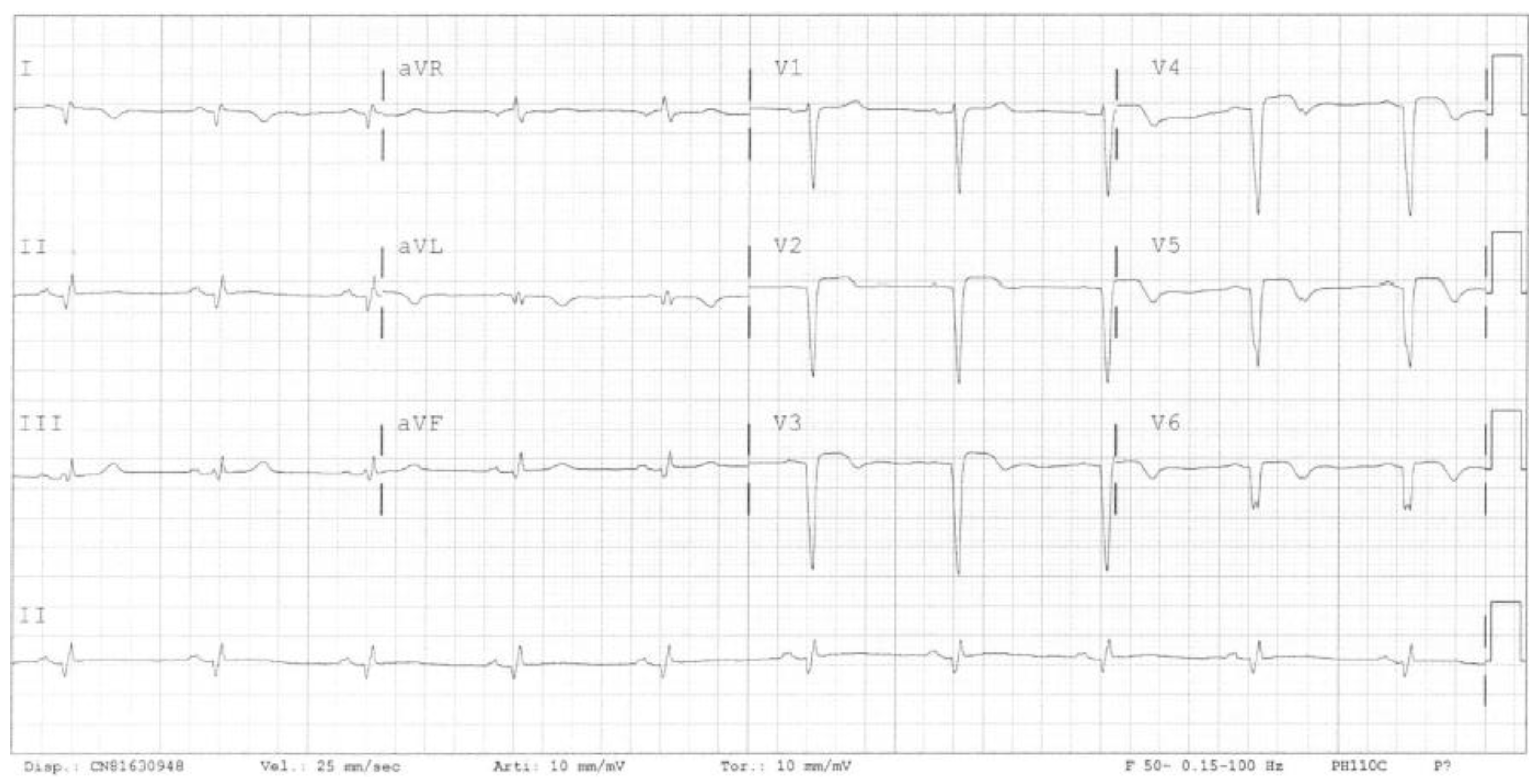
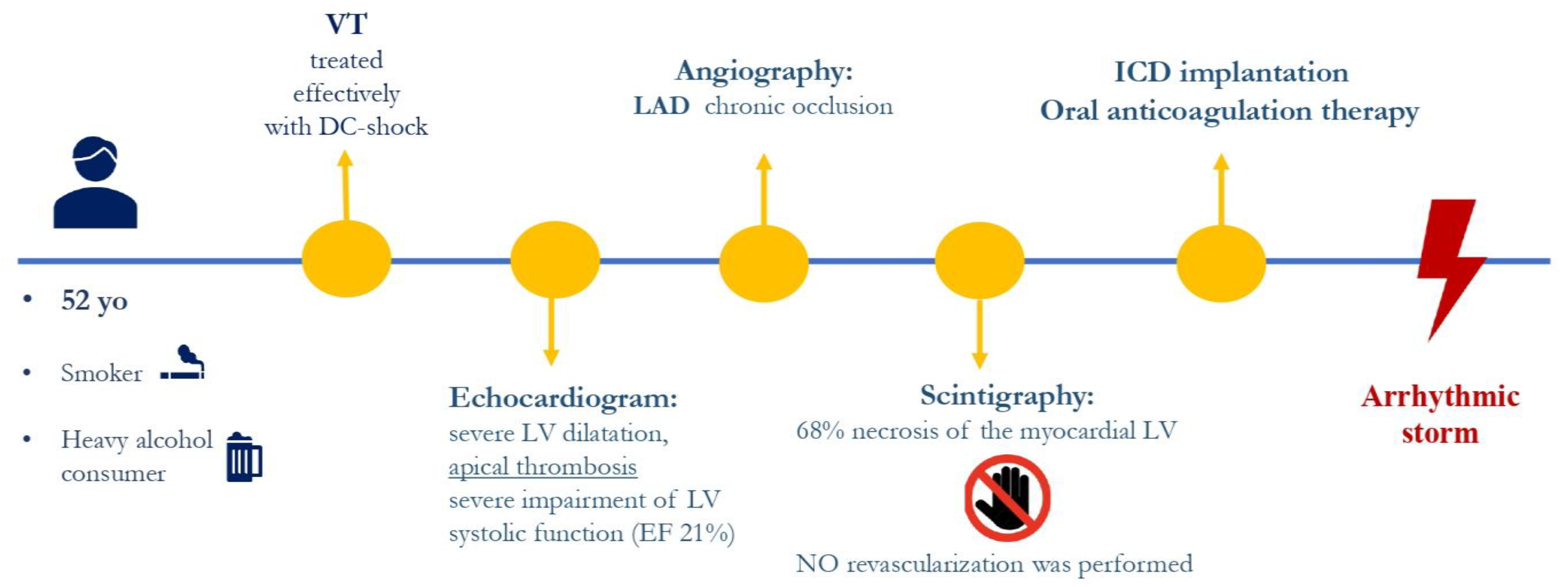
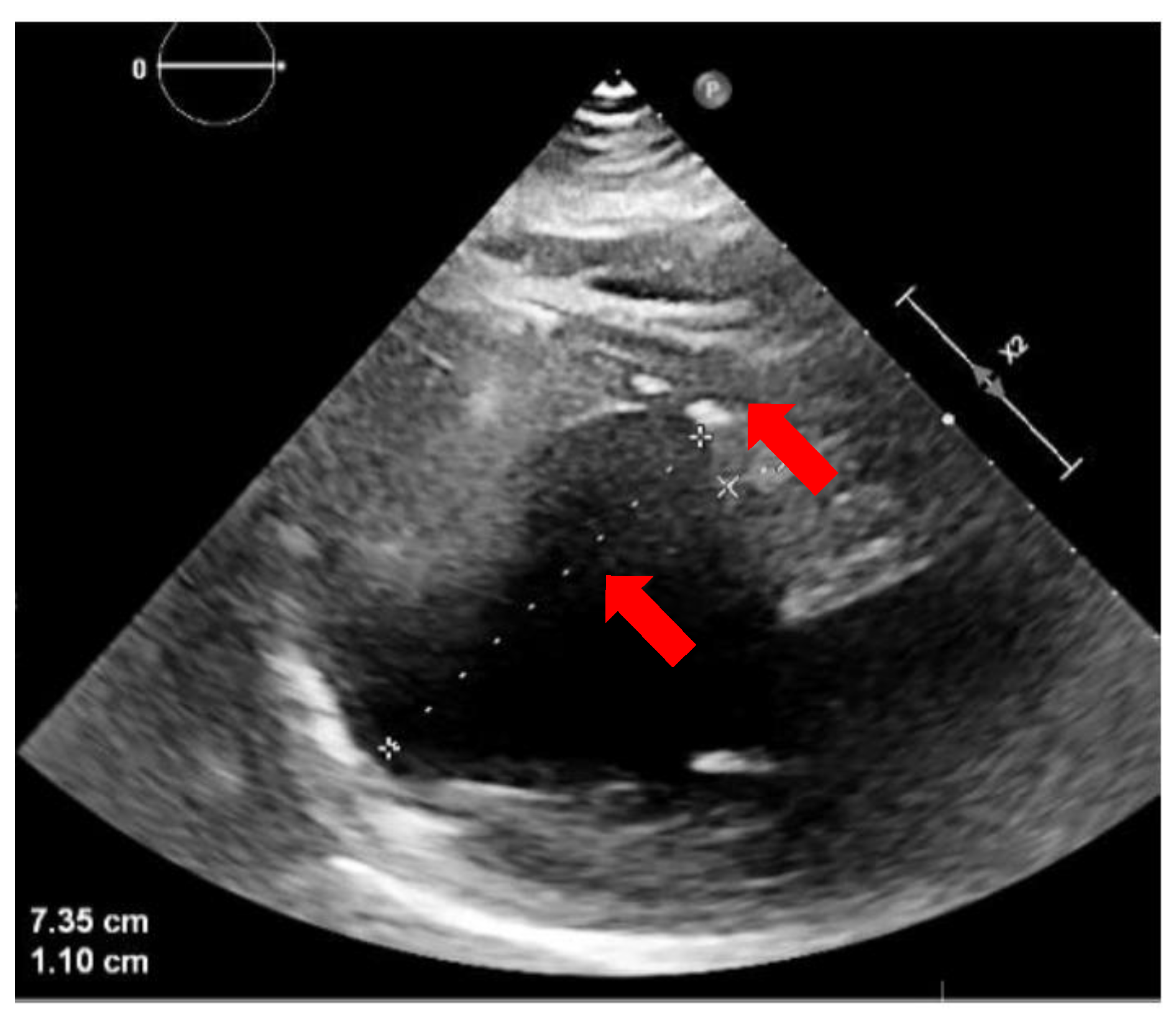
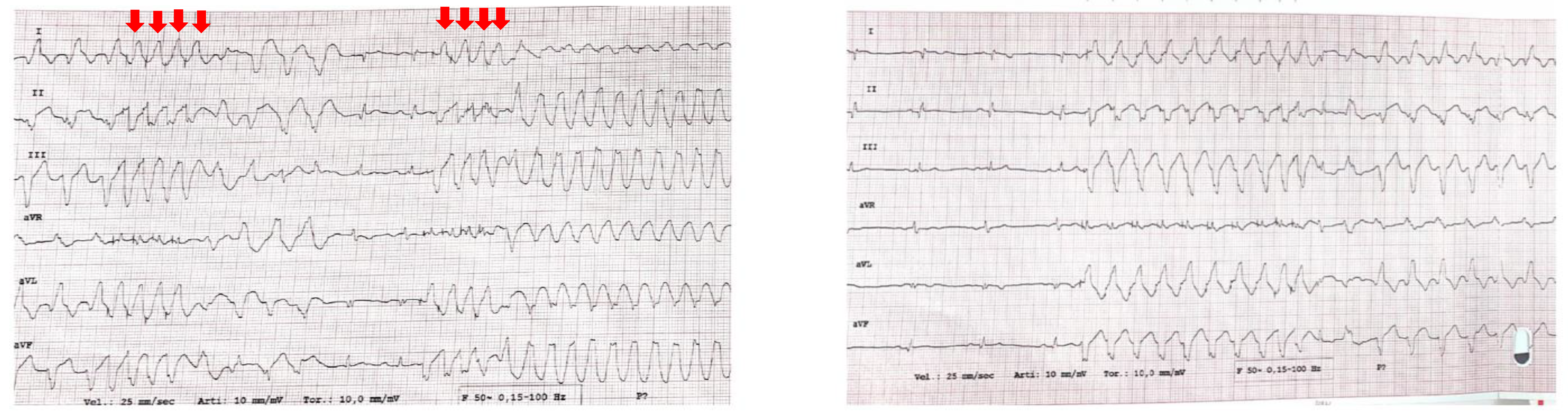
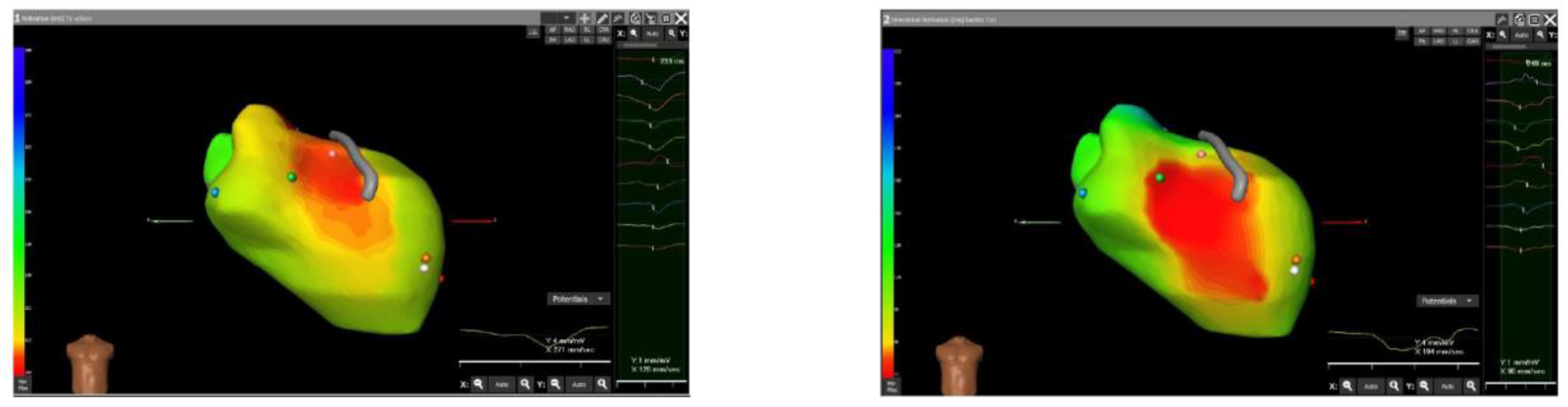
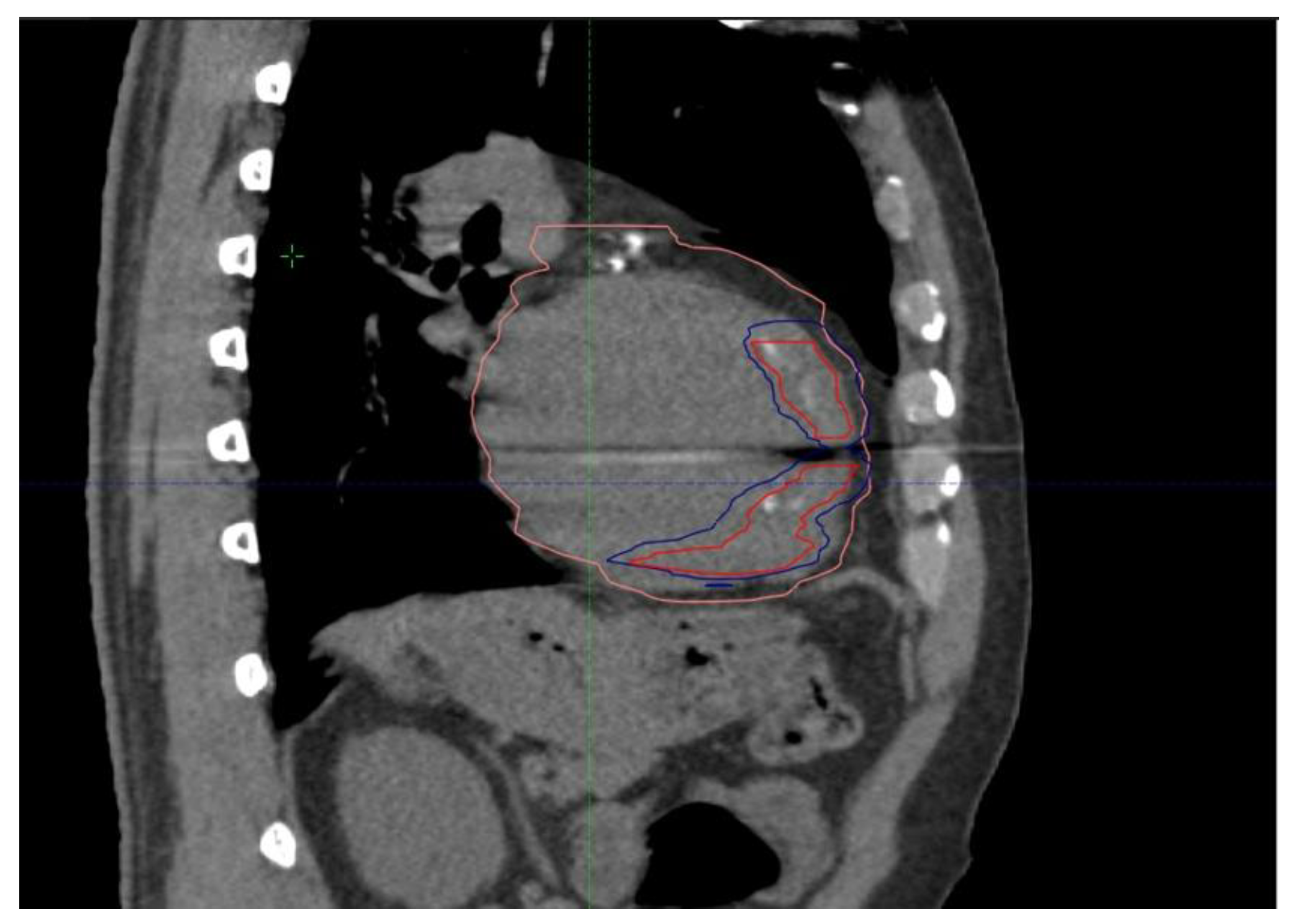
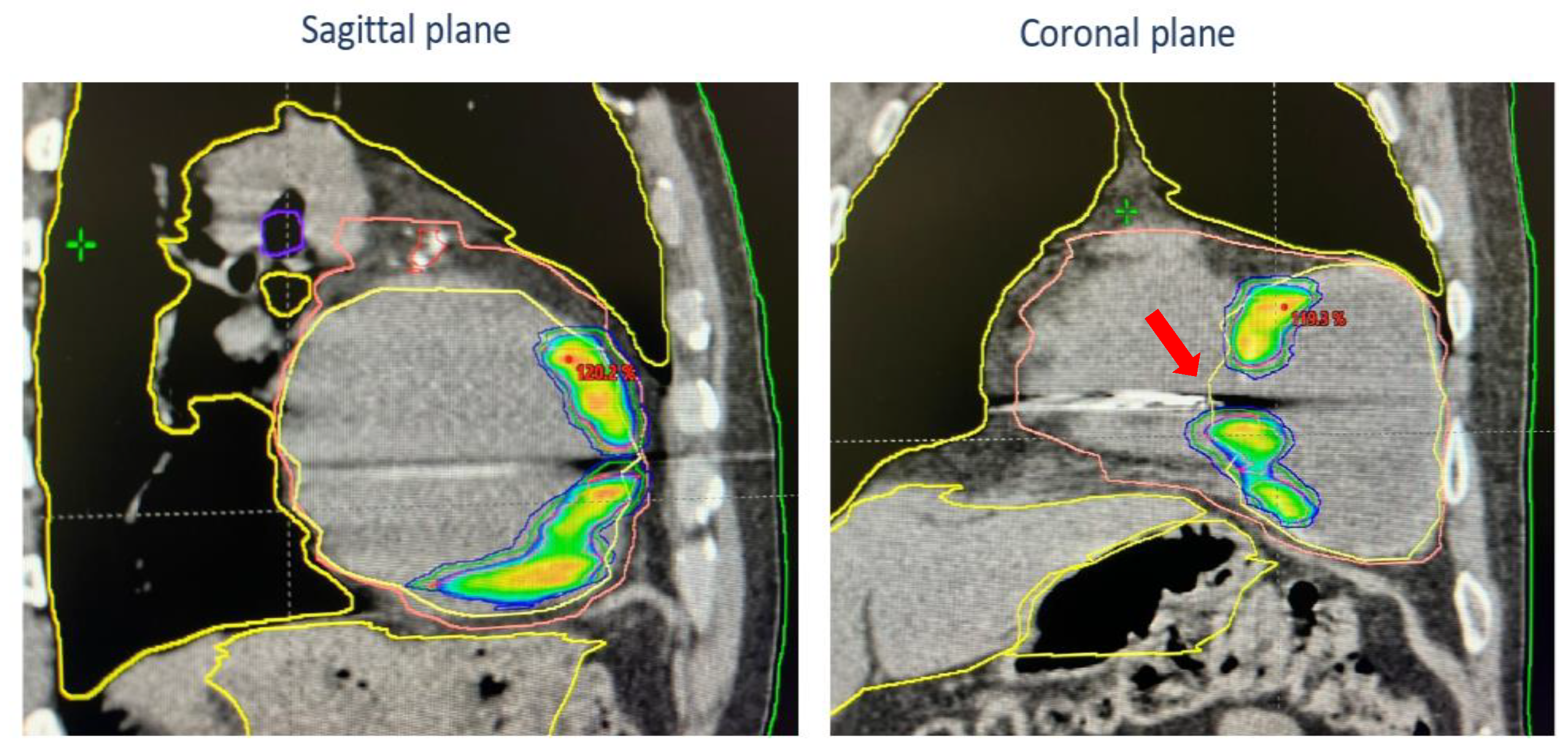
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeppenfeld, K.; Tfelt-Hansen, J.; De Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; De Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [PubMed]
- Conti, S.; Pala, S.; Biagioli, V.; Del Giorno, G.; Zucchetti, M.; Russo, E.; Marino, V.; Russo, A.D.; Casella, M.; Pizzamiglio, F.; et al. Electrical storm: A clinical and electrophysiological overview. World J. Cardiol. 2015, 7, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Exner, D.V.; Pinski, S.L.; Wyse, D.G.; Renfroe, E.G.; Follmann, D.; Gold, M.; Beckman, K.J.; Coromolas, J.; Lancaster, S.; Hallstrom, A.P. Antiarrhythmics Versus Implantable Defibrillators. Electrical storm presages nonsudden death: The antiarrhythmics versus implantable defibrillators (AVID) trial. Circulation 2001, 103, 2066–2071. [Google Scholar] [CrossRef] [PubMed]
- Bencardino, G.; DI Monaco, A.; Rio, T.; Frontera, A.; Santangeli, P.; Leo, M.; Pelargonio, G.; Perna, F.; Narducci, M.L.; Gabrielli, F.; et al. The association between ICD interventions and mortality is independent of their modality: Clinical implications. J. Cardiovasc. Electrophysiol. 2014, 25, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Carbucicchio, C.; Santamaria, M.; Trevisi, N.; Maccabelli, G.; Giraldi, F.; Fassini, G.; Riva, S.; Moltrasio, M.; Cireddu, M.; Veglia, F.; et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter defibrillators: Short- and long-term outcomes in a prospective single-center study. Circulation 2008, 117, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Deneke, T.; Shin, D.-I.; Lawo, T.; Bösche, L.; Balta, O.; Anders, H.; Bünz, K.; Horlitz, M.; Grewe, P.H.; Lemke, B.; et al. Catheter ablation of electrical storm in a collaborative hospital network. Am. J. Cardiol. 2011, 108, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Kozeluhova, M.; Peichl, P.; Cihak, R.; Wichterle, D.; Vancura, V.; Bytesnik, J.; Kautzner, J. Catheter ablation of electrical storm in patients with structural heart disease. Europace 2011, 13, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Vergara, P.; Tung, R.; Vaseghi, M.; Brombin, C.; Frankel, D.; Di Biase, L.; Nagashima, K.; Tedrow, U.; Tzou, W.S.; Sauer, W.H.; et al. Successful ventricular tachycardia ablation in patients with electrical storm reduces recurrences and improves survival. Heart Rhythm. 2018, 15, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Di Monaco, A.; Quadrini, F.; Troisi, F.; Vitulano, N.; Caruso, R.; Duni, N.; Cecere, G.; Guida, P.; Langialonga, T.; Grimaldi, M. Cardiopulmonary support in patients undergoing catheter ablation of poorly tolerated ventricular arrhythmias and electrical storm. J. Cardiovasc. Electrophysiol. 2019, 30, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Cuculich, P.S.; Schill, M.R.; Kashani, R.; Mutic, S.; Lang, A.; Cooper, D.; Faddis, M.; Gleva, M.; Noheria, A.; Smith, T.W.; et al. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N. Engl. J. Med. 2017, 377, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Di Monaco, A.; Surgo, A.; Vitulano, N.; Gregucci, F.; Ludovico, E.; Carbonara, R.; Quadrini, F.; Rubini, G.; Bonaparte, I.; et al. Linac Based STereotactic Arrhythmia Radioablation (STAR) of Ventricular Tachycardia: Case report and literature review. Clin. Case Rep. 2020, 9, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Bonaparte, I.; Gregucci, F.; Surgo, A.; Di Monaco, A.; Vitulano, N.; Ludovico, E.; Carbonara, R.; Ciliberti, M.P.; Quadrini, F.; Grimaldi, M.; et al. Linac-based STereotactic Arrhythmia Radioablation (STAR) for ventricular tachycardia: A treatment planning study. Jpn. J. Radiol. 2021, 39, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Cheniti, G.; Puyo, S.; Martin, C.A.; Frontera, A.; Vlachos, K.; Takigawa, M.; Bourier, F.; Kitamura, T.; Lam, A.; Dumas-Pommier, C.; et al. Noninvasive Mapping and Electrocardiographic Imaging in Atrial and Ventricular Arrhythmias (CardioInsight). Card. Electrophysiol. Clin. 2019, 11, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rudy, Y. Application of the method of fundamental solutions to potential-based inverse electrocardiography. Ann. Biomed. Eng. 2006, 34, 1272–1288. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.S.; Taccardi, B.; Lux, R.L.; Ershler, P.R.; Rudy, Y. Electrocardiographic imaging: Noninvasive characterization of intramural myocardial activation from inverse-reconstructed epicardial potentials and electrograms. Circulation 1998, 97, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.J.; Orini, M.; Zacur, E.; Dhillon, G.; Daw, H.; Srinivasan, N.T.; Martin, C.; Lane, J.; Mansell, J.S.; Cambridge, A.; et al. Evaluation of ECG Imaging to Map Hemodynamically Stable and Unstable Ventricular Arrhythmias. Circ. Arrhythm. Electrophysiol. 2020, 13, e007377. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Shivkumar, K.; Valderrabano, M.; Diaz, J.C.; Alviz, I.; Briceno, D.; Natale, A.; Di Biase, L. Modern mapping and ablation techniques to treat ventricular arrhythmias from the left ventricular summit and interventricular septum. Heart Rhythm. 2020, 17, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Boda-Heggemann, J.; Blanck, O.; Mehrhof, F.; Ernst, F.; Buergy, D.; Fleckenstein, J.; Tülümen, E.; Krug, D.; Siebert, F.-A.; Zaman, A.; et al. Interdisciplinary Clinical Target Volume Generation for Cardiac Radioablation: Multicenter Benchmarking for the RAdiosurgery for VENtricular TAchycardia (RAVENTA) Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Gianni, C.; Rivera, D.; Burkhardt, J.D.; Pollard, B.; Gardner, E.; Maguire, P.; Zei, P.C.; Natale, A.; Al-Ahmad, A. Stereotactic arrhythmia radioablation for refractory scar-related ventricular tachycardia. Heart Rhythm. 2020, 17, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Knutson, N.C.; Samson, P.P.; Hugo, G.D.; Goddu, S.M.; Reynoso, F.J.; Kavanaugh, J.A.; Mutic, S.; Moore, K.; Hilliard, J.; Cuculich, P.S.; et al. Radiation therapy workflow and dosimetric analysis from a phase 1/2 trial of noninvasive cardiac radioablation for ventricular tachycardia. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Neuwirth, R.; Cvek, J.; Knybel, L.; Jiravsky, O.; Molenda, L.; Kodaj, M.; Fiala, M.; Peichl, P.; Feltl, D.; Januška, J.; et al. Stereotactic radiosurgery for ablation of ventricular tachycardia. Europace 2019, 21, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Kautzner, J.; Jedlickova, K.; Sramko, M.; Peichl, P.; Cvek, J.; Ing, L.K.; Neuwirth, R.; Jiravsky, O.; Voska, L.; Kucera, T. Radiation-induced changes in ventricular myocardium after stereotactic body radiotherapy for recurrent ventricular tachycardia. JACC Clin. Electrophysiol. 2021, 7, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- van der Ree, M.H.; Blanck, O.; Limpens, J.; Lee, C.H.; Balgobind, B.V.; Dieleman, E.M.; Wilde, A.A.; Zei, P.C.; de Groot, J.R.; Slotman, B.J.; et al. Cardiac radioablation—A systematic review. Heart Rhythm. 2020, 17, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Navara, R.; Yin, T.; Szymanski, J.; Goldsztejn, U.; Kenkel, C.; Lang, A.; Mpoy, C.; Lipovsky, C.E.; Qiao, Y.; et al. Cardiac radiotherapy induces electrical conduction repro-gramming in the absence of transmural fibrosis. Nat. Commun. 2021, 12, 5558. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luo, H.; Mao, T.; Xiang, C.; Hu, H.; Zhao, J.; Wang, X.; Wang, J.; Liu, H.; Yu, L.; et al. Stereotactic arrhythmia radioablation: A novel therapy for cardiac arrhythmia. Heart Rhythm. 2023, 20, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanazzi, I.; Di Monaco, A.; Bonaparte, I.; Valenti, N.; Surgo, A.; Di Guglielmo, F.; Fiorentino, A.; Grimaldi, M. Noninvasive Mapping System for the Stereotactic Radioablation Treatment of Ventricular Tachycardia: A Case Description. J. Cardiovasc. Dev. Dis. 2024, 11, 239. https://doi.org/10.3390/jcdd11080239
Romanazzi I, Di Monaco A, Bonaparte I, Valenti N, Surgo A, Di Guglielmo F, Fiorentino A, Grimaldi M. Noninvasive Mapping System for the Stereotactic Radioablation Treatment of Ventricular Tachycardia: A Case Description. Journal of Cardiovascular Development and Disease. 2024; 11(8):239. https://doi.org/10.3390/jcdd11080239
Chicago/Turabian StyleRomanazzi, Imma, Antonio Di Monaco, Ilaria Bonaparte, Noemi Valenti, Alessia Surgo, Fiorella Di Guglielmo, Alba Fiorentino, and Massimo Grimaldi. 2024. "Noninvasive Mapping System for the Stereotactic Radioablation Treatment of Ventricular Tachycardia: A Case Description" Journal of Cardiovascular Development and Disease 11, no. 8: 239. https://doi.org/10.3390/jcdd11080239





