Cardiovascular Risk Factors Predicting Cardiovascular and Cancer Deaths in a Middle-Aged Population Followed-Up for 61 Years until Extinction
Abstract
:1. Introduction
2. Material and Methods
2.1. Population and Measurements
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veelusamy, R.; Nolan, M.; Mrphy, A.; Thavendiranathan, P.; Marwick, T.H. Screening for coronary artery disease in cancer survivors: JACC CardioOncology state-of-the-art review. Cardio Oncol. 2023, 5, 22–38. [Google Scholar]
- Shi, C.; de Wit, S.; Učambarlić, E.; Markousis-Mavrogenis, G.; Screever, E.M.; Meijers, W.C.; de Boer, R.A.; Aboumsallem, J.P. Multifactorial diseases of the heart, kidneys, lungs, and liver and incident cancer: Epidemiology and shared mechanisms. Cancers 2023, 15, 729. [Google Scholar] [CrossRef]
- Keramida, K.; Yang, E.H.; Deswal, A. Moving theory and reality closer together in cardio-oncology training. Eur. J. Heart Fail. 2024, 26, 772–775. [Google Scholar] [CrossRef]
- Mandala, E.; Lafara, K.; Kokkinovasilis, D.; Kalafatis, I.; Koukoulitsa, V.; Katodritou, E.; Lafaras, C. Applied cardio-oncology in hemato-logical malignancies: A narrative review. Life 2024, 18, 524. [Google Scholar] [CrossRef]
- Shaik, T.; Bhavsar, J.; Garg, S.; Gupta, V.; Kanagala, S.G.; Jain, R. The cardio-oncology continuum: Bridging the gap between cancer and cardiovascular care. Glob. Cardiol. Sci. Pract. 2024, 1, e202409. [Google Scholar] [CrossRef]
- Andres, M.S.; Murphy, T.; Poku, N.; Nazir, M.S.; Ramalingam, S.; Baksi, J.; Jarman, J.W.E.; Khattar, R.; Sharma, R.; Rosen, S.D.; et al. The United Kingdom’s first cardio-oncology service: A decade of growth and evolution. Cardio Oncol. 2024, 6, 310–312. [Google Scholar]
- Caro-Codón, J.; López-Fernández, T.; Álvarez-Ortega, C.; Zamora Auñón, P.; Rodríguez, I.R.; Gómez Prieto, P.; Soto, A.B.; Albendea, M.C.; Albaladejo, A.; Mediavilla, G.; et al. Cardiovascular risk factors during cancer treatment. Prevalence and prognostic relevance: Insights from the CARDIOTOX registry. Eur. J. Prev. Cardiol. 2022, 29, 859–868. [Google Scholar] [CrossRef]
- Wang, X.; Nakano, K.; Shiga, T.; Ohmoto, A.; Oyakawa, T.; Ebihara, A.; Sato, Y.; Fukuda, N.; Nishizawa, M.; Urasaki, T.; et al. Assessment of pazopanib-related heart failure in patients with advanced soft tissue sarcoma—A single institute analysis. Circ. J. 2024, 25, 228–233. [Google Scholar] [CrossRef]
- Shindo, M.; Komiyama, C.; Yamaguchi, T.; Kageyama, K.; Yamamoto, H.; Fujimoto, Y.; Uchida, N.; Kodama, T. Ponatinib-related vasospastic angina. Int. Heart J. 2024, 65, 349–353. [Google Scholar] [CrossRef]
- Tan, S.; Kader, Z.; Day, D.; Chen, D.; Nicholls, S.J.; Ramkumar, S. Cardiotoxicity in oncology guidelines: Discrepancies do matter. Heart Lung Circ. 2024, 33, 553–557. [Google Scholar] [CrossRef]
- Van’t Klooster, C.C.; Ridker, P.M.; Cook, N.R.; Aerts, J.G.; Westerink, J.; Asselbergs, F.W.; van der Graaf, Y.; Visseren, F.L.J.; on behalf of the on behalf of UCC-SMART Study Group. Prediction of lifetime and 10-Year risk of cancer in individual patients with established cardiovascular disease. Cardio Oncol. 2020, 2, 400–410. [Google Scholar] [CrossRef]
- Dzaye, O.; Berning, P.; Dardari, Z.A.; Mortensen, M.B.; Marshall, C.H.; Nasir, K.; Budoff, M.J.; Blumenthal, R.S.; Whelton, S.P.; Blaha, M.J. Coronary artery calcium is associated with in-creased risk for lung and colorectal cancer in men and women: The Multi-Ethnic Study of Atherosclerosis (MESA). Eur. Heart J. Imaging 2022, 23, 708–716. [Google Scholar] [CrossRef]
- Bell, C.F.; Lei, X.; Haas, A.; Baylis, R.A.; Gao, H.; Luo, L.; Giordano, S.H.; Wehner, M.R.; Nead, K.T.; Leeper, N.J. Risk of cancer after diagnosis of cardiovascular disease. Cardio Oncol. 2023, 5, 431–440. [Google Scholar] [CrossRef]
- Finke, D.; Heckmann, M.B.; Wilhelm, S.; Entenmann, L.; Hund, H.; Bougatf, N.; Lehmann, L.H. Coronary artery disease, left ventricular function and cardiac biomarkers determine all-cause mortality in cancer patients: A large monocenter cohort study. Clin. Res. Cardiol. 2023, 112, 203–214. [Google Scholar] [CrossRef]
- Alizadehasl, A.; Alavi, M.S.; Boudagh, S.; Alavi, M.S.; Mohebi, S.; Aliabadi, L.; Akbarian, M.; Ahmadi, P.; Mannarino, M.R.; Sahebkar, A. Lipid-lowering drugs and cancer: An updated perspective. Pharmacol. Rep. 2024, 76, 1–24. [Google Scholar] [CrossRef]
- Romann, S.W.; Finke, D.; Heckmann, M.B.; Hund, H.; Giannitsis, E.; Katus, H.A.; Frey, N.; Lehmann, L.H. Cardiological parameters predict mortality and cardiotoxicity in oncological patients. ESC Heart Fail. 2024, 11, 366–377. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Lei, Y.; Chen, Y.; Cheng, M.; Wei, X.; Liu, J.; Liu, P.; Chen, R.; Yin, X.; et al. Coronary atherosclerotic disease and cancer: Risk factors and interrelation. Front. Cardiovasc. Med. 2022, 9, 821267. [Google Scholar] [CrossRef]
- Youn, J.-C.; Chung, W.-B.; Ezekowitz, J.A.; Hong, J.H.; Nam, H.; Kyoung, D.-S.; Kim, I.-C.; Lyon, A.R.; Kang, S.-M.; Jung, H.O.; et al. Cardiovascular disease burden in adult patients with cancer: An 11-year nationwide population-based cohort study. Int. J. Cardiol. 2020, 317, 167–173. [Google Scholar] [CrossRef]
- Chi, K.; Luo, Z.; Zhao, H.; Li, Y.; Liang, Y.; Xiao, Z.; He, Y.; Zhang, H.; Ma, Z.; Zeng, L.; et al. The impact of tumor characteristics on cardiovascular disease death in breast cancer patients with CT or RT: A population-based study. Front. Cardiovasc. Med. 2023, 10, 1149633. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Qadir, H.; Thavendiranathan, P.; Austin, P.C.; Lee, D.S.; Amir, E.; Fung, K.; Anderson, G.M. Cardiovascular diseases following breast cancer: Towards a case-by-case assessment through a prediction risk score model in 943 patients by Benoite, M. et al. Am. J. Clin. Oncol. 2023, 46, 129. [Google Scholar] [CrossRef] [PubMed]
- de Vries, S.; Haaksma, M.L.; Jóźwiak, K.; Schaapveld, M.; Hodgson, D.C.; Lugtenburg, P.J.; Krol, A.D.; Petersen, E.J.; van Spronsen, D.J.; Ahmed, S.; et al. Development and validation of risk prediction models for coronary heart disease and heart failure after treatment for hodgkin lymphoma. J. Clin. Oncol. 2023, 41, 86–95. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Qin, K.; Li, J.; Li, Y.; Chen, Z.; Xu, J.; Zhu, Y. Increased incidence risks of cardiovascular disease among cancer patients: Evidence from a population-based cohort study in China. Int. J. Cardiol. 2024, 396, 131362. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt, P.; Bruthans, J.; Krajčoviechová, A.; Šulc, P.; Linhart, A.; Filipovský, J.; Mayer, O.J.; Widimský, J.J.; Blaha, M.; Abrahámová, J.; et al. Systematic coonary risk evaluation (SCORE) and 20-year risk of cardiovascular mortality and cancer. Eur. J. Intern. Med. 2020, 79, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Mars, N.; Gen, F.; Koskela, J.T.; Ripatti, P.; Kiiskinen, T.T.J.; Havulinna, A.S.; Lindbohm, J.V.; Ahola-Olli, A.; Kurki, M.; Karjalainen, J.; et al. Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers. Nat. Med. 2020, 26, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Tawfiq, E.; Selak, V.; Elwood, J.M.; Pylypchuk, R.; Tin, S.T.; Harwood, M.; Grey, C.; McKeage, M.; Wells, S. Performance of cardiovascular disease risk prediction equations in more than 14,000 survivors of cancer in New Zealand primary care: A validation study. Lancet 2023, 401, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Mensink, G.B.M.; Hoffmeister, H. The relationship between resting heart rate and all-cause, cardiovascular and cancer mortality. Eur. Heart J. 1997, 18, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Lachman, S.; Peters, R.J.; Lentjes, M.A.; Mulligan, A.A.; Luben, R.N.; Wareham, N.J.; Khaw, K.-T.; Boekholdt, S.M. Ideal cardiovascular health and risk of cardiovascular events in the EPIC-Norfolk prospective population study. Eur. J. Prev. Cardiol. 2016, 23, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Panizza, C.E.; Shvetsov, Y.B.; Harmon, B.E.; Wilkens, L.R.; Le Marchand, L.; Haiman, C.; Reedy, J.; Boushey, C.J. Testing the predictive validity of the healthy eating index-2015 in the multiethnic cohort: Is the score associated with a reduced risk of all-cause and cause-specific mortality? Nutrients 2018, 19, 452. [Google Scholar] [CrossRef]
- Si, S.; Tewara, M.A.; Ji, X.; Wang, Y.; Liu, Y.; Dai, X.; Wang, Z.; Xue, F. Body surface area, height, and body fat percentage as more sensitive risk factors of cancer and cardiovascular disease. Cancer Med. 2020, 9, 4433–4446. [Google Scholar] [CrossRef]
- Lo, K.; Huang, Y.-Q.; Shen, G.; Huang, J.-Y.; Liu, L.; Yu, Y.-L.; Chen, C.-L.; Feng, Y.Q. Effects of waist to height ratio, waist circumference, body mass index on the risk of chronic diseases, all-cause, cardiovascular and cancer mortality. Postgrad. Med. J. 2021, 97, 306–311. [Google Scholar] [CrossRef]
- Bracun, V.; Suthahar, N.; Shi, C.; de Wit, S.; Meijers, W.C.; Klip, I.T.; de Boer, R.A.; Aboumsallem, J.P. Established tumour biomarkers predict cardiovascular events and mortality in the general population. Front. Cardiovasc. Med. 2021, 8, 75885. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.-D.; Agudo, A.; Sánchez, M.-J. Evidence update on the relationship between diet and the most common cancers from the European prospective investigation into cancer and nutrition (EPIC) Study: A systematic review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Huang, N.; Jiang, M.; Holleczek, B.; Schöttker, B.; Huang, T.; Brenner, H. Mortality and morbidity risk prediction for older former smokers based on a score of smoking history: Evidence from UK Biobank and ESTHER cohorts. Age Ageing 2022, 51, afac154. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, L.; Burstein, D.S.; Getz, K.D. Considerations of competing risks analysis in cardio-oncology studies: JACC CardioOncology state-of-the-art review. Cardio Oncol. 2022, 20, 287–301. [Google Scholar]
- Reding, K.W.; Simon, M.S.; Cheng, R.K. Toward a more precise understanding of obesity and cancer and cardiovascular disease risk. Cardio Oncol. 2022, 4, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Demirel, E.; Dilek, O. A new finding for the obesity paradox? Evaluation of the relationship between muscle and adipose tissue in nuclear grade prediction in patients with clear cell renal cell carcinoma. Acta Radiol. 2023, 64, 1659–1667. [Google Scholar] [CrossRef]
- Suthahar, N.; Wang, D.; Aboumsallem, J.P.; Shi, C.; de Wit, S.; Liu, E.E.; Lau, E.S.; Bakker, S.J.; Gansevoort, R.; van der Vegt, B.; et al. Association of initial and longitudinal changes in C-reactive protein with the risk of cardiovascular disease, cancer, and mortality. Mayo Clin. Proc. 2023, 98, 549–558. [Google Scholar] [CrossRef]
- Wilcox, N.S.; Amit, U.; Reibel, J.B.; Berlin, E.; Howell, K.; Ky, B. Cardiovascular disease and cancer: Shared risk factors and mechanisms. Nat. Rev. Cardiol. 2024, 1–15. [Google Scholar] [CrossRef]
- Hao, X.; Li, D. The healthy eating index-2015 and all-cause/cause-specific mortality: A systematic review and dose–response meta-analysis. Adv. Nutr. Int. Rev. J. 2024, 15, 100166. [Google Scholar] [CrossRef]
- Makram, O.M.; Kunhiraman, H.H.; Harris, R.A.; Hedrick, C.C.; Nasir, K.; Weintraub, N.L.; Wang, X.; Guha, A. Examining the interplay between car-diovascular disease and cancer incidence: Data from NHANES III and continuous. Am. Heart J. Plus 2024, 40, 100380. [Google Scholar]
- Whelton, S.P.; Marshall, C.H.; Cainzos-Achirica, M.; Dzaye, O.; Blumenthal, R.S.; Nasir, K.; McClelland, R.L.; Blaha, M.J. Pooled cohort equations and the competing risk of cardiovascular disease versus cancer: Multi-Ethnic study of atherosclerosis. Am. J. Prev. Cardiol. 2021, 7, 100212. [Google Scholar] [CrossRef]
- Polter, E.J.; Blaes, A.; Wolfson, J.; Lutsey, P.L.; Florido, R.; Joshu, C.E.; Guha, A.; Platz, E.A.; Prizment, A. Performance of the pooled cohort equations in cancer survivors: The Atherosclerosis Risk in Communities study. J. Cancer Surviv. 2024, 18, 124–134. [Google Scholar] [CrossRef]
- Menotti, A.; Puddu, P.E. How the Seven Countries Study contributed to the launch and development of cardiovascular epide-miology in Italy. A historical perspective. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P.E.; Maiani, G.; Catasta, G. Age at death as a useful indicator of healthy aging at population level: A 50-year follow-up of the Italian rural areas of the seven countries study. Aging Clin. Exp. Res. 2018, 30, 901–911. [Google Scholar] [CrossRef]
- Menotti, A.; Puddu, P.E.; Lanti, M.; Maiani, G.; Catasta, G.; Alberti Fidanza, A. Lifestyle habits and mortality from all and specific causes of death: 40-year follow-up in the Italian Rural Areas of the Seven Countries Study. J. Nutr. Health Aging 2014, 18, 314–321. [Google Scholar] [CrossRef]
- Menotti, A.; Puddu, V. Ten-year mortality from coronary heart disease among 172,000 men classified by occupational physical activity. Scand. J. Work Environ. Health 1979, 5, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P.E. Comparison of four dietary scores as determinants of coronary heart disease mortality. Sci. Rep. 2018, 8, 15001. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P.E. Dietary fatty acids predicting long term cardiovascular mortality in a cohort of middle-aged men fol-lowed-up until extinction. Hearts 2024, 5, 196–210. [Google Scholar] [CrossRef]
- Rose, G.; Blackburn, H. Cardiovascular Survey Methods; World Health Organization: Geneva, Switzerland, 1968; pp. 1–188. [Google Scholar]
- Heymsfield, S.B.; McManus, C.; Smith, J.; Stevens, V.; Nixon, D.W. Anthropometric measurement of muscle mass: Revised equations for calculating bone-free arm muscle area. Am. J. Clin. Nutr. 1982, 36, 680–690. [Google Scholar] [CrossRef]
- Anderson, J.T.; Keys, A. Cholesterol in serum and lipoprotein fractions; its measurement and stability. Clin. Chem. 1956, 2, 145–159. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases, 8th ed.; Revision: Geneva, Switzerland, 1965; pp. 1–671. [Google Scholar]
- Puddu, P.E.; Menotti, A. Heart diseases of uncertain etiology: A new definition of heart failure for epidemiological studies. J. Cardiovasc. Dev. Dis. 2023, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Puddu, P.E.; Piras, P.; Menotti, A. Mortality time-trends of different cardiovascular diseases in a practically extinct cohort of Italian middle-aged men followed-up for 61 Years: A possible etiological explanation? J. Cardiovasc. Dev. Dis. 2024, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Conti, S.; Giampaoli, S.; Mariotti, S.; Signoretti, P. Coronary risk factors predicting coronary and other causes of death in fifteen years. Acta Cardiol. 1980, 35, 107–120. [Google Scholar] [PubMed]
- Puddu, P.E.; Piras, P.; Kafatos, A.; Adachi, H.; Tolonen, H.; Menotti, A. Competing risks of coronary heart disease mortality versus other causes of death in 10 cohorts of middle-aged men of the Seven Countries Study followed for 60 years to extinction. J. Cardiovasc. Dev. Dis. 2023, 10, 482. [Google Scholar] [CrossRef]
- Puddu, P.E.; Piras, P.; Menotti, A. Lifetime competing risks between coronary heart disease mortality and other causes of death during 50 years of follow-up. Int. J. Cardiol. 2017, 228, 359–363. [Google Scholar] [CrossRef]
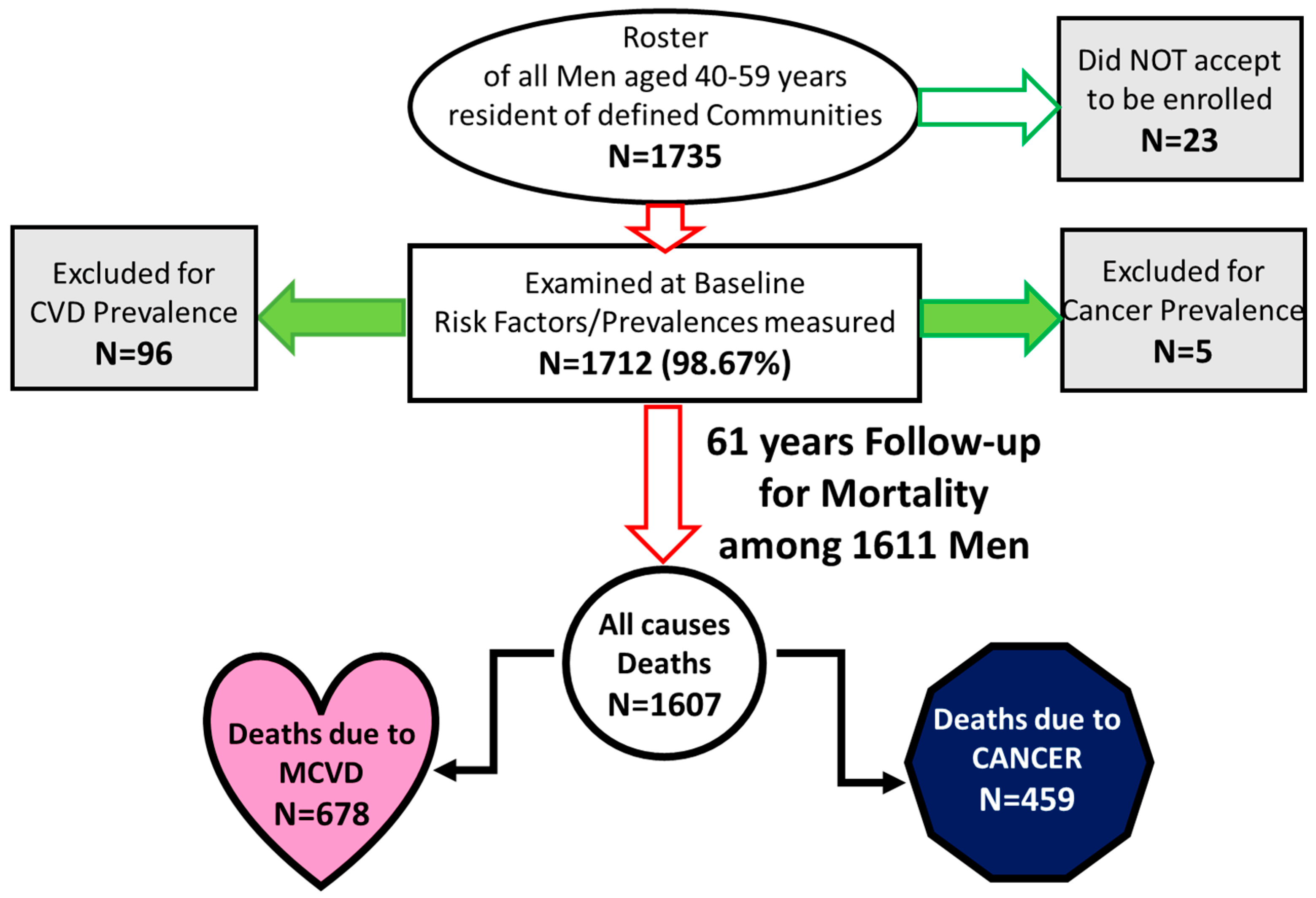
| Risk Factor | Definition or Details | Unit of Measurement | Mean and (SD) or Proportion (%) and (SE) | Bibliographic Reference | Notes |
|---|---|---|---|---|---|
| Age | Approximated to the nearest birthday | Years | 49.1 (5.1) | [44] | |
| Father history | Father dead <65 years from non-infectious nor violent causes | 0 = no 1 = yes | 21.1 (0.99)% | [44] | From questionnaire |
| Mother history | Mother dead <65 years from non-infectious nor violent causes | 0 = no 1 = yes | 20.6 (0.98)% | [44] | From questionnaire |
| Family history of heart attack | History of myocardial infarction, or equivalent term in 1st degree siblings | 0 = no 1 = yes | 37.9 (1.17)% | [44] | From questionnaire |
| Marital status | Presently married (first marriage) | 0 = no 1 = yes | 90.5 (0.71)% | [44] | From questionnaire |
| High socio-economic status HSES | Professional, business, public administrators, foreman, and high-rank clerks | 0 = no 1 = yes | 11.0 (0.76)% | [44] | |
| Sedentary physical activity | Job-related derived from questions matched with reported occupation | 0 = no 1 = yes | 9.7 (0.7)% | [45,46] | Dummy reference for physical activity |
| Moderate physical activity | Job-related derived from questions matched with reported occupation | 0 = no 1 = yes | 22.1 (1.0)% | [45,46] | Classes of physical activity validated by ergonometric procedure and energy intake |
| Vigorous physical activity | Job-related derived from questions matched with reported occupation | 0 = no 1 = yes | 68.2 (1.1)% | [45,46] | |
| Cigarette smoking | Smokers | 0 = no 1 = yes | 61.1 (1.2)% | [45] | Dummy reference for smoking habits |
| Cigarette smoking | Ex-smokers | 0 = no 1 = yes | 13.6 (0.8)% | [45] | |
| Cigarette smoking | Never smokers | 0 = no 1 = yes | 25.4 (1.1)% | [45] | |
| Non-healthy diet | Dietary history | 0 = no 1 = yes | 33.4 (1.1)% | [47,48] | Dummy reference for dietary habits |
| Intermediate Diet | Dietary history | 0 = no 1 = yes | 33.3 (1.1)% | [47,48] | Classes of diet derived from factor score of principal component analysis on 18 food groups |
| Healthy diet | Dietary history | 0 = no 1 = yes | 33.4 (1.1)% | [47,48] | |
| Body mass index | Weight/height squared | kg/m2 | 25.2 (3.7) | [49] | |
| Trunk/height ratio | (sitting height/height) × 100 | Ratio | 53.3 (1.5) | [49] | |
| Shoulder/pelvis shape (ratio) | Biacromial diameter/bicristal diameter | Ratio | 1.36 (0.1) | [49] | |
| Laterality/ linearity index | (Sum of 2 diameters/height) × 100 | 40.9 (1.8) | [49] | ||
| Subscapular skinfold | Harpenden caliper. Below tip of right scapula | mm | 11.8 (5.8) | [49] | |
| Midarm circumference | Right arm. Mathematically cleaned from skin and subcutaneous tissue using the value of tricipital skinfold thickness | mm | 268.6 (23.6) | [49,50] | |
| Systolic blood pressure | Supine Average of 2 measurements | mmHg | 143.6 (21.0) | [49] | |
| Heart rate | From ECG, average rate in lead I and V6 | beats/minute | 71.3 (12.9) | ||
| Vital capacity | Best of 2 tests Adjusted (divided) for height2 | L/m2 | 1.65 (0.24) | [49] | |
| Forced expiratory volume | Best of 2 tests Adjusted (divided) for height2 | L/m2 | 1.08 (0.24) | [49] | |
| Serum cholesterol | Method of Abel–Kendall modified by Anderson and Keys. Casual blood sample | mg/dL | 201.6 (40.8) | [51] | |
| Urine protein | Spot urines. Semiquantitative method by stix Definite present | 0 = absent 1 = present | 7.8 (0.6)% | ||
| Diabetes | Clinical diagnosis plus spot urine glucose | 0 = no 1 = yes | 4.7 (0.5)% | ||
| Baldness | Partial evident or total | 0 = no 1 = yes | 29.0 (1.1)% | ||
| Corneal arcus | Clinical judgment | 0 = no 1 = yes | 13.9 (0.8)% | ||
| Xanthelasma | Clinical judgment | 0 = no 1 = yes | 1.5 (0.3)% |
| Cancer Groups | n Cases | Proportion % Over All | Notes | n Cases after Exclusion of Prevalence |
|---|---|---|---|---|
| Stomach | 81 | 17.5 | 81 | |
| Lung | 78 | 16.8 | 77 | |
| Colon, rectum | 57 | 12.3 | Arbitrarily combined | 56 |
| Prostate | 50 | 10.8 | 49 | |
| Bladder | 26 | 5.6 | 26 | |
| Unidentified | 20 | 4.3 | 19 | |
| Pancreas | 15 | 3.2 | 15 | |
| Larynx | 14 | 3.0 | 14 | |
| Brain | 11 | 2.4 | 11 | |
| Liver | 11 | 2.4 | 11 | |
| Others | 100 | 21.6 | Covering 31 other locations | 100 |
| Total cancer deaths | 463 | 100.0 | 459 | |
| Cardiovascular disease groups | n cases | Proportion % over all | ||
| CHD | 281 | 38.7 | 270 | |
| HDUE | 216 | 29.7 | 206 | |
| STROKE | 230 | 31.6 | 226 | |
| Total MCVD deaths | 727 | 100.0 | 678 |
| Risk Factor | Coefficient | p Value | Delta | HR | 95% CLs |
|---|---|---|---|---|---|
| Age | 0.0789 | <0.0001 | 5 | 1.48 | 1.33 1.65 |
| High socio-economic status | −0.2167 | 0.2404 | 1 | 0.81 | 0.56 1.16 |
| Father early death | 0.1298 | 0.2600 | 1 | 1.14 | 0.91 1.43 |
| Mother early death | 0.2595 | 0.0239 | 1 | 1.30 | 1.03 1.62 |
| Familiarity heart attack | −0.0056 | 0.9547 | 1 | 0.99 | 0.82 1.21 |
| Marriage | 0.3029 | 0.1079 | 1 | 1.35 | 0.94 1.96 |
| Sedentary physical activity | Reference | ---- | ---- | ---- | ---- |
| Moderate physical activity | −0.1160 | 0.5559 | 1 | 0.89 | 0.61 1.31 |
| Vigorous physical activity | −0.0347 | 0.8534 | 1 | 0.97 | 0.67 1.40 |
| Unhealthy diet | Reference | ---- | ---- | ---- | ---- |
| Intermediate diet | −0.2027 | 0.0937 | 1 | 0.82 | 0.64 1.03 |
| Healthy diet | −0.3790 | 0.0084 | 1 | 0.68 | 0.52 0.91 |
| Never smoker | Reference | ---- | ---- | ---- | ---- |
| Ex-smoker | −0.1307 | 0.4652 | 1 | 0.88 | 0.62 1.25 |
| Smoker | 0.3651 | 0.0017 | 1 | 1.44 | 0.15 1.81 |
| Body mass index | 0.0276 | 0.3246 | 3.5 | 1.10 | 0.91 1.33 |
| Trunk/height ratio | 0.0025 | 0.9409 | 1.5 | 1.00 | 0.91 1.11 |
| Shoulder pelvis shape | 0.9589 | 0.1413 | 0.1 | 1.10 | 0.97 0.15 |
| Laterality/linearity index | −0.0053 | 0.8575 | 1.08 | 0.99 | 0.89 1.10 |
| Subscapular skinfold | −0.0222 | 0.1235 | 6 | 0.88 | 0.74 1.04 |
| Arm circumference | −0.0070 | 0.0150 | 25 | 0.84 | 0.73 0.97 |
| Systolic blood pressure | 0.0010 | 0.7333 | 20 | 1.02 | 0.91 1.14 |
| Heart rate | 0.0069 | 0.1055 | 13 | 1.09 | 0.98 1.22 |
| Vital capacity | 0.3685 | 0.1673 | 0.25 | 1.10 | 0.96 1.25 |
| Forced expiratory volume | −0.3327 | 0.1766 | 0.25 | 0.92 | 0.82 1.04 |
| Serum cholesterol | 0.0019 | 0.1133 | 40 | 1.08 | 0.98 1.19 |
| Urine protein | −0.2678 | 0.2189 | 1 | 0.77 | 0.50 1.17 |
| Baldness | −0.0608 | 0.5724 | 1 | 0.94 | 0.76 1.16 |
| Corneal arcus | 0.3707 | 0.0060 | 1 | 1.45 | 1.11 1.89 |
| Xanthelasma | 1.0177 | 0.0012 | 1 | 2.77 | 1.49 5.12 |
| Diabetes | 0.4358 | 0.0448 | 1 | 1.55 | 1.01 2.37 |
| Risk Factor | Coefficient | p Value | Delta | HR | 95% CLs |
|---|---|---|---|---|---|
| Age | 0.0573 | <0.0001 | 5 | 1.33 | 1.16 1.53 |
| High socio-economic status | −0.2655 | 0.2050 | 1 | 0.77 | 0.51 1.16 |
| Father early death | −0.1229 | 0.4316 | 1 | 0.88 | 0.65 1.20 |
| Mother early death | 0.1745 | 0.2440 | 1 | 1.19 | 0.89 1.60 |
| Familiarity heart attack | 0.1503 | 0.2319 | 1 | 1.16 | 0.91 1.49 |
| Marriage | −0.2924 | 0.1340 | 1 | 0.75 | 0.51 1.09 |
| Sedentary physical activity | Reference | ---- | ---- | ---- | ---- |
| Moderate physical activity | −0.4563 | 0.0275 | 1 | 0.63 | 0.42 0.95 |
| Vigorous physical activity | −0.6661 | 0.0009 | 1 | 0.51 | 0.35 0.76 |
| Unhealthy diet | Reference | ---- | ---- | ---- | ---- |
| Intermediate diet | −0.3743 | 0.0162 | 1 | 0.69 | 0.51 0.93 |
| Healthy diet | −0.4787 | 0.0099 | 1 | 0.62 | 0.43 0.89 |
| Never smoker | Reference | ---- | ---- | ---- | ---- |
| Ex-smoker | 0.1093 | 0.5968 | 1 | 1.12 | 0.74 1.67 |
| Smoker | 0.2870 | 0.0514 | 1 | 1.33 | 1.00 1.78 |
| Body mass index | 0.0039 | 0.9102 | 3.5 | 1.01 | 0.80 1.28 |
| Trunk/height ratio | 0.0014 | 0.9737 | 1.5 | 1.00 | 0.88 1.14 |
| Shoulder pelvis shape | −0.2723 | 0.7438 | 0.1 | 0.97 | 0.83 1.15 |
| Laterality/linearity index | 0.0377 | 0.3394 | 1.8 | 1.07 | 0.93 1.23 |
| Subscapular skinfold | −0.0151 | 0.3714 | 6 | 0.91 | 0.75 1.11 |
| Arm circumference | −0.0034 | 0.3493 | 25 | 0.92 | 0.77 1.10 |
| Systolic blood pressure | 0.0137 | <0.0001 | 20 | 1.32 | 1.16 1.49 |
| Heart rate | −0.0038 | 0.4714 | 13 | 0.95 | 0.83 1.09 |
| Vital capacity | −0.5223 | 0.1277 | 0.25 | 0.88 | 0.74 1.04 |
| Forced expiratory volume | −0.4094 | 0.2069 | 0.25 | 0.90 | 0.77 1.04 |
| Serum cholesterol | 0.0062 | <0.0001 | 40 | 1.28 | 1.14 1.45 |
| Urine protein | 0.0749 | 0.7487 | 1 | 1.08 | 0.68 1.70 |
| Baldness | 0.1835 | 0.1607 | 1 | 1.20 | 0.93 1.55 |
| Corneal arcus | 0.0606 | 0.7464 | 1 | 1.06 | 0.74 1.53 |
| Xanthelasma | 0.6478 | 0.1630 | 1 | 1.91 | 0.77 4.75 |
| Diabetes | 0.1282 | 0.6628 | 1 | 1.14 | 0.64 2.02 |
| Risk Factor | Coefficient | p Value | Delta | HR | 95% CLs |
|---|---|---|---|---|---|
| Age | 0.1674 | <0.0001 | 5 | 2.31 | 1.94 2.74 |
| High socio-economic status | −0.0849 | 0.7504 | 1 | 0.92 | 0.54 1.55 |
| Father early death | 0.2831 | 0.0895 | 1 | 1.33 | 0.96 1.84 |
| Mother early death | 0.2658 | 0.1179 | 1 | 1.30 | 0.93 1.82 |
| Familiarity heart attack | 0.0839 | 0.5636 | 1 | 1.09 | 0.82 1.45 |
| Marriage | 0.0386 | 0.8803 | 1 | 1.04 | 0.63 1.72 |
| Sedentary physical activity | Reference | ---- | ---- | ---- | ---- |
| Moderate physical activity | −0.2207 | 0.4299 | 1 | 0.80 | 0.46 1.39 |
| Vigorous physical activity | −0.3741 | 0.1596 | 1 | 0.69 | 0.41 1.16 |
| Unhealthy diet | Reference | ---- | ---- | ---- | ---- |
| Intermediate diet | 0.1184 | 0.5443 | 1 | 0.89 | 0.61 1.30 |
| Healthy diet | 0.0309 | 0.8857 | 1 | 1.03 | 0.68 1.57 |
| Never smoker | Reference | ---- | ---- | ---- | ---- |
| Ex-smoker | 0.3568 | 0.1205 | 1 | 1.43 | 0.91 2.24 |
| Smoker | 0.5073 | 0.0034 | 1 | 1.66 | 1.18 2.33 |
| Body mass index | −0.0247 | 0.5656 | 3.5 | 0.92 | 0.68 1.23 |
| Trunk/height ratio | 0.0866 | 0.0894 | 1.5 | 1.14 | 0.98 1.32 |
| Shoulder pelvis shape | 0.2434 | 0.8025 | 0.1 | 1.02 | 0.85 1.24 |
| Laterality/linearity index | 0.0624 | 0.1565 | 1.8 | 1.12 | 0.96 1.31 |
| Subscapular skinfold | −0.0096 | 0.6421 | 6 | 0.94 | 0.74 1.20 |
| Arm circumference | −0.0055 | 0.2255 | 25 | 0.87 | 0.70 1.09 |
| Systolic blood pressure | 0.0145 | 0.0003 | 20 | 1.34 | 1.14 1.56 |
| Heart rate | −0.0150 | 0.0257 | 13 | 0.82 | 0.39 0.98 |
| Vital capacity | −0.0992 | 0.8060 | 0.25 | 0.98 | 0.80 1.19 |
| Forced expiratory volume | −0.4286 | 0.2439 | 0.25 | 0.90 | 0.75 1.08 |
| Serum cholesterol | 0.0014 | 0.4680 | 40 | 1.06 | 0.91 1.22 |
| Urine protein | 0.5421 | 0.0311 | 1 | 1.72 | 1.05 2.81 |
| Baldness | 0.1374 | 0.3721 | 1 | 1.15 | 0.85 1.55 |
| Corneal arcus | 0.2371 | 0.2479 | 1 | 1.32 | 0.85 1.89 |
| Xanthelasma | 0.6092 | 0.4005 | 1 | 1.81 | 0.44 7.61 |
| Diabetes | 0.3401 | 0.3362 | 1 | 1.41 | 0.70 2.81 |
| Risk Factor | Coefficient | p Value | Delta | HR | 95% CLs |
|---|---|---|---|---|---|
| Age | 0.1110 | <0.0001 | 5 | 1.74 | 1.49 2.04 |
| High socio-economic status | 0.1765 | 0.4284 | 1 | 1.19 | 0.77 1.85 |
| Father early death | 0.1379 | 0.3992 | 1 | 1.15 | 0.83 1.58 |
| Mother early death | 0.0904 | 0.5895 | 1 | 1.09 | 0.79 1.52 |
| Familiarity heart attack | 0.1033 | 0.4546 | 1 | 1.11 | 0.85 1.45 |
| Marriage | −0.1881 | 0.4075 | 1 | 0.83 | 0.53 1.29 |
| Sedentary physical activity | Reference | ---- | ---- | ---- | ---- |
| Moderate physical activity | −0.1058 | 0.6736 | 1 | 0.90 | 0.55 1.47 |
| Vigorous physical activity | −0.3466 | 0.1698 | 1 | 0.71 | 0.43 1.16 |
| Unhealthy diet | Reference | ---- | ---- | ---- | ---- |
| Intermediate diet | 0.0210 | 0.9074 | 1 | 1.02 | 0.72 1.46 |
| Healthy diet | −0.0344 | 0.8686 | 1 | 0.97 | 0.64 1.45 |
| Never smoker | Reference | ---- | ---- | ---- | ---- |
| Ex-smoker | 0.5446 | 0.0099 | 1 | 1.72 | 1.14 2.61 |
| Smoker | 0.3625 | 0.0317 | 1 | 1.44 | 1.03 2.00 |
| Body mass index | −0.0484 | 0.2267 | 3.5 | 0.84 | 0.64 1.11 |
| Trunk/height ratio | 0.0711 | 0.1455 | 1.5 | 1.11 | 0.96 1.28 |
| Shoulder pelvis shape | 0.1870 | 0.8453 | 0.1 | 1.02 | 0.84 1.23 |
| Laterality/linearity index | 0.1157 | 0.0074 | 1.8 | 1.23 | 1.06 1.43 |
| Subscapular skinfold | −0.0121 | 0.5247 | 6 | 0.93 | 0.74 1.16 |
| Arm circumference | −0.0007 | 0.8709 | 25 | 0.98 | 0.80 1.21 |
| Systolic blood pressure | 0.0126 | 0.0007 | 20 | 1.29 | 1.11 1.49 |
| Heart rate | 0.0025 | 0.6717 | 13 | 1.03 | 0.89 1.20 |
| Vital capacity | −0.0136 | 0.0106 | 0.25 | 0.78 | 0.64 0.94 |
| Forced expiratory volume | 0.3464 | 0.3557 | 0.25 | 1.09 | 0.91 1.31 |
| Serum cholesterol | 0.0034 | 0.0506 | 40 | 1.15 | 1.00 1.31 |
| Urine protein | 0.2200 | 0.3901 | 1 | 1.25 | 0.75 2.06 |
| Baldness | 0.1434 | 0.3235 | 1 | 1.15 | 0.87 1.53 |
| Corneal arcus | 0.2574 | 0.1882 | 1 | 1.29 | 0.88 1.90 |
| Xanthelasma | 0.4620 | 0.4380 | 1 | 1.59 | 0.49 5.10 |
| Diabetes | 0.4972 | 0.0966 | 1 | 1.64 | 0.91 2.96 |
| Risk Factor | Coefficient | p Value | Delta | HR | 95% CLs |
|---|---|---|---|---|---|
| Age | 0.1047 | <0.0001 | 5 | 1.69 | 1.55 1.84 |
| High socio-economic status | −0.0632 | 0.6315 | 1 | 0.94 | 0.73 1.22 |
| Father early death | 0.0760 | 0.4145 | 1 | 1.08 | 0.90 1.30 |
| Mother early death | 0.1745 | 0.0608 | 1 | 1.19 | 0.99 1.43 |
| Familiarity heart attack | 0.1134 | 0.1470 | 1 | 1.12 | 0.96 1.31 |
| Marriage | −0.1685 | 0.1877 | 1 | 0.84 | 0.66 1.09 |
| Sedentary physical activity | reference | ---- | ---- | ---- | ---- |
| Moderate physical activity | −0.2919 | 0.0342 | 1 | 0.75 | 0.57 0.98 |
| Vigorous physical activity | −0.5052 | 0.0002 | 1 | 0.60 | 0.46 0.78 |
| Unhealthy diet | Reference | ---- | ---- | ---- | ---- |
| Intermediate diet | −0.1945 | 0.0529 | 1 | 0.82 | 0.68 1.00 |
| Healthy diet | −0.2000 | 0.0829 | 1 | 0.82 | 0.65 1.03 |
| Never smoker | Reference | ---- | ---- | ---- | ---- |
| Ex-smoker | 0.3261 | 0.0083 | 1 | 1.39 | 1.09 1.77 |
| Smoker | 0.3758 | 0.0001 | 1 | 1.46 | 1.21 1.75 |
| Body mass index | −0.0205 | 0.3562 | 3.5 | 0.93 | 0.80 1.08 |
| Trunk/height ratio | 0.0466 | 0.0897 | 1.5 | 1.07 | 0.99 1.16 |
| Shoulder pelvis shape | 0.0604 | 0.9089 | 0.1 | 1.01 | 0.91 1.12 |
| Laterality linearity index | 0.0726 | 0.0028 | 1.8 | 1.14 | 1.05 1.24 |
| Subscapular skinfold | −0.0135 | 0.2109 | 6 | 0.92 | 0.81 1.05 |
| Arm circumference | −0.0031 | 0.1833 | 25 | 0.93 | 0.82 1.04 |
| Systolic blood pressure | 0.0137 | <0.0001 | 20 | 1.32 | 1.21 1.43 |
| Heart rate | −0.0048 | 0.1523 | 13 | 0.94 | 0.86 1.02 |
| Vital capacity | −0.5499 | 0.0117 | 0.25 | 0.87 | 0.78 0.97 |
| Forced expiratory volume | −0.1667 | 0.3856 | 0.25 | 0.96 | 0.87 1.06 |
| Serum cholesterol | 0.0040 | <0.0001 | 40 | 1.17 | 1.09 1.27 |
| Urine protein | 0.2571 | 0.0716 | 1 | 1.29 | 0.98 1.71 |
| Baldness | 0.1551 | 0.0587 | 1 | 1.17 | 0.99 1.37 |
| Corneal arcus | 0.1876 | 0.0963 | 1 | 1.21 | 0.97 1.50 |
| Xanthelasma | 0.5740 | 0.0784 | 1 | 1.78 | 0.94 3.36 |
| Diabetes | 0.3028 | 0.0926 | 1 | 1.35 | 0.95 1.93 |
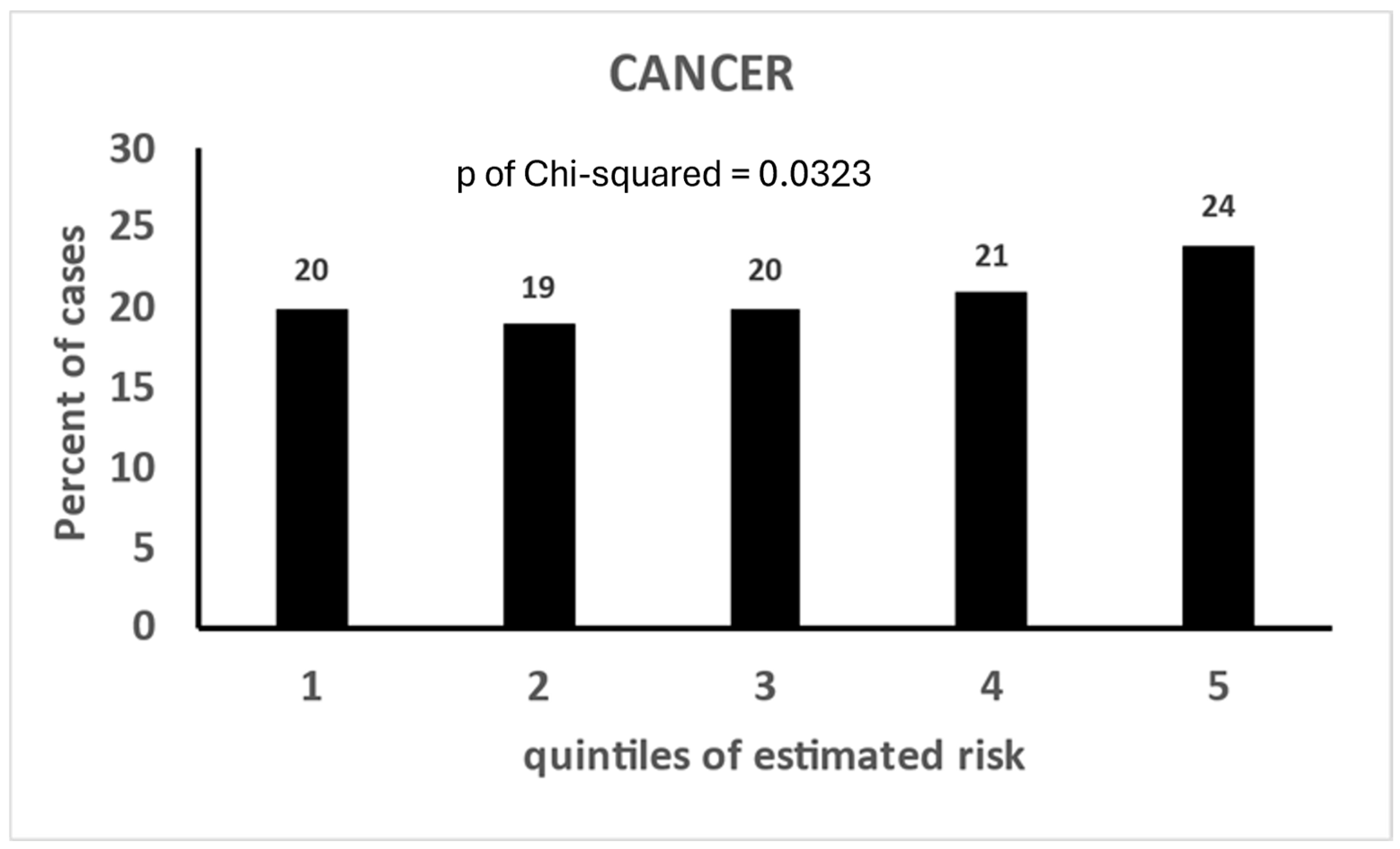
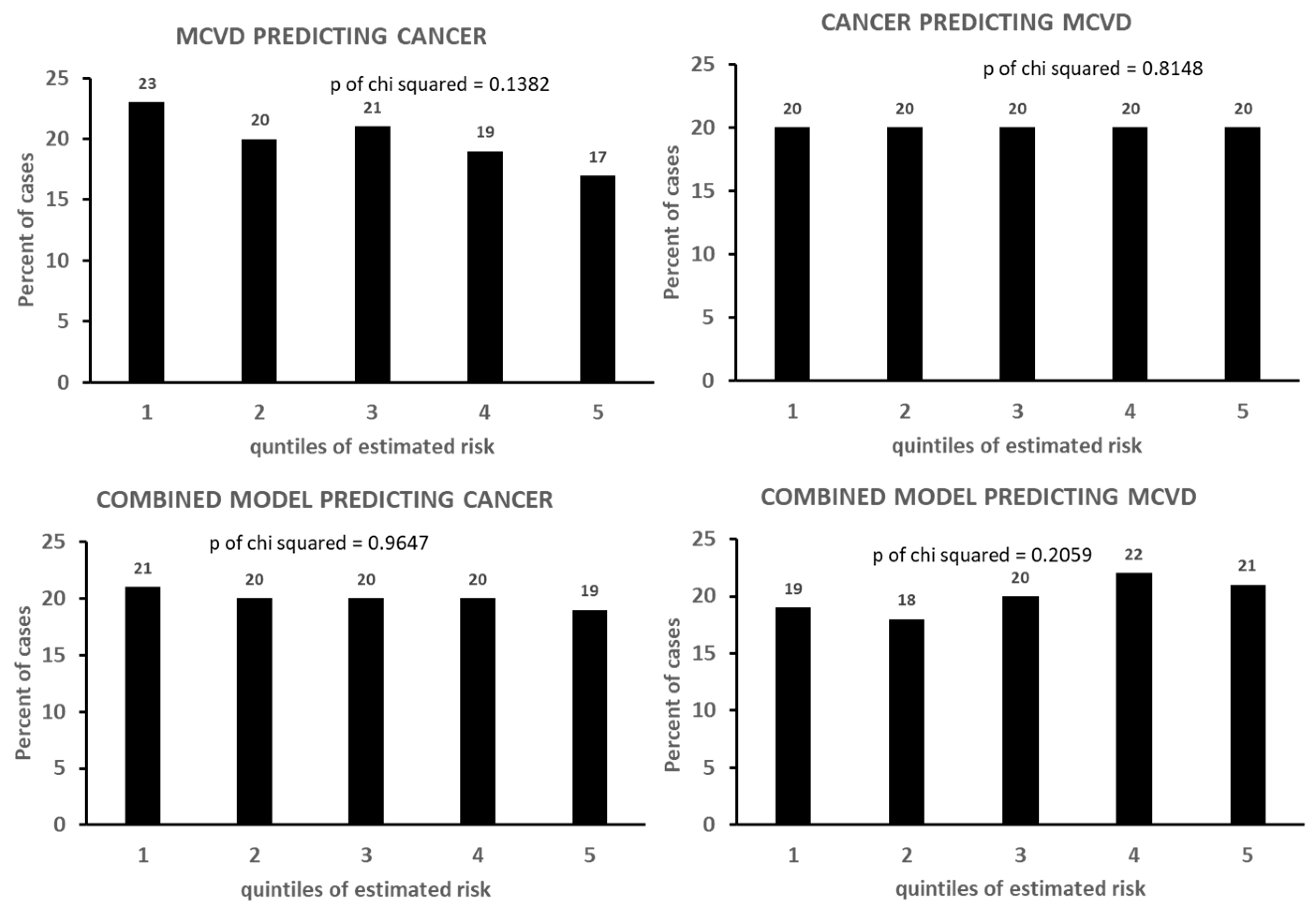
| End-Points of Models | Quintiles of Estimated Risk | p of Chi-Squared | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| CHD | 15 | 16 | 20 | 23 | 26 | 0.0099 |
| HDUE | 19 | 18 | 19 | 21 | 23 | 0.7370 |
| STROKE | 17 | 14 | 20 | 25 | 24 | 0.0428 |
| MCVD | 18 | 18 | 20 | 22 | 22 | 0.0407 |
| Risk Factor | Direct Model | Inverse Model | ||
|---|---|---|---|---|
| Coefficient | p Value | Coefficient | p Value | |
| Age | 0.1026 | <0.0001 | 0.0804 | <0.0001 |
| High socio-economic status | −0.2382 | 0.0550 | −0.3672 | 0.0440 |
| Father early death | 0.0442 | 0.6400 | 0.0562 | 0.6400 |
| Mother early death | 0.1190 | 0.1900 | 0.1664 | 0.1600 |
| Familiarity heart attack | 0.0776 | 0.3100 | −0.0732 | 0.4700 |
| Marriage | −0.1304 | 0.3400 | 0.3288 | 0.0700 |
| Sedentary physical activity | Reference | ---- | Reference | ---- |
| Moderate physical activity | −0.1297 | 0.3800 | 0.0729 | 0.7100 |
| Vigorous physical activity | −0.3949 | 0.0044 | −0.2067 | 0.2700 |
| Unhealthy diet | Reference | ---- | Reference | ---- |
| Intermediate diet | −0.1506 | 0.1200 | −0.1925 | 0.1200 |
| Healthy diet | −0.1898 | 0.0990 | −0.3592 | 0.0170 |
| Never smoker | Reference | ---- | Reference | ---- |
| Ex-smoker | 0.4620 | 0.0001 | −0.0072 | 0.9700 |
| Smoker | 0.3930 | <0.0001 | 0.4074 | <0.0001 |
| Body mass index | −0.0126 | 0.5900 | 0.0419 | 0.1700 |
| Trunk/height ratio | 0.0319 | 0.2300 | −0.0156 | 0.6600 |
| Shoulder pelvis shape | 0.2554 | 0.5900 | 1.0524 | 0.1300 |
| Laterality linearity index | 0.0665 | 0.0052 | −0.0328 | 0.3100 |
| Subscapular skinfold | −0.0221 | 0.0410 | −0.0221 | 0.1500 |
| Arm circumference | −0.0041 | 0.0820 | −0.0083 | 0.0055 |
| Systolic blood pressure | 0.0146 | <0.0001 | 0.0010 | 0.7500 |
| Heart rate | 0.0055 | 0.1200 | 0.0167 | 0.0001 |
| Vital capacity | −0.7609 | 0.0008 | 0.2246 | 0.4000 |
| Forced expiratory volume | −0.0914 | 0.6500 | −0.3103 | 0.2000 |
| Serum cholesterol | 0.0037 | 0.0001 | 0.0020 | 0.1200 |
| Urine protein | 0.2002 | 0.1800 | −0.2769 | 0.2200 |
| Baldness | 0.1379 | 0.0860 | −0.1041 | 0.3600 |
| Corneal arcus | 0.0398 | 0.7400 | 0.2548 | 0.0680 |
| Xanthelasma | 0.7281 | 0.0200 | 1.1972 | 0.0001 |
| Diabetes | 0.1883 | 0.4100 | 0.3409 | 0.0770 |
| Risk Factor | Delta | Direct Model | Inverse Model | ||
|---|---|---|---|---|---|
| HR | 95% CLs | HR | 95% CLs | ||
| Age | 1 | 1.67 | 1.52 1.83 | 1.49 | 1.33 1.68 |
| High socio-economic status | 1 | 0.76 | 0.58 1.01 | 0.69 | 0.48 0.99 |
| Father early death | 1 | 1.05 | 0.87 1.26 | 1.06 | 0.84 1.33 |
| Mother early death | 1 | 1.13 | 0.94 1.34 | 1.18 | 0.94 1.49 |
| Familiarity heart attack | 1 | 1.08 | 0.93 1.26 | 0.93 | 0.76 1.13 |
| Marriage | 1 | 0.88 | 0.67 1.15 | 1.39 | 0.97 1.98 |
| Sedentary physical activity | Reference | ---- | ---- | ---- | ---- |
| Moderate physical activity | 1 | 0.88 | 0.66 1.17 | 1.08 | 0.74 1.57 |
| Vigorous physical activity | 1 | 0.67 | 0.51 0.88 | 1.23 | 0.85 1.78 |
| Unhealthy diet | Reference | ---- | ---- | ---- | ---- |
| Intermediate diet | 1 | 0.86 | 0.71 1.04 | 0.82 | 0.65 1.05 |
| Healthy diet | 1 | 0.83 | 0.66 1.04 | 0.70 | 0.52 0.94 |
| Never smoker | Reference | ---- | ---- | ---- | ---- |
| Ex-smoker | 1 | 1.59 | 1.26 2.00 | 0.99 | 0.70 1.41 |
| Smoker | 1 | 1.48 | 1.24 1.77 | 1.50 | 1.19 1.89 |
| Body mass index | 3.5 | 0.96 | 0.81 1.12 | 1.16 | 0.94 1.43 |
| Trunk/height ratio | 1.5 | 1.05 | 0.97 1.14 | 0.98 | 0.88 1.08 |
| Shoulder pelvis shape | 0.1 | 1.03 | 0.94 1.12 | 1.11 | 0.97 1.27 |
| Laterality linearity index | 1.8 | 1.13 | 1.04 1.23 | 0.94 | 0.84 1.06 |
| Subscapular skinfold | 6 | 0.88 | 0.77 0.99 | 0.88 | 0.73 1.05 |
| Arm circumference | 25 | 0.90 | 0.80 1.01 | 0.81 | 0.70 0.94 |
| Systolic blood pressure | 20 | 1.34 | 1.22 1.47 | 1.02 | 0.91 1.15 |
| Heart rate | 13 | 1.07 | 0.98 1.18 | 1.24 | 1.11 1.39 |
| Vital capacity | 0.25 | 0.83 | 0.74 0.92 | 1.06 | 0.93 1.20 |
| Forced expiratory volume | 0.25 | 0.98 | 0.88 1.08 | 0.93 | 0.82 1.04 |
| Serum cholesterol | 40 | 1.16 | 1.08 1.25 | 1.08 | 0.98 1.20 |
| Urine protein | 1 | 1.22 | 0.91 1.63 | 0.76 | 0.49 1.18 |
| Baldness | 1 | 1.15 | 0.98 1.34 | 0.90 | 0.72 1.12 |
| Corneal arcus | 1 | 1.04 | 0.82 1.32 | 1.29 | 0.98 1.70 |
| Xanthelasma | 1 | 2.07 | 1.12 3.82 | 3.31 | 1.81 6.06 |
| Diabetes | 1 | 1.21 | 0.77 1.88 | 1.14 | 0.96 20.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menotti, A.; Puddu, P.E.; Piras, P. Cardiovascular Risk Factors Predicting Cardiovascular and Cancer Deaths in a Middle-Aged Population Followed-Up for 61 Years until Extinction. J. Cardiovasc. Dev. Dis. 2024, 11, 240. https://doi.org/10.3390/jcdd11080240
Menotti A, Puddu PE, Piras P. Cardiovascular Risk Factors Predicting Cardiovascular and Cancer Deaths in a Middle-Aged Population Followed-Up for 61 Years until Extinction. Journal of Cardiovascular Development and Disease. 2024; 11(8):240. https://doi.org/10.3390/jcdd11080240
Chicago/Turabian StyleMenotti, Alessandro, Paolo Emilio Puddu, and Paolo Piras. 2024. "Cardiovascular Risk Factors Predicting Cardiovascular and Cancer Deaths in a Middle-Aged Population Followed-Up for 61 Years until Extinction" Journal of Cardiovascular Development and Disease 11, no. 8: 240. https://doi.org/10.3390/jcdd11080240







