The Functional Significance of Cardiac Looping: Comparative Embryology, Anatomy, and Physiology of the Looped Design of Vertebrate Hearts
Abstract
1. Introduction
2. Overview of the Early Morphogenesis of Vertebrate Hearts
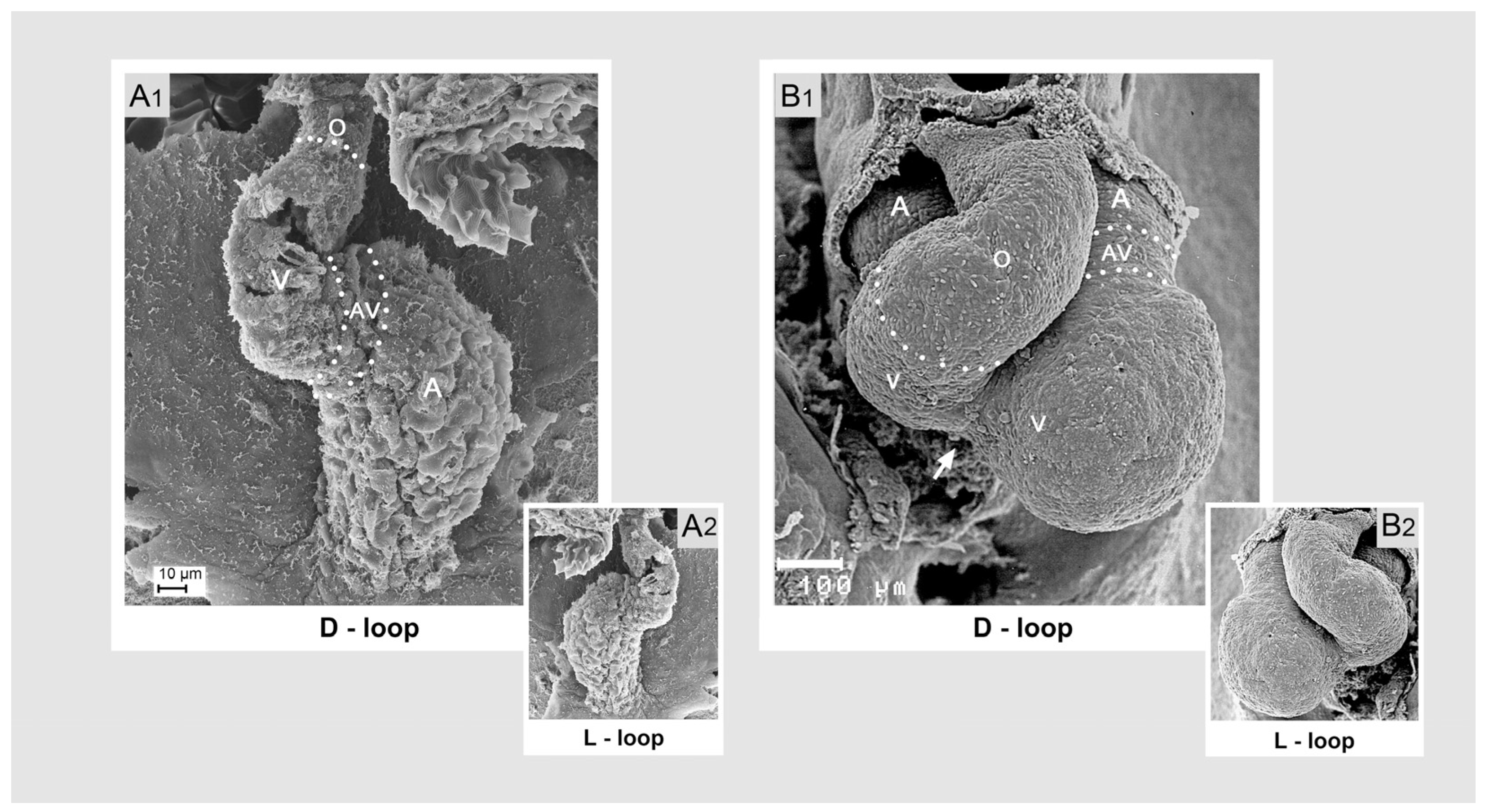
Comparative Aspects of Cardiac Looping among Vertebrates
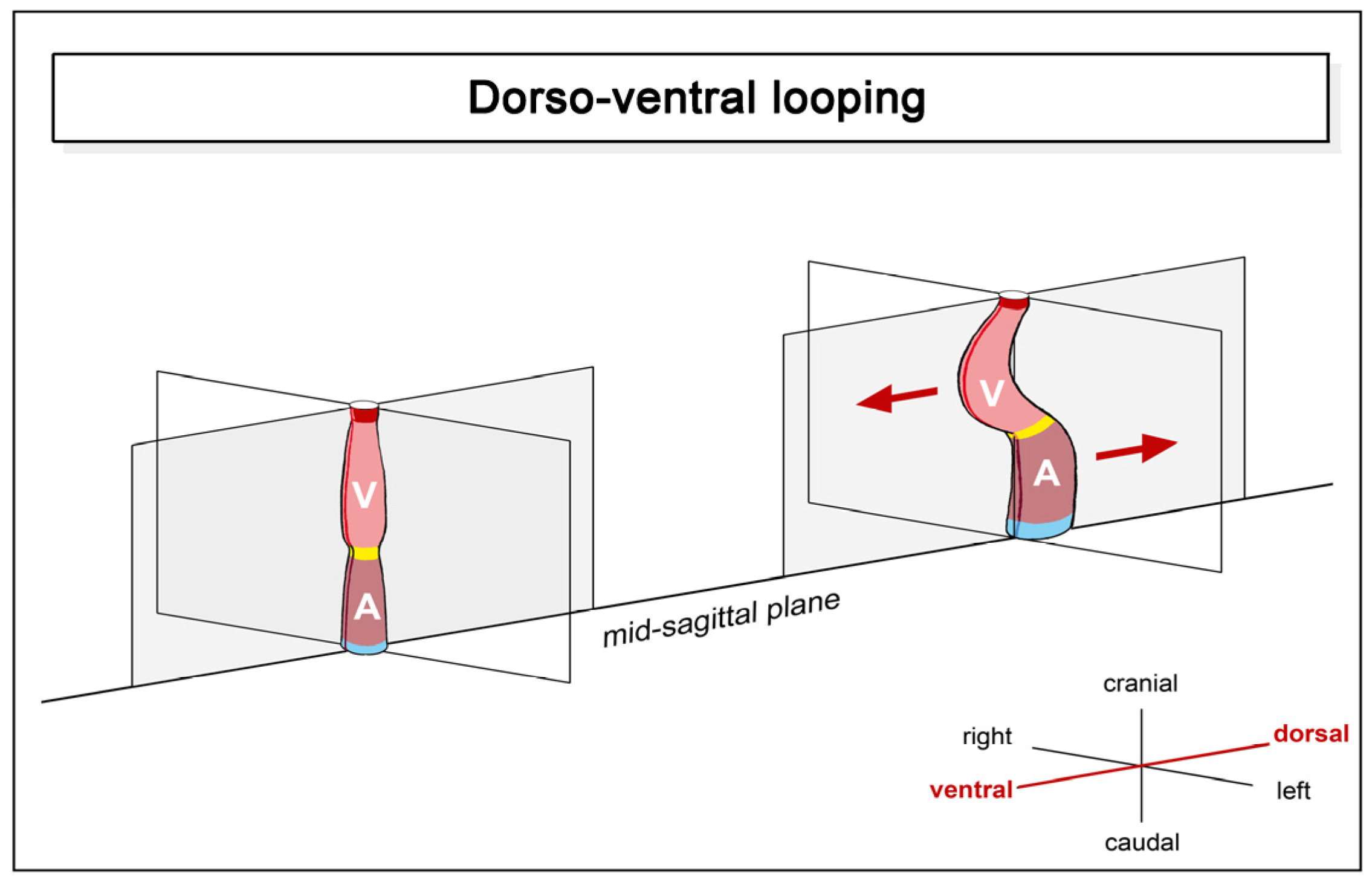
- (1)
- positional and morphological changes along the original dorsoventral heart axis.
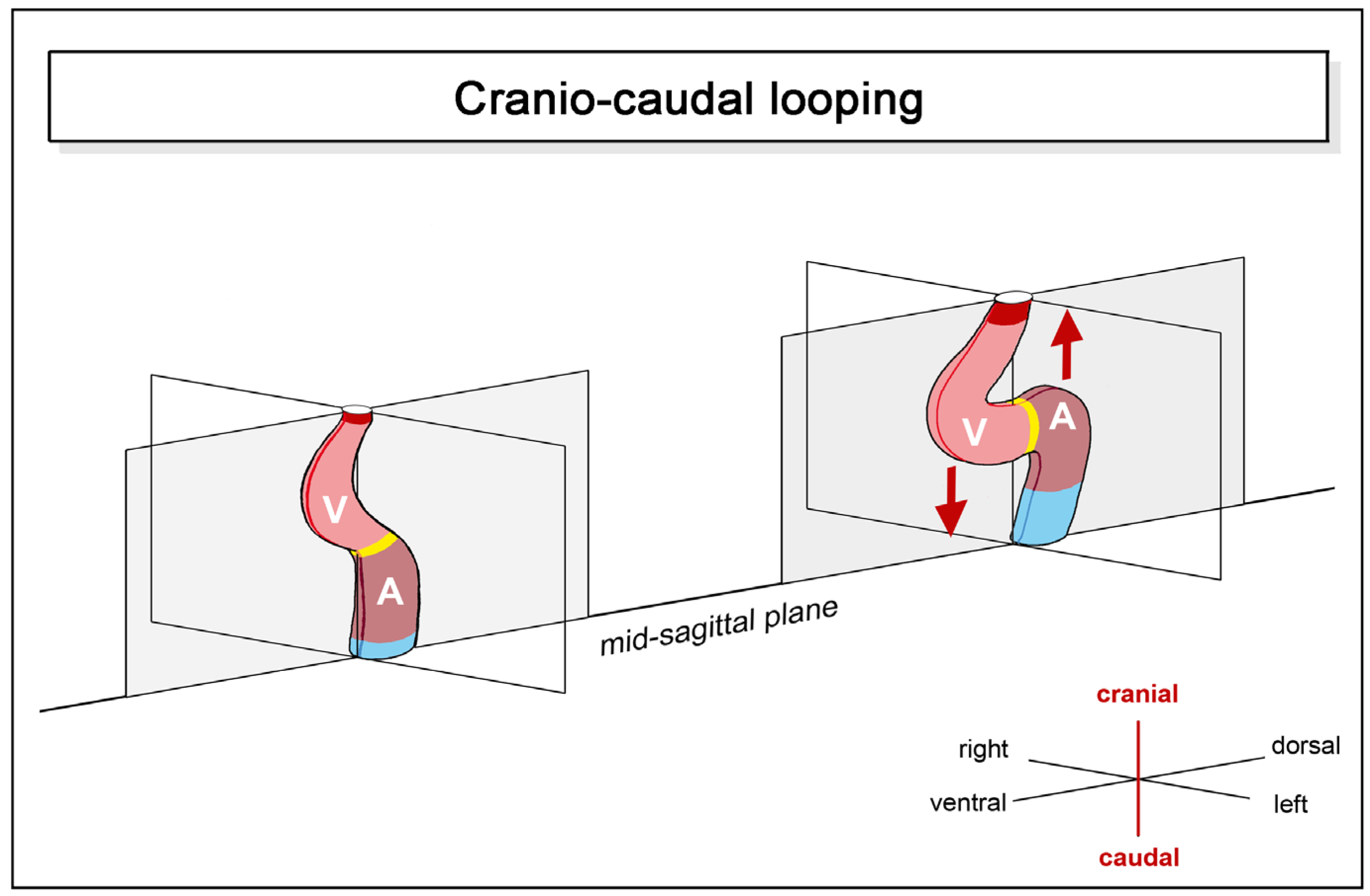
- (2)
- positional and morphological changes along the craniocaudal body axis.
- (3)
- positional and morphological changes along the so-called left-right body axis, which lead to a bilaterally asymmetric heart shape. This component may be named “lateral looping” or “chiral looping”.
- (4)
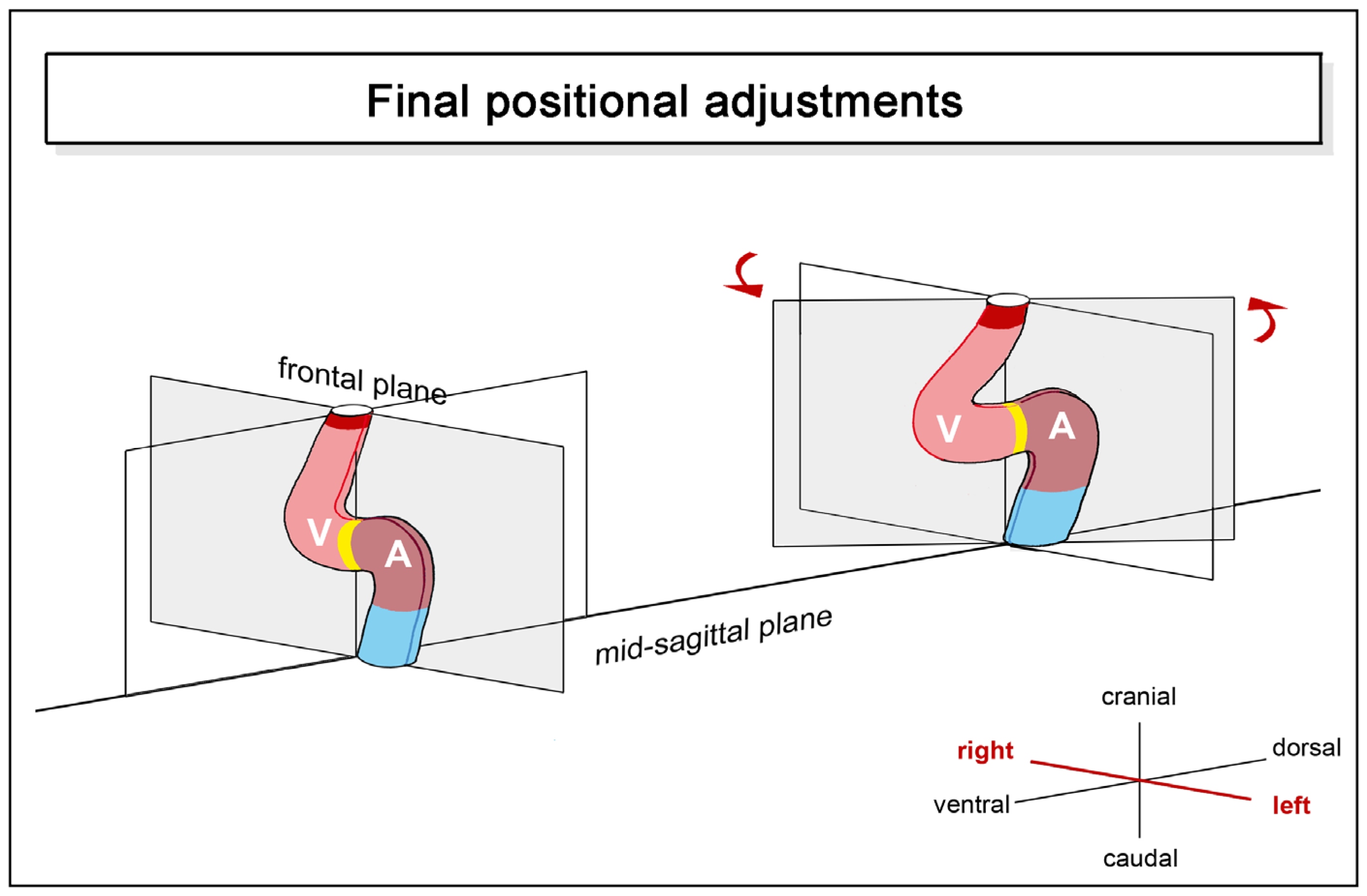
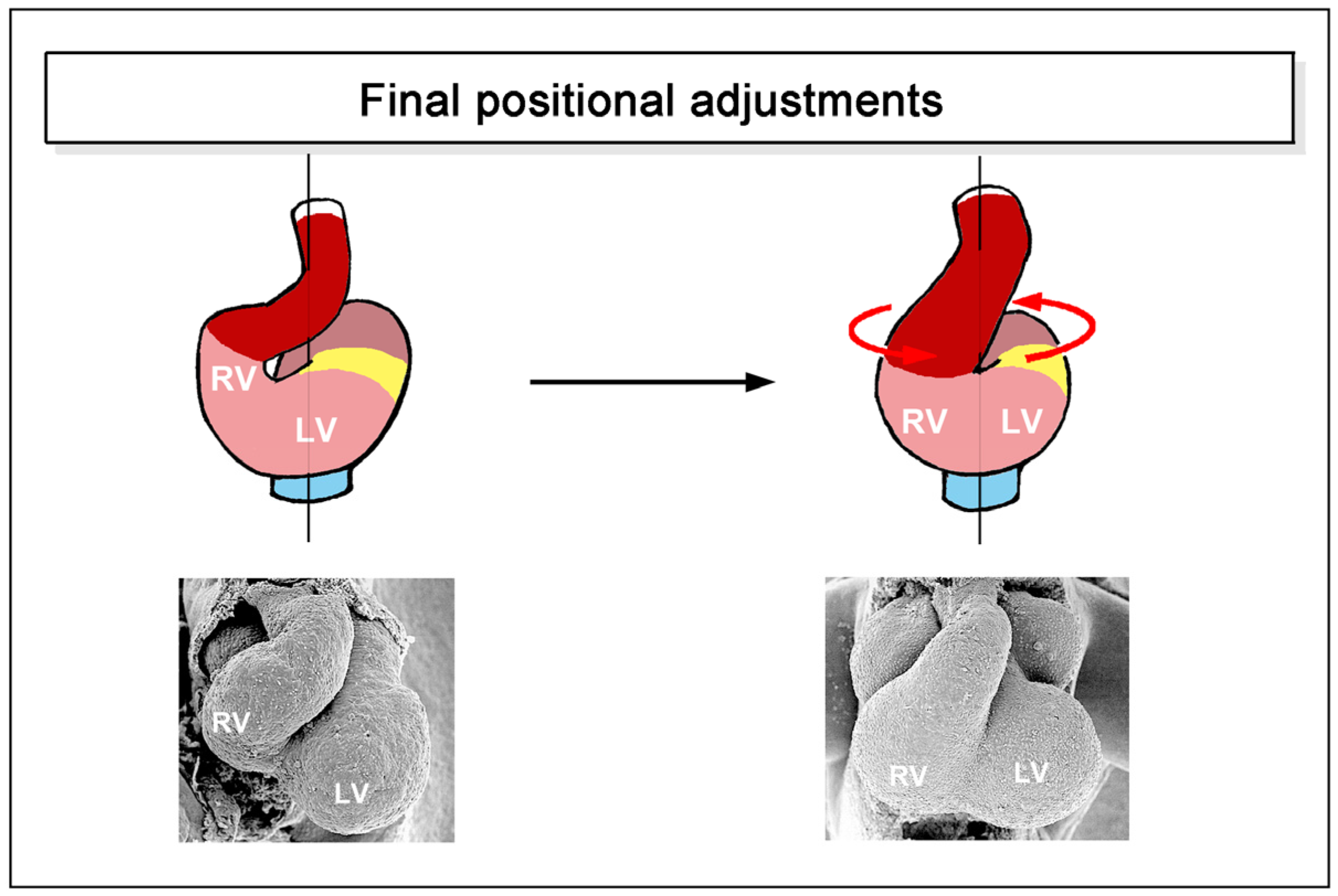
- positional and morphological changes that reduce the degree of lateral/chiral looping reached earlier stages of cardiac looping. This component may be named “final positional adjustments”.
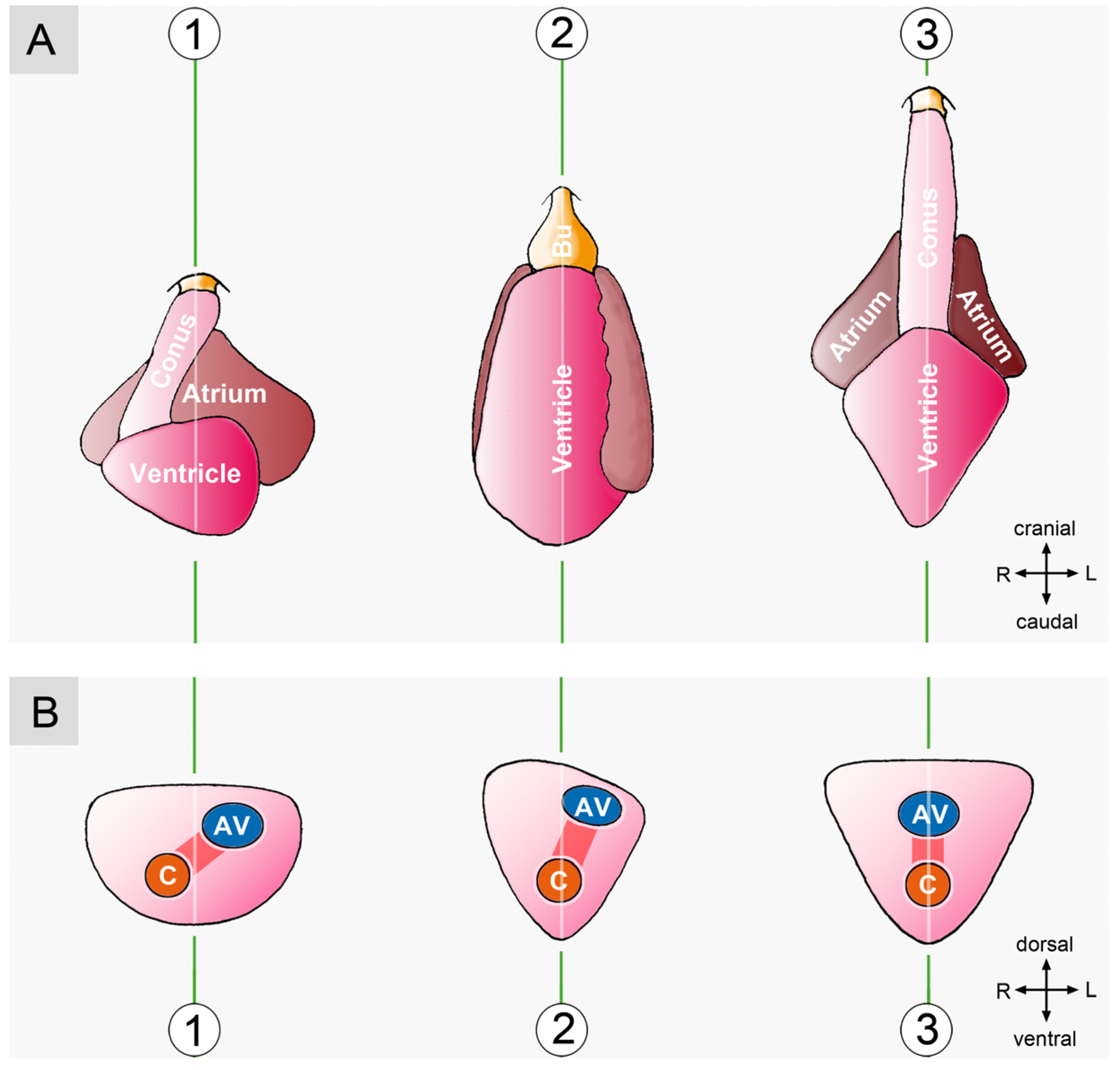
3. Comparative Anatomy of the Looped Design of Mature Vertebrate Hearts
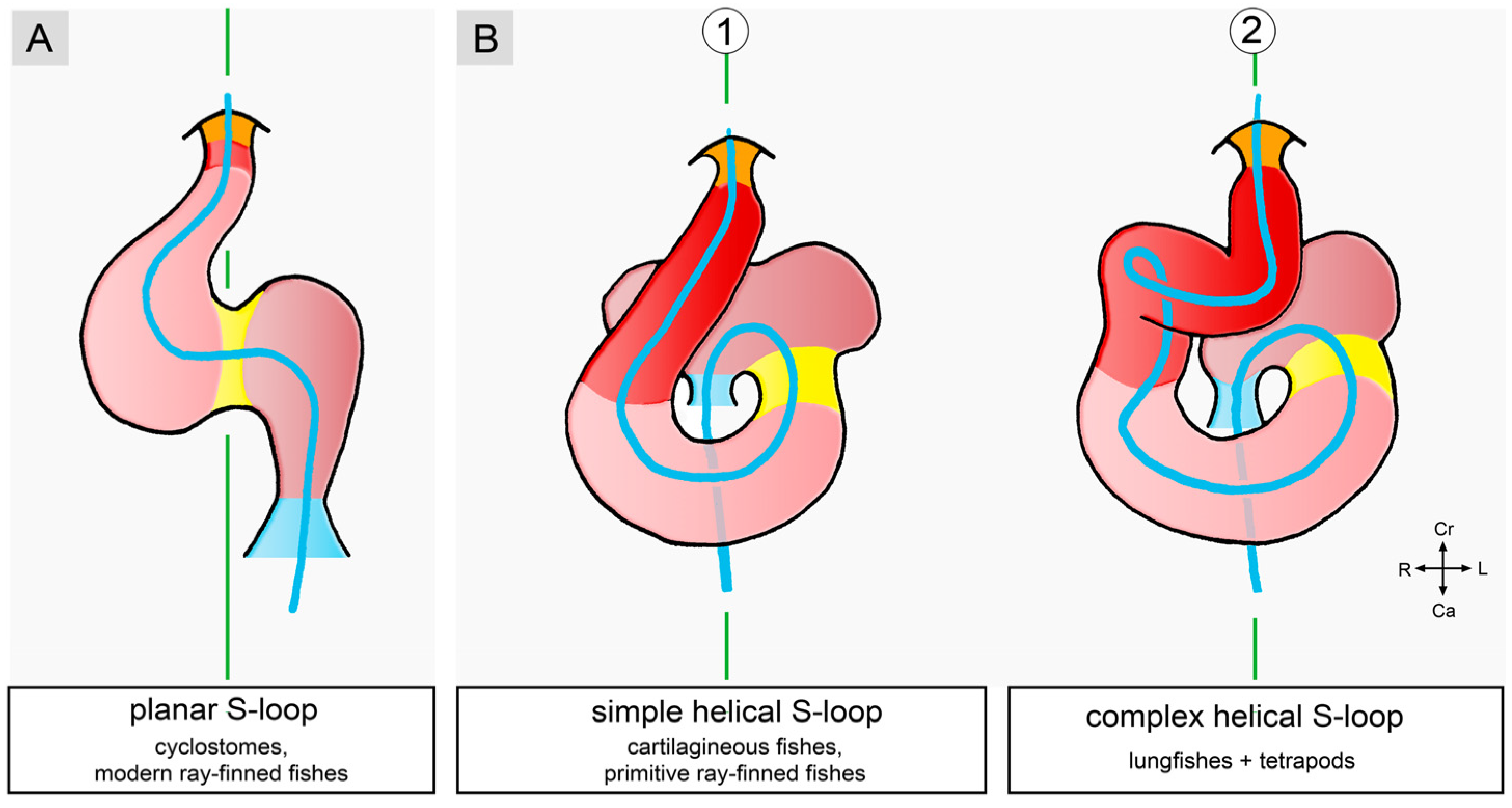
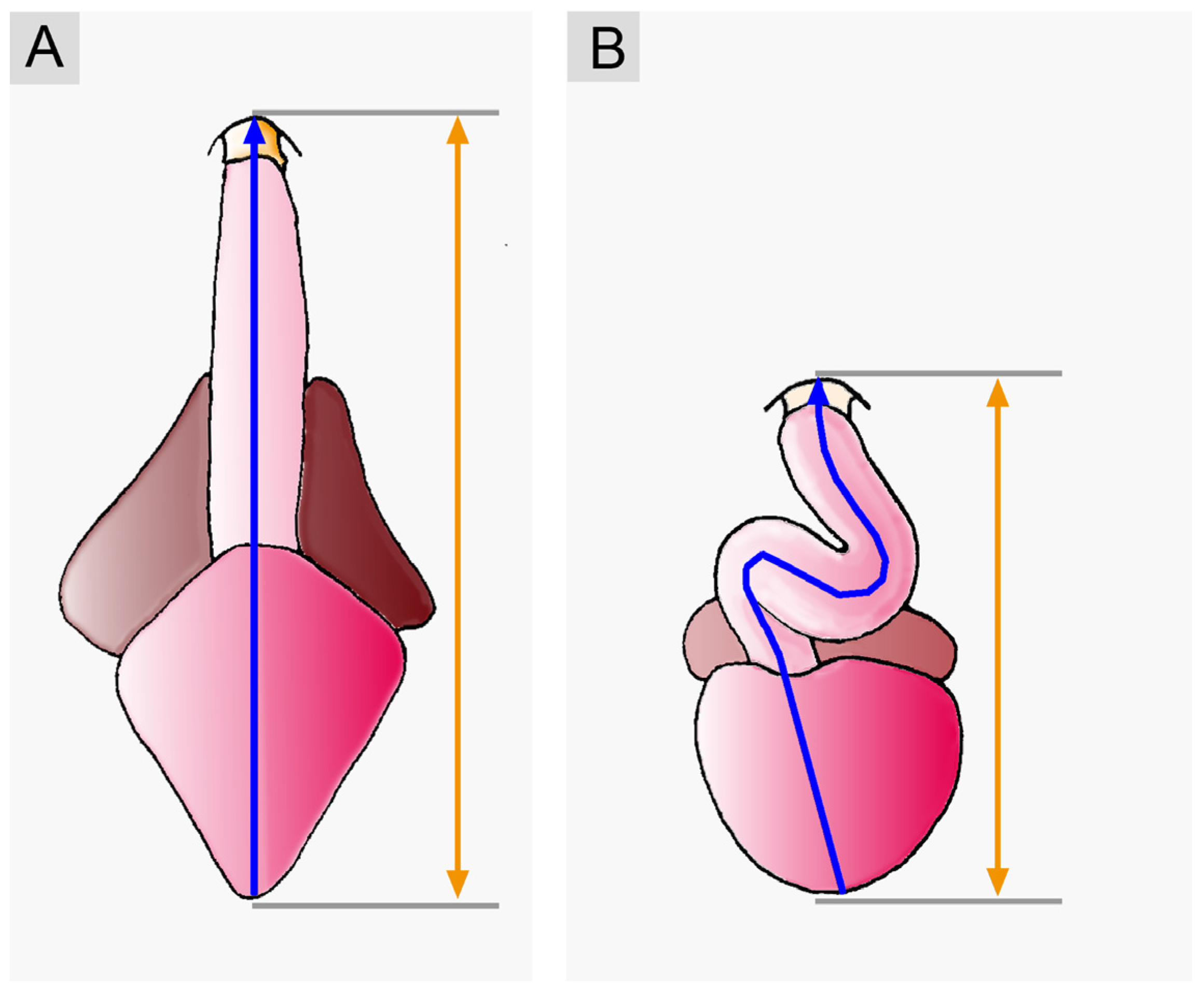
3.1. Comparative Anatomy of the Sigmoid Routing of the Flow Path(s) of Mature Vertebrate Hearts
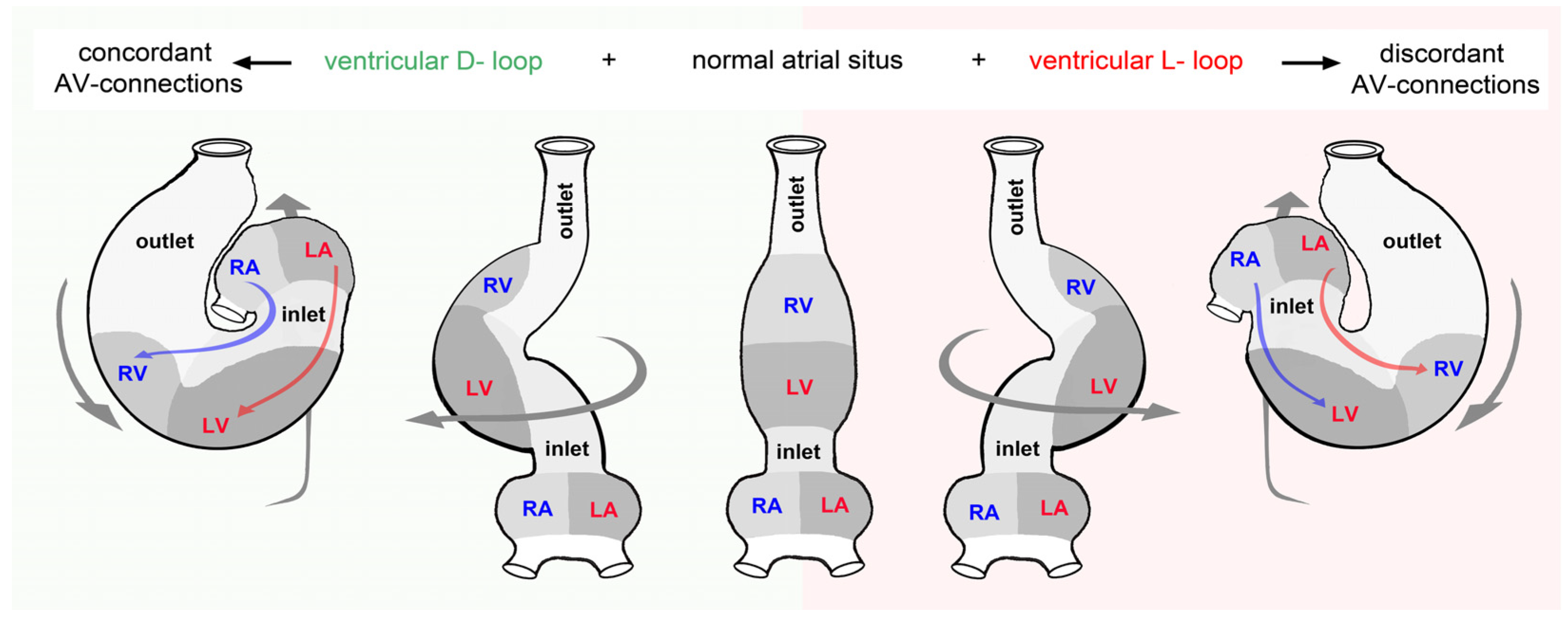
3.2. Comparative Anatomy of the Bilaterally Asymmetric (Chiral) Routing of the Flow Path(s) of Mature Vertebrate Hearts
3.2.1. Hearts with a Visually Conspicuous Bilateral Asymmetry
3.2.2. Hearts with a Visually Obscured Bilateral Asymmetry
3.2.3. Hearts with a Nearly Perfect Bilateral Symmetry
4. The Functional Significance of the Looped Design of Embryonic and Mature Vertebrate Hearts
4.1. A Closer View on the Functional Design of Embryonic Vertebrate Hearts
4.2. A Closer View on the Functional Design of Mature Vertebrate Hearts
4.2.1. Looping Dictates the Alignments and Separations of the Systemic and Pulmonary Flow Paths
4.2.2. Looping Might Optimize the Cardiac Pumping Efficiency
4.2.3. A Critical Evaluation of the Proposed Functional Advantages of the Sigmoid Routing of the Cardiac Flow Path(s)
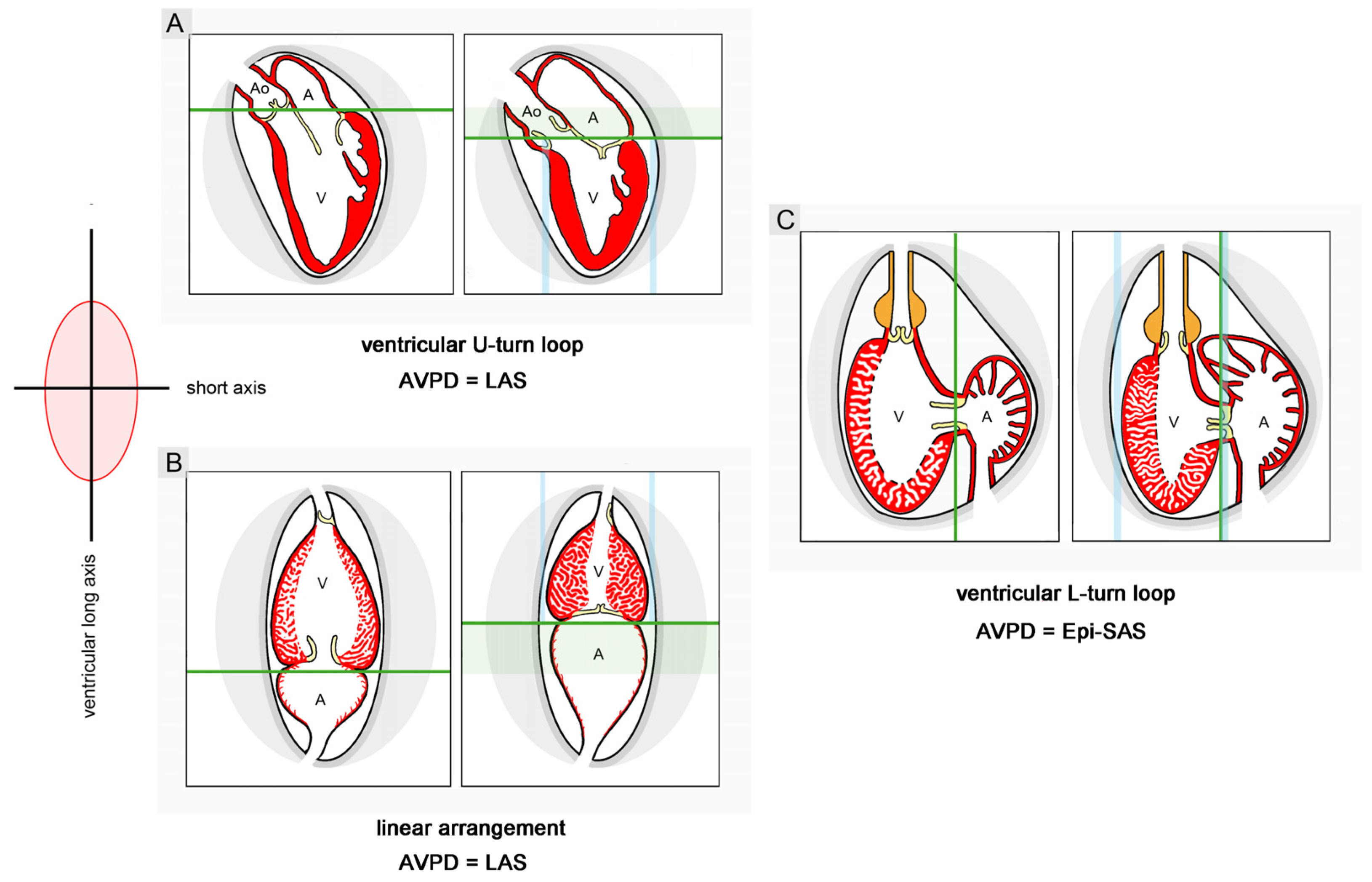
Does Looping Really Minimize the Loss of Momentum of the Inflowing Blood?
Does Looping Really Enhance Ventriculoatrial Coupling?
Implications for the Usage of Fishes in Cardiovascular Research
5. Summary and Conclusions
- (1)
- the looped configuration of the flow path of vertebrate hearts is roughly characterized by an s-shaped (sigmoid) component and a bilaterally asymmetric (chiral) component.
- (2)
- the fully looped heart tube of vertebrate embryos regularly shows sigmoid as well as chiral routing of its flow path.
- (3)
- data from physical pump models suggest that the looped design of valveless embryonic heart tubes might improve the pumping efficiency.
- (4)
- among the mature hearts of vertebrates, sigmoid and chiral routing of the flow path(s) is regularly found only in the multi-chambered hearts of lungfish and tetrapods. Here, the bilaterally asymmetric (chiral) anatomy is regarded as the main determinant of the alignment and functional or structural separation of the systemic and pulmonary flow paths.
- (5)
- among the two-chambered hearts of fish, only sigmoid routing of the flow path is a regular feature, while a bilaterally asymmetric (chiral) configuration is not a regular feature. Among fish, we can find species with bilaterally asymmetric hearts as well as species with bilaterally symmetric hearts.
- (6)
- the presence of bilateral symmetry in the mature hearts of some fishes seems to be the consequence of a process of back-rotation of the heart during the post-embryonic period of development.
- (7)
- the presence of a nearly perfect bilateral symmetry in the high-performance hearts of several active fish, suggests that chiral routing of the cardiac flow path may not significantly improve the pumping function of two-chambered hearts. If the pumping function of two-chambered hearts may profit from a looped design, such a benefit should be attributed to the sigmoid routing of the cardiac flow path, which is a feature found in the mature heart of all vertebrates.
- (8)
- the bilaterally asymmetric (chiral) anatomy of some fish hearts seems to represent no more than the solution to a packing problem.
- (9)
- it was frequently stated that the evolution of the vertebrate heart was characterized by an increase in the degree of sigmoid routing of the cardiac flow path(s). The lowest degree was ascribed to the hearts of basal vertebrates (jawless fish). A moderate degree was ascribed to the hearts of jawed fish, while high degrees were ascribed exclusively to the hearts of lungfish and tetrapods. Original data from fish, however, show that, among jawed fish, we can find a spectrum of various degrees of sigmoid routing of the cardiac flow path. This spectrum ranges from low degrees, resulting from an atrial position that is dorso-caudal to the ventricle, to high degrees, resulting from an atrial position that is dorso-cranial to the ventricle. Thus, a high degree of sigmoid routing of the cardiac flow path(s) is not exclusively found in the mature hearts of tetrapods.
- (10)
- it has been postulated that sigmoid routing of the cardiac flow path(s) may produce effects supporting the long-axis displacement of the AVP, while a linear routing of the cardiac flow path(s) may produce effects suppressing the long-axis displacement of the AVP, particularly in the exercising state. Data from snail hearts and physical models cast doubt on the validity of this hypothesis. The relation between the looped design and the pumping function of vertebrate hearts remains an enigma.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Von Baer, K.E. Untersuchungen Über die Entwickelungsgeschichte der Fische Nebst Einem Anhange Über die Schwimmblase; F.C.W. Vogel: Leipzig, Germany, 1835. [Google Scholar]
- Bischoff, T.L.W. Entwickelungsgeschichte des Säugethier- und Menscheneies; L. Voß: Leipzig, Germany, 1842. [Google Scholar]
- His, W. Anatomie Menschlicher Embryonen III; F.C.W. Vogel: Leipzig, Germany, 1885. [Google Scholar]
- Sobotta, J. Über die Entwickelung des Blutes, des Herzens und der grossen Gefässstämme der Salmoniden. Anat. Hefte 1902, 19, 579–688. [Google Scholar] [CrossRef]
- Bremer, J.L. Part I. An interpretation of the development of the heart. Part II. The left aorta of reptiles. Am. J. Anat. 1928, 42, 307–369. [Google Scholar] [CrossRef]
- Patten, B.M. The formation of the cardiac loop in the chick. Am. J. Anat. 1922, 30, 373–397. [Google Scholar] [CrossRef]
- Senior, H.D. The development of the heart in shad (Alosa Sapadissima, Wilson). Am. J. Anat. 1909, 9, 211–262. [Google Scholar] [CrossRef]
- Tschermak, A. Physiologische Untersuchungen am embryonalen Fischherzen. Sitzgsber Akad Wiss Wien Math-Naturwiss Kl 3 1909, 118, 17. [Google Scholar]
- Robertson, J.I. The development of the heart and vascular system of Lepidosiren paradoxa. J. Cell Sci. 1913, 59, 53–132. [Google Scholar] [CrossRef]
- Tschermak, A. Über das Verhalten des embryonalen Fischherzens gegenüber dem konstanten Strom. Z. Exper. Med. 1929, 68, 452–474. [Google Scholar] [CrossRef]
- Icardo, J.M.; Guerrero, A.; Durán, A.C.; Domezain, A.; Colvee, E.; Sana-Coma, V. The development of the sturgeon heart. Anat. Embryol. 2004, 208, 439–449. [Google Scholar] [CrossRef]
- Rodríguez, C.; Sans-Coma, V.; Grimes, A.C.; Fernández, B.; Arqué, J.M.; Durán, A.C. Embryonic development of the bulbus arteriosus of the primitive heart of jawed vertebrates. Zool. Anz. 2013, 252, 359–366. [Google Scholar] [CrossRef]
- Cooke, J. Developmental mechanism and evolutionary origin of vertebrate left/right asymmetries. Biol. Rev. 2004, 79, 377–407. [Google Scholar] [CrossRef]
- Palmer, A.R. Symmetry breaking and the evolution of development. Science 2004, 306, 828–833. [Google Scholar] [CrossRef]
- Ramsdell, A.F. Left-right asymmetry and congenital cardiac defects: Getting to the heart of the matter in vertebrate left-right axis determination. Dev. Biol. 2005, 288, 1–20. [Google Scholar] [CrossRef]
- Stainier, D.Y.R.; Fishman, M.C. The zebrafish as a model system to study cardiovascular development. Trends Cardiovasc. Med. 1994, 4, 207–212. [Google Scholar] [CrossRef]
- Smith, K.A.; Uribe, V. Getting to the heart of left-right asymmetry: Contributions from the zebrafish model. J. Cardiovasc. Dev. Dis. 2021, 8, 64. [Google Scholar] [CrossRef]
- Lombardo, V.A.; Heise, M.; Moghtadaei, M.; Bornhorst, D.; Männer, J.; Abdelilah-Seyfried, S. Morphogenetic control of zebrafish cardia looping by Bmp signaling. Development 2019, 146, dev180091. [Google Scholar] [CrossRef]
- Tessadori, F.; Tsingos, E.; Colizzi, E.S.; Kruse, F.; van den Brink, S.C.; van den Boogaard, M.; Christoffels, V.M.; Merks, R.M.H.; Bakkers, J. Twisting of the zebrafish heart tube during cardiac looping is a tbx5-dependent and tissue-intrinsic process. eLife 2021, 10, e61733. [Google Scholar] [CrossRef]
- Gabriel, G.C.; Lo, C.W. Left-right patterning in congenital heart disease and beyond. Am. J. Genet. C Semin. Med. Genet. 2020, 184, 90–96. [Google Scholar] [CrossRef]
- Nakano, H.; Fajardo, V.M.; Nakano, A. The role of glucose in physiological and pathological heart formation. Dev. Biol. 2021, 475, 222–233. [Google Scholar] [CrossRef]
- Rahman, T.; Zhang, H.; Fan, J.; Wan, Q. Cell chirality in cardiovascular development and disease. APL Bioeng. 2020, 4, 031503. [Google Scholar] [CrossRef]
- Desgrange, A.; Le Garrec, J.F.; Meilac, S.M. Left-right asymmetry in heart development and disease: Forming the right loop. Development 2018, 145, dev162776. [Google Scholar] [CrossRef]
- Kilner, P.J.; Yang, G.Z.; Wilkes, A.J.; Mohladdin, R.H.; Firmin, D.N.; Yacoub, M.H. Asymmetric redirection of flow through the heart. Nature 2000, 404, 759–761. [Google Scholar] [CrossRef]
- Kilner, P.J.; Yang, G.Z.; Firmin, D.N. Morphodynamics of flow through sinuous curvatures of the heart. Biorheology 2002, 39, 409–417. [Google Scholar]
- Amodeo, A.; Olivero, M.; Versacci, P.; Marino, B. Spiral shapes in heart and shells: When form and function do matter. Eur. J. Cardio-Thorac. Surg. 2012, 41, 473–475. [Google Scholar] [CrossRef]
- Hiermeier, F.; Männer, J. Kinking and torsion can significantly improve the efficiency of valveless pumping in periodically compressed tubular conduits. Implications for understanding of the form-function relationship of embryonic heart tubes. J. Cardiovasc. Dev. Dis. 2017, 4, 19. [Google Scholar] [CrossRef]
- Sherrid, M.V.; Männer, J.; Swistel, D.G.; Olivotto, I.; Halpern, D.G. On the cardiac loop and its failing: Left ventricular outflow tract obstruction. J. Am. Heart Ass 2020, 9, e014857. [Google Scholar] [CrossRef]
- Männer, J. When does the human embryonic heart start beating? A review of contemporary and historical sources of knowledge about the onset of blood circulation in man. J. Cardiovasc. Dev. Dis. 2022, 9, 187. [Google Scholar] [CrossRef]
- Simões-Costa, M.S.; Vasconcelos, M.; Sampaio, A.C.; Cravo, R.M.; Linhares, V.L.; Hochgreb, T.; Yan, C.Y.I.; Davidson, B.; Xavier-Neto, J. The evolutionary origin of cardiac chambers. Dev. Biol. 2005, 277, 1–15. [Google Scholar] [CrossRef]
- Männer, J.; Wessel, A.; Yelbuz, T.M. How does the tubular embryonic heart work? Looking for the physical mechanism driving unidirectional blood flow in the valveless embryonic heart tube. Dev. Dyn. 2010, 239, 1035–1046. [Google Scholar] [CrossRef]
- Abu Issa, R.; Kirby, M.L. Heart field: From mesoderm to heart tube. Ann. Rev. Cell Dev. Biol. 2007, 23, 45–68. [Google Scholar] [CrossRef]
- Meilhac, S.M.; Lescroart, F.; Blanpain, C.; Buckingham, M.E. Cardiac cell lineages that form the heart. Cold Spring Harb. Perspect. Med. 2014, 4, a013888. [Google Scholar] [CrossRef]
- Männer, J. The anatomy of cardiac looping: A step towards the understanding of the morphogenesis of several forms of congenital cardiac malformations. Clin. Anat. 2009, 22, 21–35. [Google Scholar] [CrossRef]
- Männer, J. Cardiac looping in the chick embryo: A morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat. Rec. 2000, 259, 248–262. [Google Scholar] [CrossRef]
- Männer, J. On rotation, torsion, lateralization, and handedness of the embryonic heart loop: New insights from a simulation model for the heart loop of chick embryos. Anat. Rec. A 2003, 278A, 481–492. [Google Scholar] [CrossRef]
- Bayraktar, M.; Männer, J. Cardiac looping may be driven by compressive loads resulting from unequal growth of the heart and pericardial cavity. Observations on a physical simulation model. Front. Physiol. 2014, 5, 112. [Google Scholar] [CrossRef]
- Le Garrec, J.-F.; Dominguez, J.N.; Desgrange, A.; Ivanovitch, K.D.; Raphael, E.; Bangham, A.; Torres, M.; Coen, E.; Mohun, T.; Meilhac, S.M. A predictive model of asymmetric morphogenesis from 3D reconstructions of mouse heart looping dynamics. eLife 2017, 6, e28951. [Google Scholar] [CrossRef]
- Burggren, W.W. Cardiac design in lower vertebrates: What can phylogeny reveal about ontogeny? Experentia 1988, 44, 919–930. [Google Scholar] [CrossRef]
- Bettex, D.A.; Prêtre, R.; Chassot, P.G. Is our heart a well-designed pump? The heart along evolution. Eur. Heart J. 2014, 35, 2322–2332. [Google Scholar] [CrossRef]
- Männer, J. On the form problem of embryonic heart loops, its geometrical solutions, and a new biophysical concept of cardiac looping. Ann. Anat. 2013, 195, 312–323. [Google Scholar] [CrossRef]
- Omer, S.O.; Alhabshan, F.M.; Jijeh, A.M.Z.; Caimbon, N.C.; Enriquez, C.C.; Männer, J.; Yelbuz, T.M. Is transposition of the great arteries associated with shortening of the intrapericardial portions of the great arterial trunks? An echocardiographic analysis on newborn infants with simple transposition of the great arteries to explore an animal model.based hypothesis on human beings. J. Am. Heart Assoc. 2021, 10, e019334. [Google Scholar] [CrossRef]
- Ziermann, J.M.; Freitas, R.; Diogo, R. Muscle development in the shark Scyliorhinus canicula: Implications for the evolution of the gnathostome head and paired appendage musculature. Front. Zool. 2017, 14, 31. [Google Scholar] [CrossRef]
- Kunz, Y. Morphologische Studien über die embryonale und postembryonale Entwicklung bei Teleostiern mit besonderer Berücksichtigung des Dottersystems und der Leber. Rev. Suisse Zool. 1964, 71, 445–525. [Google Scholar] [CrossRef]
- Kunz-Ramsay, Y. Developmental Biology of Teleost Fishes; Springer: New York, NY, USA, 2013. [Google Scholar]
- Singelman, C.; Holtzman, N.G. Analysis of postembryonic heart development and maturation in the zebrafish Danio rerio. Dev. Dyn. 2012, 241, 1993–2004. [Google Scholar] [CrossRef]
- Pernkopf, E.; Wirtinger, W. Die Transposition der Herzostien—Ein Versuch der Erklärung dieser Erscheinung. I. Teil. Die Pheronomie der Herzentwicklung. Z. Anat. Entwickl. Gesch. 1933, 100, 563–711. [Google Scholar] [CrossRef]
- Icardo, J.M.; Colvee, E.; Schorna, S.; Lauriano, E.R.; Fudge, D.S.; Glover, C.N.; Zaccone, G. Morphological analysis of the hagfish heart I: The ventricle, the arterial connection and the ventral aorta. J. Morphol. 2015, 277, 326–340. [Google Scholar] [CrossRef]
- Icardo, J.M. Heart morphology and anatomy. In The Cardiovascular System: Morphology, Control and Function. Fish Physiology Vol. 36 Part A; Gamperl, A.K., Gillis, T.E., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–54. [Google Scholar]
- Muramatsu, B.; Suzuki, D.G.; Suzuki, M.; Higashiyama, H. Gross anatomy of the Pacific hagfish, Eptatretus burgeri, with special reference to the coelomic viscera. Anat. Rec. 2024, 307, 155–171. [Google Scholar] [CrossRef]
- Santer, R.M. Morphology and innervation of the fish heart. In Advances in Anatomy, Embryology and Cell Biology; Springer: Berlin/Heidelberg, Germany, 1985; Volume 89. [Google Scholar]
- Richardson, M.K.; Admiraal, J.; Wright, G.M. Developmental anatomy of lampreys. Biol. Rev. 2010, 85, 1–33. [Google Scholar] [CrossRef]
- Anthony, J.; Millot, J.; Robineau, D. Le cœur et l’aorte ventrale de Latimeria chalumnae (Poisson coelacanthidé). CR Acad. Sci. 1965, 261, 223–226. [Google Scholar]
- Millot, J.; Anthony, J.; Robineau, D. Anatomie de Latimeria chalumnae. Apparell digestif—Apparel respiratoire—Apparel urogenital—Glandes endocrines—Apparel circulatoire—Teguments—Écailles—Conclusion générales. CNRS 1978, 3, 198. [Google Scholar]
- Gregory, J.A.; Graham, J.B.; Cech, J.J., Jr.; Dalton, N.; Michaels, J.; Lai, N.C. Pericardial and peritoneal canal relationships to cardiac function in the white sturgeon (Acipenser transmontanus). Comp. Biochem. Physiol. A 2004, 138, 203–213. [Google Scholar] [CrossRef]
- Boas, J.E.V. Über Herz und Arterienbogen bei Ceratodus und Protopterus. Morphol. JB 1880, 6, 321–354. [Google Scholar]
- Home, E., XXVII. Additions to an account of the anatomy of Squalus maximus, contained in a former paper; with observations on the structure of the branchial artery. Phil Trans. R. Soc. 1813, 103, 227–241. [Google Scholar]
- Liem, K.F. Tetrapod parallelisms and other features in the functional morphology of the blood vascular system of Fluta alba Zuiew (pisces: Teleostei). J. Morphol. 1961, 108, 131–143. [Google Scholar] [CrossRef]
- Lai, N.C.; Graham, J.B.; Dalton, N.; Shabetai, R.; Bhargava, V. Echocardiographic and hemodynamic determinations of the ventricular filling pattern in some teleost fishes. Physiol. Zool. 1998, 71, 157–167. [Google Scholar] [CrossRef]
- Carlsson, M.; Ugander, M.; Mosen, H.; Buhre, T.; Arheden, H. Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy, athletes, and patients with dilated cardiomyopathy. AJP Heart Circ. Physiol. 2007, 292, H1452–H1459. [Google Scholar] [CrossRef]
- Ludwig, W. Das Rechts-Links-Problem im Tierreich und beim Menschen; Springer: Berlin/Heidelberg, Germany, 1932. [Google Scholar]
- Mercola, M. Embryological basis for cardiac left-right asymmetry. Sem. Cell Dev. Biol. 1999, 10, 109–116. [Google Scholar] [CrossRef]
- Kathiriya, I.S.; Srivastava, D. Left-right asymmetry and cardiac looping: Implications for cardiac development and congenital heart disease. Am. J. Med. Genet. (Semin. Med. Genet.) 2000, 97, 271–279. [Google Scholar] [CrossRef]
- Fishman, M.C.; Chien, K.R. Fashioning the vertebrate heart: Earliest embryonic decisions. Development 1999, 124, 2099–2117. [Google Scholar] [CrossRef]
- Blum, M.; Ott, T. Animal left-right asymmetry. Curr. Biol. 2018, 28, R293–R305. [Google Scholar] [CrossRef]
- Soares, K.D.A.; Toledo-Piza, M. Branching patterns of the afferent branchial arteries and their phylogenetic significance in rays (Batoidea). Sci. Rep. 2021, 11, 23236. [Google Scholar] [CrossRef]
- Van Mierop, L.H.S.; Kutsche, L.M. Comparative anatomy and embryology of the ventricles and arterial pole of the vertebrate heart. In Congenital Heart Disease. Causes and Processes; Nora, J.J., Takao, A., Eds.; Futura: New York, NY, USA, 1984; pp. 459–479. [Google Scholar]
- Zaccone, G.; Grimes, A.C.; Farrell, A.P.; Dabrowski, K.; Marino, F. Morphology, innervation and its phylogenetic step in the heart of the longnose gar Lepisosteus osseus. Acta Zool. 2012, 93, 381–389. [Google Scholar] [CrossRef]
- Santer, R.M.; Walker, M.G.; Emerson, L.; Witthames, P.R. On the morphology of the heart ventricle in marine teleost fish (Teleostei). Comp. Biochem. Physiol. Part A Physiol. 1983, 76A, 453–457. [Google Scholar] [CrossRef]
- Tiedemann, D.F. Anatomie des Fischherzens; Thomann: Landshut, Germany, 1809. [Google Scholar]
- Tota, B. Heart. In Sharks, Skated, and Rays. The Biology of Elasmobranch Fish; Hamlett, W.C., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1999; pp. 238–272, Chapter 10. [Google Scholar]
- Icardo, J.M.; Colvee, E.; Cerra, M.C.; Tota, B. Structure of the conus arteriosus of the sturgeon (Acipenser naccarii) heart I: The conus valves and subendocardium. Anat. Rec. 2002, 267, 17–27. [Google Scholar] [CrossRef]
- Szidon, J.; Lahiri, S.; Lev, M.; Fishman, A.P. Heart and circulation of the African lungfish. Circ. Res. 1969, 25, 23–38. [Google Scholar] [CrossRef]
- Hatta, A.P. Über die Entwicklung des Gefäßsystems des Neunauges, Lampetra mitsukurii Hatta. Zool. JB (Anat.) 1923, 44, 1–264. [Google Scholar]
- Piavis, G.W. Embryology. In Biology of Lampreys; Hardisty, M.W., Potter, I.C., Eds.; Academic Press: London, UK, 1971; pp. 361–400. [Google Scholar]
- Icardo, J.M. Conus arteriosus of the teleost heart: Dismissed, but not missed. Anat. Rec. 2006, 288A, 900–908. [Google Scholar] [CrossRef]
- Grimes, A.C.; Kirby, M.L. The outflow tract of the heart of fishes: Anatomy, genes, and evolution. J. Fish. Biol. 2009, 74, 983–1036. [Google Scholar] [CrossRef]
- Lorenzale, M.; Lopez-Unzu, M.A.; Rodríguez, C.; Fernández, B.; Durán, A.C.; Sans-Coma, V. The anatomical components of the cardiac outflow tract of chondrichthyans and actinopterygians. Biol. Rev. 2018, 93, 1604–1619. [Google Scholar] [CrossRef]
- Drechsel, J. Myokardauffaserungsversuche am Fisch- und Vogelherzen. Z. Anat. Entwickl. Gesch. 1935, 104, 403–423. [Google Scholar] [CrossRef]
- Victor, S.; Nayak, V.M.; Rajasingh, R. Evolution of the ventricles. Tex. Heart Inst. 1999, 26, 168–175. [Google Scholar]
- Schäfer, K.J. Untersuchungen zur Frage der bilateral asymmetrischen Morphologie des adulten Zebrafischherzens. Ph.D. Thesis, Georg-August-University, Goettingen, Germany, 2023. [Google Scholar]
- Sánchez-Quintana, D.; García-Martínez, V.; Climent, V.; Hurlé, J.M. Myocardial fiber and connective tissue architecture in the fish heart ventricle. J. Exp. Zool. 1996, 275, 112–124. [Google Scholar] [CrossRef]
- Ballard, W.W. Stages and rates of normal development in the holostean fish, Amia calva. J. Exp. Zool. 1986, 238, 337–354. [Google Scholar] [CrossRef]
- Clowes, C.; Boylan, M.G.S.; Ridge, L.A.; Barnes, E.; Wright, J.A.; Hentges, K.E. The functional diversity of essential genes required for mammalian cardiac development. Genesis 2014, 52, 713–737. [Google Scholar] [CrossRef]
- Sabin, F.R. Origin and development of the primitive vessels of the chick and of the pig. Contr Embryol. Carnegie Inst. 1917, 6, 61–124. [Google Scholar]
- Jones, E.A.V. The initiation of blood flow and flow induced events in the early vascular development. Semin. Cell Dev. Biol. 2011, 22, 1028–1035. [Google Scholar] [CrossRef]
- Kalogirou, S.; Malissovas, N.; Moro, E.; Argenton, F.; Stainier, D.Y.R.; Beis, D. Intracardiac flow dynamics regulate atrioventricular valve morphogenesis. Cardiovasc. Res. 2014, 104, 49–60. [Google Scholar] [CrossRef]
- Berndt, C.; Poschmann, G.; Stühler, K.; Holmgren, A.; Bräutigam, L. Zebrafish heart development is regulated via glutaredoxin 2 dependent migration and survival of neural crest cells. Redox Biol. 2014, 2, 673–678. [Google Scholar] [CrossRef]
- Forouhar, A.S.; Liebling, M.; Hickerson, A.; Nasiraei-Moghaddam, A.; Tsai, H.J.; Hove, J.R.; Fraser, S.E.; Dickinson, M.E.; Gharib, M. The embryonic vertebrate heart tube is a dynamic suction pump. Science 2006, 312, 751–753. [Google Scholar] [CrossRef]
- Bisphoric, N.H. Evolution of the heart from bacteria to man. Ann. N. Y. Acad. Sci. 2005, 1047, 13–29. [Google Scholar] [CrossRef]
- Johansen, K. Air-breathing fishes. Sci. Am. 1968, 219, 102–111. [Google Scholar] [CrossRef]
- Grant, R.P. The morphogenesis of corrected transposition and other anomalies of cardiac polarity. Circulation 1964, 29, 71–83. [Google Scholar] [CrossRef]
- Meredith, M.A.; Hutchins, G.M.; Moore, G.W. Role of the left interventricular sulcus in formation of the interventricular septum and crista supraventricularis in normal human cardiogenesis. Anat. Rec. 1979, 194, 417–428. [Google Scholar] [CrossRef]
- Moorman, A.F.M.; Christoffels, V.M. Cardiac chamber formation: Development, genes, and evolution. Physiol. Rev. 2003, 83, 1223–1267. [Google Scholar] [CrossRef]
- Lewis, F.T.; Abbott, M.E. Reversed torsion of the human heart. Anat. Rec. 1915, 9, 103–105. [Google Scholar] [CrossRef][Green Version]
- Lewis, F.T.; Abbott, M.E. Reversed torsion of the ventricular bend of the embryonic heart in the explanation of certain forms of cardiac anomaly. Bull. Int. Assoc. Med. Mus. 1916, 6, 111–115. [Google Scholar]
- De la Cruz, M.V.; Anselmi, G.; Cisneros, F.; Reinhold, M.; Portillo, B.; Espino-Vela, J. An embryologic explanation for the corrected transposition of the great vessels: Additional description of the main anatomic features of this malformation and its varieties. Am. Heart J. 1959, 57, 104–117. [Google Scholar] [CrossRef]
- Van Praagh, R.; Van Praagh, S.; Vlad, P.; Keith, J.D. Anatomic types of congenital dextrocardia: Diagnostic and embryologic implications. Am. J. Cardiol. 1964, 13, 510–531. [Google Scholar] [CrossRef]
- Anderson, R.H.; Webb, S.; Brown, N.A. Defective lateralisation in children with congenitally malformed hearts. Cardiol. Young 1998, 8, 512–531. [Google Scholar] [CrossRef]
- Stainier, D.Y.R.; Lee, R.K.; Fishman, M.C. Cardiovascular development in the zebrafish. I. Myocardial fate map and heart tube formation. Development 1993, 119, 31–40. [Google Scholar] [CrossRef]
- Yang, G.Z.; Merrifield, R.; Masood, S.; Kilner, P.J. Flow and myocardial interaction: An imaging perspective. Phil. Trans. R. Soc. B 2007, 362, 1329–1341. [Google Scholar] [CrossRef][Green Version]
- Sandblom, E.; Axelsson, M. The venous circulation: A piscine perspective. Comp. Biochem. Physiol. A 2007, 148, 785–801. [Google Scholar] [CrossRef]
- Farrell, A.P. From hagfish to tuna: A perspective on cardiac function in fish. Physiol. Zool. 1991, 64, 1137–1164. [Google Scholar] [CrossRef]
- Henein, M.Y.; Gibson, D.G. Normal long axis function. Heart 1999, 81, 111–113. [Google Scholar] [CrossRef]
- Carlsson, M.; Ugander, M.; Heiberg, E.; Arheden, H. The quantitative relationship between longitudinal and radial function in left, right, and total heart pumping in humans. AJP Heart Circ. Physiol. 2007, 293, H636–H644. [Google Scholar] [CrossRef]
- Steding-Ehrenborg, K.; Carlsson, M.; Stephensen, S.; Arheden, H. Atrial aspiration from pulmonary and caval veins is caused by ventricular contraction and secures 70% of the total stroke volume independent of resting heart rate and heart size. Clin. Physiol. Funct. Imaging 2013, 33, 233–240. [Google Scholar] [CrossRef]
- Arutunyan, A.H. Atrioventricular plane displacement is the sole mechanism of atrial and ventricular refill. AJP Heart Circ. Physiol. 2015, 308, H1317–H1320. [Google Scholar] [CrossRef]
- Watanabe, H.; Sugiura, S.; Hisada, T. The looped heart does not save energy by maintaining the momentum of blood flowing in the ventricle. AJP Heart Circ. Physiol. 2008, 294, H2191–H2196. [Google Scholar] [CrossRef]
- Kilner, P.J. Letter to the editor: “Postulated functional advantages of a looped as opposed to a linearly arranged heart”. AJP Heart Circ. Physiol. 2010, 298, H726. [Google Scholar] [CrossRef]
- Maksuti, E.; Bjällmark, A.; Broomé, M. Modelling the heart with the atrioventricular plane as a piston unit. Med. Eng. Phys. 2015, 37, 87–92. [Google Scholar] [CrossRef]
- Jordan, H.J.; Hirsch, G.C. Übungen aus der Vergleichenden Physiologie; Springer: Berlin/Heidelberg, Germany, 1927. [Google Scholar]
- Biering, P. Untersuchungen über das Kreislaufsystem bei den Weichtieren II. Z. Vergl. Physiol. 1929, 10, 465–484. [Google Scholar] [CrossRef]
- Schwartzkopff, J. Über die Leistung des isolierten Herzens der Weinbergschnecke (Helix pomatia L.) im künstlichen Kreislauf. Z. Vergl. Physiol. 1954, 36, 543–594. [Google Scholar] [CrossRef]
- Jones, H.D. Circulatory pressures in Helix pomatia. Comp. Biochem. Physiol. A 1971, 39, 289–295. [Google Scholar] [CrossRef]
- Von Skramlik, E. Weichtieren. In Ergebnisse der Biologie; Frisch, K., Ruhland, W., Koehler, O., Stubbe, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1941; Volume 18, pp. 88–286. [Google Scholar]
- Van Aardt, W.J.; Vosloo, A. Modifications to an optocardiographic method for measuring of heart rate in a range of invertebrate species. S. Afr. J. Zool. 1996, 32, 97–100. [Google Scholar]
- Nopp, H. Temperaturbezogene Regulationen des Sauerstoffverbrauchs und der Herzschlagrate bei einigen Pulmonaten. Z. Vergl. Physiol. 1965, 50, 641–659. [Google Scholar] [CrossRef]
- Wünnenberg, W. Diurnal rhythms of the heart rate in the snail Helix pomatia L. Comp. Biochem. Physiol. A 1991, 99, 415–417. [Google Scholar] [CrossRef]
- Romero, S.M.; Hoffmann, A. Heart rate and behavioral patterns of Megalobulimus sanctipauli (mollusca, gastropoda, pulmonata). Braz. J. Med. Biol. Res. 1991, 24, 223–227. [Google Scholar]
- Renwrantz, L.; Spielvogel, F. Heart rate and hemocyte number as stress indicators in disturbed hibernating vineyard snails, Helix pomatia. Comp. Biochem. Physiol. Part A 2011, 160, 467–473. [Google Scholar] [CrossRef]
- Noll, A. Untersuchungen über das Kreislaufsystem bei den Weichtieren III. Z. Vergl. Physiol. 1929, 10, 761–777. [Google Scholar] [CrossRef]
- Ugander, M.; Carlsson, M.; Arheden, H. Short axis epicardial volume change is a measure of cardiac left ventricular short axis function, which is independent of myocardial wall thickess. AJP Heart Circ. Physiol. 2010, 298, H530–H535. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Itazawa, Y. Differences in blood oxygen levels in the outflow vessels of the heart of an air-breathing fish, Channa argus: Do separate blood streams exist in a teleostean heart? J. Comp. Physiol. 1983, 149, 435–440. [Google Scholar] [CrossRef]
- Munshi, J.S.D.; Olson, K.R.; Roy, P.K.; Ghosh, U. Scanning electron microscopy of the heart of the climbing perch. J. Fish. Biol. 2001, 59, 1170–1180. [Google Scholar] [CrossRef]
- Park, M.H.; Imbrie-Moore, A.M.; Zhu, Y.; Wilkerson, R.J.; Wang, H.; Park, G.H.; Wu, C.A.; Pandya, P.K.; Mullis, D.M.; Marin-Cuartas, M.; et al. The critical biomechanics of aortomitral angle and systolic anterior motion: Engineering native ex vivo simulation. Ann. Biomed. Eng. 2023, 51, 794–805. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Männer, J. The Functional Significance of Cardiac Looping: Comparative Embryology, Anatomy, and Physiology of the Looped Design of Vertebrate Hearts. J. Cardiovasc. Dev. Dis. 2024, 11, 252. https://doi.org/10.3390/jcdd11080252
Männer J. The Functional Significance of Cardiac Looping: Comparative Embryology, Anatomy, and Physiology of the Looped Design of Vertebrate Hearts. Journal of Cardiovascular Development and Disease. 2024; 11(8):252. https://doi.org/10.3390/jcdd11080252
Chicago/Turabian StyleMänner, Jörg. 2024. "The Functional Significance of Cardiac Looping: Comparative Embryology, Anatomy, and Physiology of the Looped Design of Vertebrate Hearts" Journal of Cardiovascular Development and Disease 11, no. 8: 252. https://doi.org/10.3390/jcdd11080252
APA StyleMänner, J. (2024). The Functional Significance of Cardiac Looping: Comparative Embryology, Anatomy, and Physiology of the Looped Design of Vertebrate Hearts. Journal of Cardiovascular Development and Disease, 11(8), 252. https://doi.org/10.3390/jcdd11080252





